To establish clinical guidelines for the clinical use and interpretation of motor evoked potentials (MEP) in diagnosing and monitoring patients with multiple sclerosis (MS). Recommendations for MEP use and interpretation will help us rationalise and optimise resources used in MS patient diagnosis and follow up.
MethodWe completed an extensive literature review and pooled our own data to produce a consensus statement with recommendations for the clinical use of MEPs in the study of MS.
ResultsMEPs, in addition to spinal and cranial magnetic resonance imaging (MRI), help us diagnose and assess MS patients whose disease initially presents as spinal cord syndrome and those with non-specific brain MRI findings, or a normal brain MRI and clinical signs of MS.
ConclusionsWhenever possible, a multimodal evoked potential study should be performed on patients with suspected MS in order to demonstrate involvement of the motor pathway which supports a diagnosis of dissemination in space.
Establecer una guía clínica para la utilización clínica del estudio de potenciales evocados motores (PEM) en el diagnóstico y el seguimiento de la esclerosis múltiple (EM). Disponer de unas recomendaciones para la utilización clínica de los PEM contribuye a racionalizar y optimizar los recursos en el proceso diagnóstico y de seguimiento en los pacientes con EM.
MétodoHemos llevado a cabo una extensa revisión de la literatura médica y puesto en común nuestros propios datos para consensuar recomendaciones para el uso clínico de los PEM en el estudio de la EM.
ResultadosLos PEM contribuyen, junto con la resonancia magnética medular o cerebral, al diagnóstico y evaluación de los pacientes cuyo inicio clínico es un síndrome medular, que presentan hallazgos de neuroimagen poco específicos o que presentan criterios clínicos de EM con neuroimagen cerebral normal.
ConclusionesEs aconsejable realizar un estudio de potenciales evocados multimodales en pacientes con sospecha de EM para documentar la afectación de la vía motora como apoyo al diagnóstico de diseminación en espacio.
Brain evoked potentials generated by exteroceptive stimuli reflect synchronised activity by neuronal and axonal groups in the central nervous system (CNS) resulting from the arrival of nerve impulses after stimulation of a peripheral nerve or its receptors. Depending on the type of stimulus, evoked potentials are categorised as visual (VEP), auditory (AEP), or somatosensory (SSEP). Motor evoked potentials (MEP) occur when the brain's motor area is stimulated. They result from the activation of a sufficient number of motor units.
Although the knowledge that the cerebral cortex may be excited with electrical stimuli has been around for more than 100 years, transcranial electrical stimulation (TES) is not widely used in daily clinical practice. This is because the scalp and skull are highly resistant to the passage of electrical current; also, the level of current intensity required to generate MEPs causes pain. In 1985, Barker introduced the transcranial magnetic stimulation technique (TMS) as an alternative method. In this process, a high-voltage capacitor discharges into a coil of copper wire placed on the subject's head. The resulting magnetic field is perpendicular to the coil. It can reach the brain since neither the skull nor the scalp offers resistance to the magnetic field. The magnetic field reaches 1 to 2 teslas (T) in about 50μs and decreases to 0 in the following 50μs. This induces an electrical current in adjacent structures, including the brain. The stimulus is not painful and it causes the patient minimal local discomfort.
Exactly which cortical structures are stimulated by TMS remains a topic for debate. Studies in animals (and in humans undergoing surgical procedures) have shown that TES may directly activate axons of cortical motor neurons, thereby producing a descending discharge, or D-wave (direct wave), in the pyramidal tract. Increased intensity of the stimulus results in stimulation of the cortical interneurons, which in turn causes trans-synaptic depolarisation of cortical motor neurons. This is reflected by the presence of I-waves (indirect waves) in the pyramidal tract. Anaesthetics and hypothermia affect I-waves more than D-waves. TMS preferentially activates cortical motor neurons via trans-synaptic action on excitatory interneurons; this primarily results in I-waves. However, TMS may also produce D-waves in cases of high-intensity stimulation in which the coil is placed in a specific position and at a certain angle. MEPs result from the activation of spinal cord α motor neurons after the arrival of descending pulses. In certain stimulation modes, TMS has an inhibitory effect on voluntary motor activity or on MEPs caused by another stimulus.1–3
The magnetic field used to activate motor neurons is generated by a device that includes a capacitor and an inductor (coil). The quick discharge of current into a coil generates a magnetic field perpendicular to that coil. The rapidly changing magnetic field is not attenuated by the bony structures and tissues covering the brain. Upon reaching the cerebral cortex, it induces an electrical current perpendicular to the magnetic field generated by the coil. This electrical current activates the excitable areas of the cerebral cortex.
