Myasthenia gravis (MG) is an antibody-mediated autoimmune disease characterised by fluctuating, fatigable muscle weakness, frequently involving bulbar and respiratory muscles. Considering the severity of respiratory involvement in MG, routine evaluation of respiratory function is essential. The aim of this study was to identify a useful clinical marker of respiratory involvement in patients with MG.
MethodsWe performed an observational study of patients with MG. All cases were evaluated with the single-breath count test, peak expiratory flow (PEF), a modified Medical Research Council dyspnoea scale (mMRC), and a neck strength assessment. The results of these parameters were correlated with forced vital capacity (FVC), maximal inspiratory pressure (MIP), and maximal expiratory pressure (MEP).
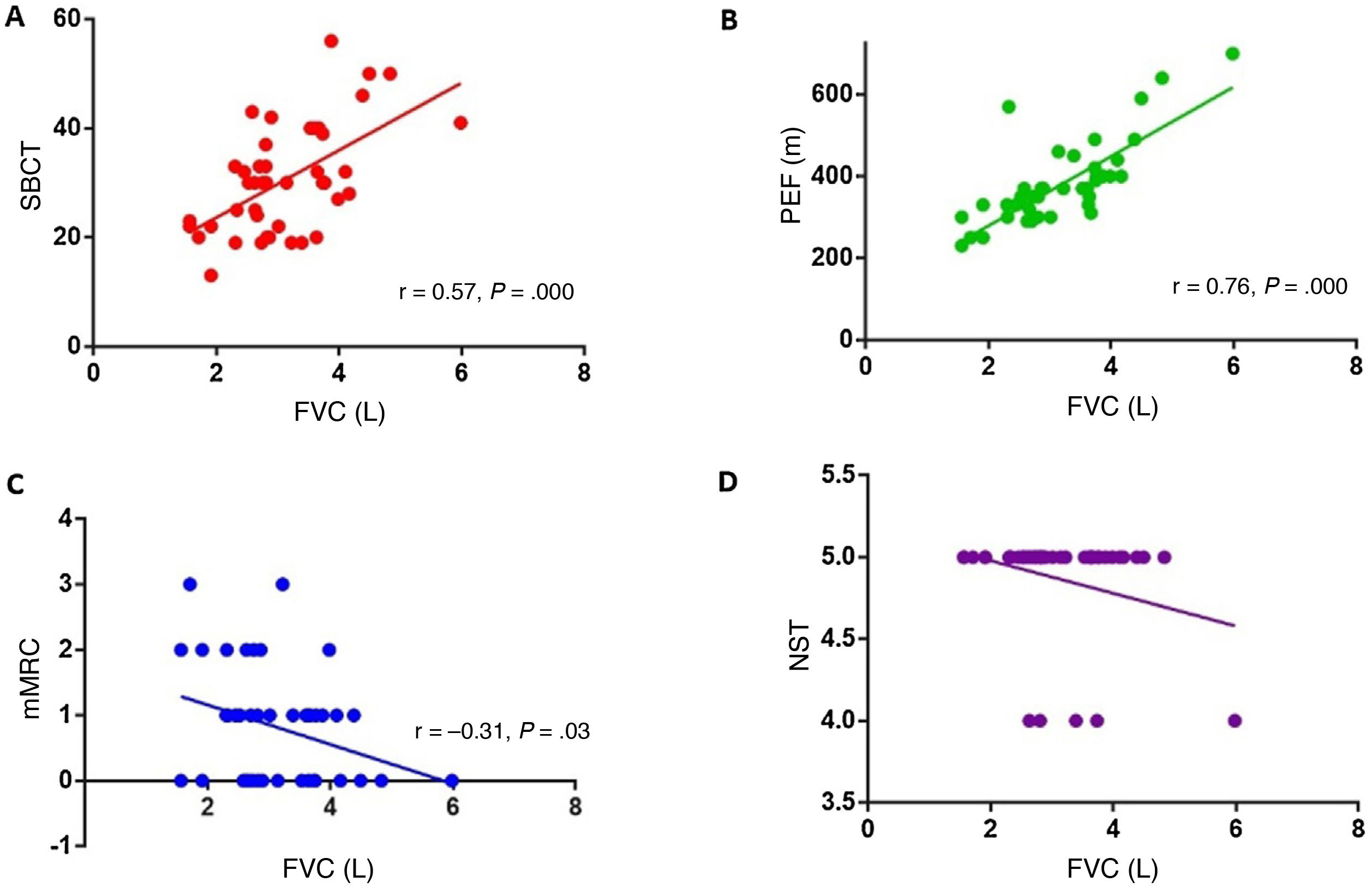
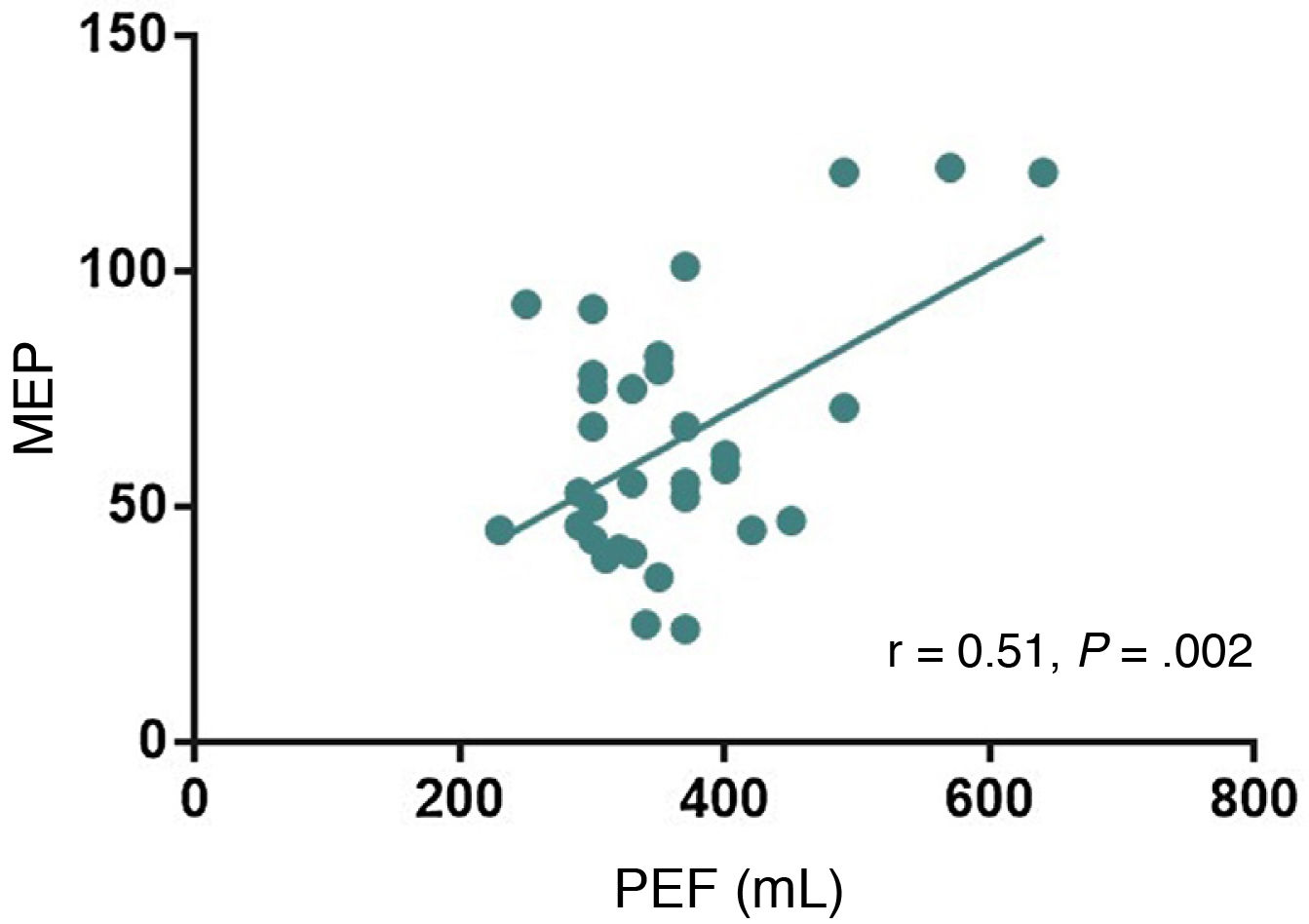
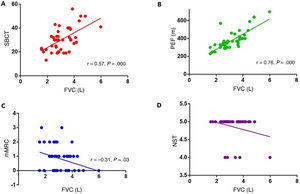
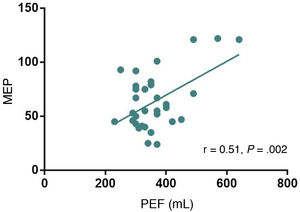
ResultsThe study included 45 patients with MG: 2 patients classified as grade I on the Myasthenia Gravis Foundation of America classification at the time of evaluation, 35 classified as grade II, 7 classified as grade III, and one classified as grade IV. Positive correlations were found between single-breath count test scores and FVC values (r = 0.57, P = .000), and between PEF and FVC values (r = 0.76, P = .000). Severity of dyspnoea according to the mMRC scale showed a negative correlation with FVC values (r = -0.31, P = .03). PEF also showed a significant correlation with MEP (r = 0.51, P = .002).