Types of stimulusCommercial devices for magnetic stimulation may generate monophasic pulses (Magstim®, Novametrix, Medtronic®, Dantec; Digitimer®) or biphasic pulses (Cadwell®). ‘Monophasic pulse’ refers to the initial phase of the stimulus, the interval considered to be physiologically active, with a peak occurring approximately 50μs after onset. Biphasic pulses have a longer duration; here, the second phase is considered active since its amplitude is higher than that of the initial phase. When focal coils are used, stimulus type determines directionality of brain activation. Doctors should therefore know what type of stimulus is employed.
Types of stimulation coils:
- -
Round. The round coil has a standard diameter of 90mm. This is the most effective coil for stimulating the motor cortex. Electrical current follows a unidirectional path in the coil, meaning that when the coil is placed on the vertex with side A up, current flows in the anti-clockwise direction, and when side B is placed up, current flows clockwise. This is an important consideration because the brain will generate an electrical flow moving opposite to the direction of the coil current. In addition, electrical current moving in a posterior–anterior direction is more likely to activate the motor cortex. Therefore, when side A is up, the induced current mainly stimulates the left hemisphere, whereas placing the coil with side B up will predominantly stimulate the right hemisphere. Large round coils induce extensive activation rather than focal activation.
- -
Figure-of-eight coils. Figure-of-eight coils are essentially 2 intersecting round coils. Since these coils generate 2 adjacent magnetic fields, the highest amount of energy is concentrated at the midpoint. Here, stimulation will be the most effective, and it can even be relatively focal. These coils are very useful for cortical muscle maps.
- -
Double cone. Double cone coils are similar to eight-shaped coils in that they consist of 2 conjoined round coils, but in this case, the coils meet at a 90° angle. The magnetic field they elicit produces more intense electrical activation of deep cortical structures. Double cone coils are useful for studying motor pathways to the lower limbs.
Studying evoked potentials (EP) is a reliable way of measuring presence or absence of dysfunction in the pathway being analysed. EPs are useful for detecting clinically silent lesions, which is an important aid to diagnosing MS. In addition, the type of EP alteration detected provides doctors with an idea of the characteristics of the underlying lesion. A longer conduction time is usually seen in demyelinating lesions, whereas axonal loss is usually responsible for decreased amplitude or absence of response.
The main purpose of an EP study is to ascertain whether sensory or motor pathways are affected where unclear symptoms are present. EPs also provide complementary data about the pathophysiology of the lesion underlying the patient's symptoms. However, visual EPs are the only ones currently recommended for use in diagnosing multiple sclerosis (MS).4,5
In addition, advances in MRI techniques, which are highly sensitive for detecting subclinical lesions, mean that EPs are not as helpful in diagnosing MS as they once were. In fact, the revised version of the McDonald criteria for MS diagnosis allows doctors to establish an early diagnosis of the disease based on MRI findings of dissemination in space and time. An EP battery including all modes does increase the sensitivity of an MS diagnosis (60%–85%), but it does not improve specificity (50%–85%).6 Logically enough, the specificity of EPs is very limited since the pathophysiological anomalies they detect may correspond to a number of different aetiologies. Results should therefore be assessed in their clinical context.
The usefulness of EP studies during MS follow-up is currently being debated. Recent data support using global EP scales that include MEP. These scales have a good diagnostic utility in MS and their results are correlated to disease progression. These characteristics will allow doctors to use the scales to assess neurodegeneration, predict future disability, and monitor the effects of disease-modifying drugs.7–20
Recommendations for use of motor evoked potential studies in patients with a first clinical episode of probable demyelinating originA multimodal EP battery including MEP is useful in all cases in which clinical symptoms are compatible with a first episode of demyelinating disease. MEPs have been shown to be useful for predicting the clinical course of early demyelinating episodes.21 MEP studies should be considered a priority for patients experiencing an initial episode of probable demyelinating disease, especially if symptoms include medullary syndrome.22 These studies can identify the presence and location of CNS dysfunction.
Recommendations for use of MEP studies in relapsing-remitting MSIt could be argued that EPs are not necessary for diagnosing patients with relapsing-remitting MS, which is characterised by relapses appearing in different localisations and at different moments. However, diagnostic tests, including brain MRI and EPs, are recommended in order to confirm the diagnosis and possible clinical correlations.20
MEP tests should be performed in the following cases:
- 1.
Patients with a clinical diagnosis of MS and normal results from brain MRI. In these cases, finding subclinical lesions compatible with demyelination, and which may affect the corticospinal tract, will support the clinical diagnosis.
- 2.
Patients with suspected MS whose brain MRI findings are inconclusive and cannot confirm this diagnosis. Detecting subclinical lesions compatible with demyelination, and which may affect the corticospinal tract, will support the diagnosis of MS in these cases.
In cases of primary progressive MS, complementary tests should be completed to exclude those processes that may cause progressive disability (intraspinal tumour, spinal dural arteriovenous fistula, etc.) and confirm the presence of demyelinating lesions. In this context, MEP studies and other neurophysiological tests may help doctors identify dysfunction and its possible pathophysiological mechanisms.
Technical recommendations for performing motor evoked potential studiesThe magnetic stimulator is connected to a standard EMG unit in routine studies. Doctors require an external triggering system that synchronises stimulator discharge with the moment when the EMG acquires the signal. The bandpass filter was between 50 and 2000Hz. The oscilloscope sweep and the amplifier gain should provide a complete view of the MEP, and they must be adjusted for each individual case. We recommend an initial sweep of at least 100ms and a gain of approximately 1mV per division.
The stimulator coil must be positioned correctly to make contact with the specific area being assessed (scalp for cortical stimulation or spinal column for spinal cord). Although the study may be performed by a single examiner, having an assistant to keep the coil in place is recommended in daily practice.
Cortical stimulation is performed first of all. Upper limbs are usually assessed using a round or double coil (butterfly) for cortical stimulation and a round coil for cervical stimulation. Lower limbs may be examined using a round coil or, better yet, a double cone coil.
For hand muscle studies, the coil should be placed 5cm lateral to the vertex along the auricular line, and turned 45° to the parasagittal plane. In leg muscle studies, the best location for the coil is the vertex. When using round coils, we must be aware of which face of the coil is inducing stimulation. As mentioned above, the left hemisphere is stimulated more when the electrical current flows anti-clockwise (A) since the resulting current in the brain will flow in the opposite direction, that is, depolarisation will take place posterior to anterior.
MEPs from any muscle may be recorded by using surface electrodes. The most common studies performed on the upper limbs involve the abductor pollicis brevis or first dorsal interosseous muscle; lower limb studies most frequently involve the anterior tibialis. The study may be performed on both sides simultaneously, with the patient in a comfortable sitting position.
Once cortical stimulation has been completed, we perform cervical stimulation for the upper limbs and lumbar stimulation for the lower limbs. Cervical stimulation is carried out with the coil above the C7 spinous process at the midline or 2cm lateral to the midline. This stimulus is able to activate cervical nerve roots at the intervertebral foramina (foramen stimulation). To stimulate the lumbosacral roots, the circular coil is placed along the midline over the selected vertebral body. However, the utility of lumbosacral magnetic stimulation is controversial. Lumbosacral stimulation is more difficult to perform and its localisation is less selective than is the case for cervical stimulation. Electrical stimulation and central motor conduction time (CMCT) with F-wave latency seem to be better methods for determining radicular nerve conduction time at the lumbosacral level.23
Determining motor threshold and amplitude of motor evoked potentialsStimulus intensity has an effect on MEP amplitude and latency. On the other hand, motor pathway excitability changes over time and differs from person to person. It is therefore necessary to establish reference values for intensity in clinical practice. To do so, we first define the motor threshold, that is, the minimum intensity of a magnetic stimulus that will generate MEPs. Researchers have published several methods for defining this intensity, but the best-accepted criteria are as follows: minimum intensity delivering MEPs with an amplitude of at least 100μV in response to 50% of the stimuli. There should be an interval of at least 3seconds between stimuli in order to avoid a stimulus affecting responses to the subsequent stimulus.
Motor thresholds vary according to the muscle being assessed. Thresholds are lower for hand muscles than for axial muscles or proximal muscles of the arms or legs. The motor threshold during muscle contraction is significantly lower than the resting threshold.