ConclusionsPEF, the single-breath count test, and the mMRC scale are useful measures for evaluating respiratory function in patients with MG.
La miastenia gravis (MG) es una enfermedad autoinmune mediada por anticuerpos. El cuadro clínico se caracteriza por debilidad muscular fluctuante y fatigable, con frecuente afectación de músculos fonodeglutorios y respiratorios. Dada la severidad que implica el compromiso respiratorio en la MG, su evaluación rutinaria es esencial.
Nuestro objetivo fue identificar un marcador semiológico útil en la pesquisa del compromiso respiratorio en pacientes con MG.
MétodosSe realizó un trabajo observacional en pacientes con diagnóstico de MG. Los pacientes fueron evaluados con test de cuenta máxima, pico flujo espiratorio (PEF), cuestionario de disnea modificado (mMRC) y valoración de fuerza del cuello. Los resultados de estos parámetros fueron correlacionados con la medición de CVF (capacidad vital forzada) y presiones bucales estáticas máximas (PiMáx y PeMáx).
ResultadosCuarenta y cinco pacientes con MG fueron incluidos, dos pacientes tenían MGFA grado I, 35 grado II, siete grado III y uno grado IV al momento de la evaluación. Se halló una correlación positiva entre el test de cuenta máxima y la CVF (r = 0,57, p = 0,000), y entre el PEF y la CVF (r = 0,76, p = 0,000). El grado de disnea, según el mMRC, mostró una correlación negativa con la CVF (r = -0,31 p = 0,03). A su vez, el PEF correlacionó con la PeMáx de forma positiva, estadísticamente significativa (r = 0,51, p = 0,002).
ConclusionesEl PEF, el test de cuenta máxima y el mMRC fueron útiles para evaluar la función respiratoria en pacientes con MG.
Myasthenia gravis (MG) is an antibody-mediated autoimmune disease affecting neuromuscular transmission. Most patients present anti–acetylcholine receptor (AChR) antibodies. A variable proportion of patients negative for these antibodies present seropositivity for other antibodies of confirmed pathogenicity, such as anti–muscle specific tyrosine kinase (MuSK) or anti–low density lipoprotein 4 antibodies. The action of these antibodies causes the destruction of the postsynaptic membrane, with simplification of the motor endplate and a reduction in the number of ion channels and receptors.1,2
The clinical picture is characterised by fluctuating muscle weakness and fatigability. The ocular, limb, and bulbar muscles are most frequently affected, causing such symptoms as ptosis, diplopia, limb weakness, dysarthria, and dysphagia.3,4 Such respiratory symptoms as dyspnoea or orthopnoea are also frequently reported by these patients.5,6
Bulbar and/or respiratory muscle weakness may lead to speech and swallowing alterations, abnormal accumulation of secretions in the airway, and/or inadequate lung expansion. This may result in such severe respiratory complications as atelectasis and aspiration pneumonia.7 An MG exacerbation causing respiratory failure requiring invasive or non-invasive ventilatory support is classed as a myasthenic crisis. Up to 20% of patients present myasthenic crises at some point over the progression of the disease.8,9
Given the severity of respiratory muscle weakness, routine evaluation of these muscles is crucial in all patients diagnosed with MG. Pulmonary function testing is the technique of choice for identifying respiratory disorders of neuromuscular origin. These tests enable measurement of forced vital capacity (FVC) and mouth pressures: maximal inspiratory pressure (MIP) and maximal expiratory pressure (MEP). MIP evaluates the strength of inspiratory muscles: mainly the diaphragm, but also the external intercostal and accessory muscles. MEP reflects the strength of expiratory muscles (internal intercostal and abdominal muscles), and is correlated with cough strength and removal of secretions. In healthy adults, FVC decreases by less than 10% when measured with the patient in a decubitus rather than a seated position; greater decreases reflect diaphragmatic weakness.10–12
Spirometry equipment is not always available at neurology consultations. As a result, simple, accessible manoeuvres enabling neurologists to estimate the degree of respiratory impairment in MG would be a valuable tool.
ObjectivesThis study aimed to evaluate the correlation between pulmonary function test parameters (FVC, MIP, and MEP) and a battery of selected semiological tests (single-breath count test, neck strength test, modified Medical Research Council dyspnoea scale [mMRC], peak expiratory flow [PEF]) in patients with MG.
Material and methodsWe conducted a cross-sectional correlational study in patients diagnosed with MG evaluated by the neuromuscular diseases and neurophysiology consultation of the neurology department at Hospital Ramos Mejía, in Buenos Aires, between 2016 and 2018.
The sample included patients older than 18 years with generalised MG. All patients gave written informed consent to participate and the study was approved by our hospital’s bioethics committee.
Diagnosis of MG was based on compatible clinical signs and positive results in at least one of the following diagnostic studies: 1) positive results for anti-AChR or anti-MuSK antibodies; 2) repetitive nerve stimulation test showing a decrement of at least 10% in motor potentials; 3) single-fibre electromyography study showing increased jitter; or 4) clear clinical response to acetylcholinesterase inhibitors.13
We excluded patients with concomitant respiratory disease, facial weakness preventing correct performance of the spirometry examination, or cognitive or psychiatric comorbidities preventing proper interpretation of instructions.
Patients who agreed to participate were initially assessed at the neuromuscular diseases clinic. We recorded data on sex, age, age of MG onset, and disease severity at the time of assessment, according to the Myasthenia Gravis Foundation of America (MGFA) clinical classification. Disease impact at the time of assessment was characterised with the Myasthenia Gravis Activities of Daily Living (MG-ADL) questionnaire, the Myasthenia Gravis Composite (MGC) score, and the subjective sensation of dyspnoea reported by patients according to the mMRC scale.14 We also administered the single-breath count test and measured PEF.
In the single-breath count test, patients were asked to take a deep breath and to count aloud at an average rate of 2 numbers per second, until they needed to take another breath. The highest value from 2 attempts was recorded (Appendix, video 1).
In the PEF determination, patients were instructed to inhale until reaching total lung capacity (TLC), then to place the mouthpiece of the peak flow meter in their mouth to measure forced expiration. The manoeuvre was repeated 3 times and the highest value was recorded (Appendix, video 2).15
The patient was subsequently examined by a pulmonologist blinded to the results of the previous tests. Each patient underwent pulmonary function testing: FVC (seated and supine positions) and static mouth pressures (MIP and MEP).
Spirometry testing was conducted in the pulmonology laboratory after calibration with a 3-litre syringe according to the ATS/ERS criteria. Patients’ noses were blocked with a noseclip and they were instructed to place the disposable mouthpiece in their mouths and to breathe normally until reaching the functional residual capacity. Next, they were instructed to inhale deeply until reaching TLC, then perform a forced expiration, meeting acceptability criteria for the manoeuvre. The manoeuvre was performed as many times as needed to obtain 3 acceptable results; the highest of the 3 values was recorded. The procedure was repeated with the patient in the supine position.
MIP and MEP were measured; for MEP, the patient was asked first to breathe normally and subsequently to inhale deeply until reaching TLC, then perform a forced expiration, maintaining the same pressure for at least 1.5 seconds. To measure MIP, we first asked the patient to exhale until reaching residual volume, then to perform a deep forced inspiration, maintaining the same pressure for at least 1.5 seconds. Both manoeuvres were performed 3 times and the best value was selected.