MEP amplitude depends on several factors, including coil position, subject's attention, and tonic facilitation of the muscle in question. As a result, this measurement has little value in clinical practice. It may be useful to compare MEP amplitude with compound motor action potential amplitude for the same muscle. In general, however, MEP amplitude can only be determined in distal muscles or when it decreases significantly.
Determining central motor conduction timeCortical stimulation yields MEPs once a certain intensity above the motor threshold has been reached. MEP latency includes CMCT (from the motor cortex to the spinal cord) and peripheral motor conduction time (from the spinal cord to the muscle). Therefore, CMCT is calculated by subtracting peripheral motor conduction time from MEP latency. There are 2 methods of doing so: spinal magnetic stimulation or F-wave.
- –
Spinal magnetic stimulation. As mentioned previously, magnetic stimulation can be applied to the cervical or lumbar regions to activate nerve roots in the intervertebral foramen. Use of this method means that CMCT will include the intraspinal segment. A theoretical example would be receiving a MEP with a latency of 22ms due to cortical magnetic stimulation of the first dorsal interosseous muscle, and finding an MEP with a latency of 14ms due to cervical magnetic stimulation of the same muscle (peripheral motor conduction time). The difference, 8ms, would correspond to the CMCT.
- –
Measuring peripheral motor conduction time using F-waves. F-waves are produced by antidromic activation of motor fibres resulting in motor neuron backfiring. The F-wave resulting from cubital nerve stimulation in the wrist includes antidromic conduction time (from the wrist to the motor neuron) and orthodromic conduction time (from the motor neuron to the muscle). Therefore, contrary to what happens with magnetic stimulation, conduction time in the intraspinal segment of the motor root is included when we use the F-wave to calculate peripheral motor conduction time ([F+M−1]/2). In the example described above, if MEP latency following cortical stimulation is 22ms, the F-wave of the cubital nerve will have a latency of 28ms, and if the M-wave of the cubital nerve has a latency of 3ms, the peripheral conduction time measured by the F-wave will be [28+3−1]/2=15ms and central motor conduction time will be 22−15=7ms instead of the 8ms calculated using magnetic stimulation. The difference between the two methods is the root motor conduction time.
The silent period is examined in order to study the inhibitory effect of cortical stimuli on voluntary activity. This period is defined as the time during which EMG activity is silenced in a muscle that has been activated voluntarily. The patient is asked to sustain a tonic muscle contraction (at 10–20% of maximum contraction). Making a pinching motion with the thumb and index finger is a suitable task for the first dorsal interosseous muscle. The intensity of the stimuli should exceed the threshold (for example, 120%). A focal coil will elicit silent periods in the muscles contralateral and ipsilateral to the stimulated hemisphere.
Cortical mapMuscles of the human body correspond to areas of the motor cortex, as has been known since Penfield's studies. Cortical stimulation allows researchers to define approximate cortical boundaries for a specific muscle using focal stimulation coils at a controlled intensity. In this process, the scalp is marked with a 1cm grid and Cz is used as the starting point (point 0.0). The coil is systematically placed on each of the pre-marked points in order to generate a map of the different MEP amplitudes corresponding to each coil position. The point with the greatest MEP amplitude is referred to as the motor hotspot for the muscle in question. For example, the motor hotspot of the left first dorsal interosseous muscle may be 5.2, that is, 5cm lateral and 2cm anterior to Cz.
Recommended practical protocolThe protocol recommended by clinical studies of MS is as follows:
Upper limbs:
- -
Place electrodes on the first dorsal interosseous muscle and abductor of the thumb.
- -
Determine resting motor threshold with contralateral focal stimulation (>50% of stimuli are effective, that is, an EP amplitude of more than 100μV will be generated by at least 50% of a series of stimuli at one point).
- -
Cortical stimulus is calibrated at 120% of the resting motor threshold.
- -
Cortical stimulus is calibrated at 120% of the threshold with facilitation of muscle contraction.
- -
Cervical stimulation follows similar intensity guidelines.
- -
Measuring CMCT: difference between conduction time for cortical stimulation and that for cervical stimulation.
Lower limbs:
- -
Place electrodes on the tibialis anterior.
- -
Determine the resting motor threshold with contralateral focal stimulation (>50% of stimuli are effective at one point).