Statistical analysis was conducted with the SPSS statistics software, version 20.0 for Windows (IBM Corp.; Armonk, NY, USA). Correlations between variables were evaluated using the Pearson coefficient; the threshold for statistical significance was set at P < .05. We conducted a stepwise linear regression analysis of FVC and the variables showing a significant correlation with FVC. The threshold for significance was set at P < .05.
ResultsThe study included 45 patients, 35 (78%) of whom were women. Mean age at the time of assessment was 40.7 years (range, 18-82), and mean age at MG onset was 32.5 years (12-82). Most patients (89%) were seropositive for anti-AChR antibodies, 2 (4%) presented anti-MuSK antibodies, and 3 (7%) were double-seronegative.
Regarding MGFA class, 2 patients had class I MG at the time of assessment, 35 had class II, 7 had class III, and one had class IV. Of the 43 patients with generalised symptoms, 33 (73.3%) presented predominantly axial and/or limb muscle involvement (a) and 10 (22.2%) had predominantly oropharyngeal and/or respiratory muscle involvement (b).
In the mMRC scale, 26 patients (57.7%) reported a subjective sensation of breathlessness; 17 of these reported feeling breathless when hurrying on level ground or walking up a slight hill (grade 1); 7 felt breathless when walking at the same speed as other people of the same age (grade 2); and 2 reported stopping for breath every 100 metres, despite walking at their own pace on level ground (grade 3).
In the respiratory function tests, the mean FVC was 3.11 L, 87.4% predicted (range, 1.5 L [54% predicted] to 5.9 L [116% predicted]); 14 patients (31.1%) presented FVC values below 80% predicted. FVC measurements with patients in the supine position revealed a > 10% decrease with respect to FVC in a seated position in 11 patients (24.4%).
MIP and MEP were measured in 34 patients (75.5%). Mean MEP was 63.2 (range, 24-122) and mean MIP was –52.7 (range, –85 to –1).
We analysed the association between FVC and the different semiological tests conducted at the consultation (single-breath count test, PEF, neck strength test, dyspnoea scale). Statistically significant positive correlations were identified between FVC and single-breath count test performance (r = 0.57; P = .000; Fig. 1A) and between FVC and PEF (r = 0.76; P = .000; Fig. 1B). Dyspnoea grade, assessed with the mMRC scale, showed a significant negative correlation with FVC (r = –0.31; P = .03; Fig. 1C). Thus, more severe dyspnoea was associated with lower FVC in the spirometry test. No association was found between neck strength and FVC (Fig. 1D).
A) Correlation between the single-breath count test (SBCT) and forced vital capacity (FVC) (r = 0.56; P = .000). B) Correlation between peak expiratory flow (PEF) and FVC (r = 0.76; P = .000). C) Correlation between the modified Medical Research Council dyspnoea scale (mMRC) and FVC (r = –0.31; P = .03). D) Correlation between the neck strength test (NST) and FVC.
We also analysed the association between FVC and MG severity, as measured with the MG-ADL and the MGC, finding no significant correlation. We also analysed the association with the scale item related to respiratory impairment, scored from 0 to 3, with a higher score signalling greater severity. This parameter also showed no significant correlation with FVC.
A regression analysis was conducted to evaluate the association between FVC and the single-breath count test, PEF, and the mMRC scale, yielding 2 models. The first explained 58% of variance in FVC, retaining PEF (ß = 0.007; P < .001). The second model explained 62% of variance, and was statistically significant: F (2, 42) = 35.520 (P < .001). The model retained the variables PEF (ß = .006; P < .001) and single-breath count test (ß = 0.023; P = .030).
By MGFA class, we observed FVC greater than 80% predicted in all patients with class I MG (2/2), 77.1% of patients with class II MG (27/35), and 28.6% of patients with class III MG (2/7); the only patient with class IV MG did not present FVC greater than 80% predicted (P = .02).
Analysis of the relationship between MEP and the single-breath count test, neck strength test, mMRC scale, and PEF showed a significant positive correlation between PEF and MEP (r = 0.51; P = .002; Fig. 2); the remaining semiological tests showed no correlation with MEP.
MIP showed no correlation with any of the parameters analysed.
DiscussionIn patients with MG, respiratory impairment results in greater disease severity and may even require management in the intensive care unit. While it is not a common form of presentation, up to 60% of patients may present respiratory disorders at some point over the course of the disease.16,17 Respiratory symptoms are associated with restrictive alterations in breathing due to reduced respiratory muscle strength.
Spirometry is the study of choice to determine the presence of neuromuscular respiratory disorders in patients with MG. This examination is recommended for screening for and establishing the severity of respiratory impairment and to monitor the evolution of the disease.16–18 In our sample, 24/45 patients (53.3%) presented respiratory impairment according to spirometry parameters: 14/45 (31.1%) presented FVC below 80% predicted; 7/45 (15%) presented a > 10% decrease in FVC when tested in a supine position; and 3/34 (8.8%) had MEP below 80% predicted.
We assessed the value of the neck strength test, the single-breath count test, PEF, and the mMRC dyspnoea scale for detecting respiratory impairment in the neurology consultation.
Previous studies have found the single-breath count test to be useful for determining respiratory status in patients with obstructive and restrictive respiratory disorders.19,20 Another study showed that the single-breath count test is positively correlated with FVC in patients with MG.21 Our study replicates these findings in a population of patients with MG with a younger mean age than those reported in that article, suggesting that the technique may be useful in a broader age range.
Routine application of the single-breath count test in the assessment of patients with MG is attractive due to the tool’s simplicity, brevity, and low cost. An additional advantage is its value for assessing respiratory impairment in patients with facial weakness preventing correct performance of spirometry test manoeuvres, which would limit the reliability of results.
PEF was the other parameter correlated with spirometry results (FVC and MEP). It is important to monitor expiratory muscle strength in patients with neuromuscular diseases, as alterations in these muscles may lead to atelectasis, mucus retention, and respiratory infections. According to our results, PEF is significantly correlated with FVC and with MEP. Studies of patients with amyotrophic lateral sclerosis and Duchenne muscular dystrophy have shown a correlation between PEF and MEP, suggesting that PEF may be a valuable measure of expiratory muscle weakness and bulbar involvement in these patients.22–24 Even in these diseases, PEF of at least 270 L/minute was associated with a lower rate of respiratory infection.23 To our knowledge, no previous study has evaluated the association between PEF and respiratory function examination in patients with MG.
The statistical analysis showed that PEF and the single-breath count test predicted more than half of variance in FVC in our sample.
The neck strength test is a typically hierarchical measurement in the assessment of patients with neuromuscular diseases, probably due to the role of neck flexor muscles in respiration. Unlike the results reported by Elsheikh et al.,21 we observed no association between neck flexor muscle weakness and reduced FVC.
To establish the association between complaints of dyspnoea and neuromuscular weakness in the spirometry test, we used the item from the MG-ADL and MGC evaluating respiratory impairment, as well as the mMRC scale. The scale was initially proposed in 2012 in the Global Strategy for the Diagnosis, Management and Prevention of Chronic Obstructive Pulmonary Disease to stratify patients with the disease by clinical severity.14 The scale allows for estimation of the degree of respiratory effort based on patients’ limitations in activities of daily living.25,26 A total of 57.7% of patients in our sample reported a subjective sensation of breathlessness. We found no association between reported dyspnoea, as assessed with the MG-ADL and MGC, and reduced FVC. However, we did identify a negative correlation between mMRC results and FVC, with more severe dyspnoea being associated with lower FVC. While no previous study has applied this scale to patients with neuromuscular disorders, our results suggest that it may be a useful tool in everyday practice.
The MGC and MG-ADL scales assess the most commonly affected functional activities in patients with MG. Both scales are easily reproducible and are widely used in neurology consultations.27,28 In 2012, the MGFA task force recommended the MGC as a quantitative tool for determining improvements or worsening in patients with generalised MG.29 We evaluated the correlation between scores on the MGC and MG-ADL and spirometry parameters, identifying no significant association. This underscores the importance of having other easily accessible tests in neurology consultations to screen for respiratory involvement in patients with MG.
ConclusionsDetermining the risk of respiratory muscle involvement in MG is fundamental in everyday practice, as this screening offers prognostic information and has therapeutic implications. PEF and the single-breath count test, and to a lesser extent the mMRC dyspnoea scale, are useful tools for estimating respiratory function in MG. On account of their simplicity and accessibility, these instruments may be appropriate for assessing respiratory function in these patients.
FundingThis study received no funding of any kind.
Conflicts of interestThe authors have no conflicts of interest to declare.