- -
Cortical stimulus is calibrated at 120% of the resting motor threshold.
- -
Cortical stimulus is calibrated at 120% of the threshold with facilitation of muscle contraction. Lumbar stimulation follows similar intensity guidelines.
- -
Measuring CMCT: difference between conduction time for cortical stimulation and for lumbar stimulation.
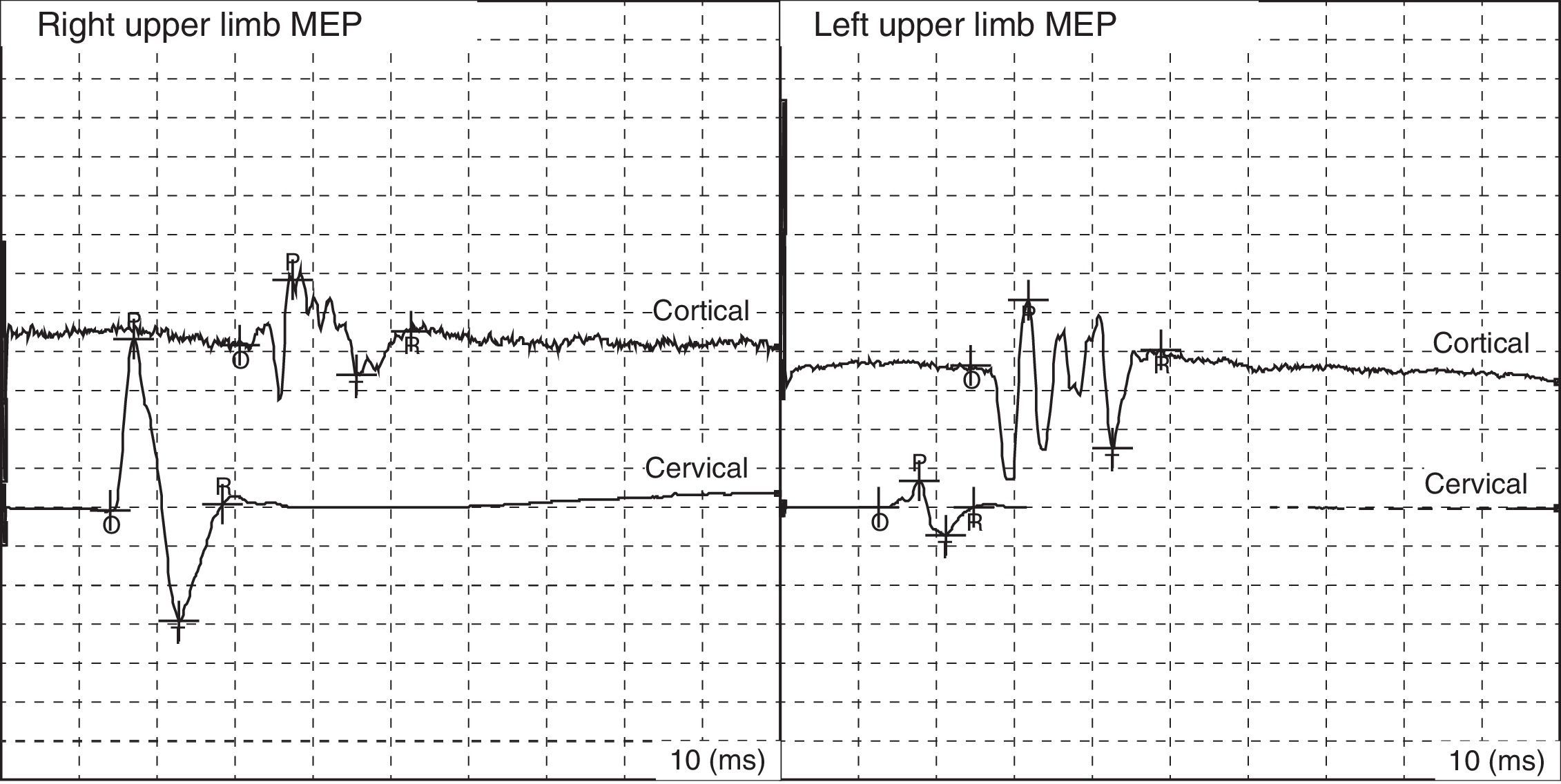
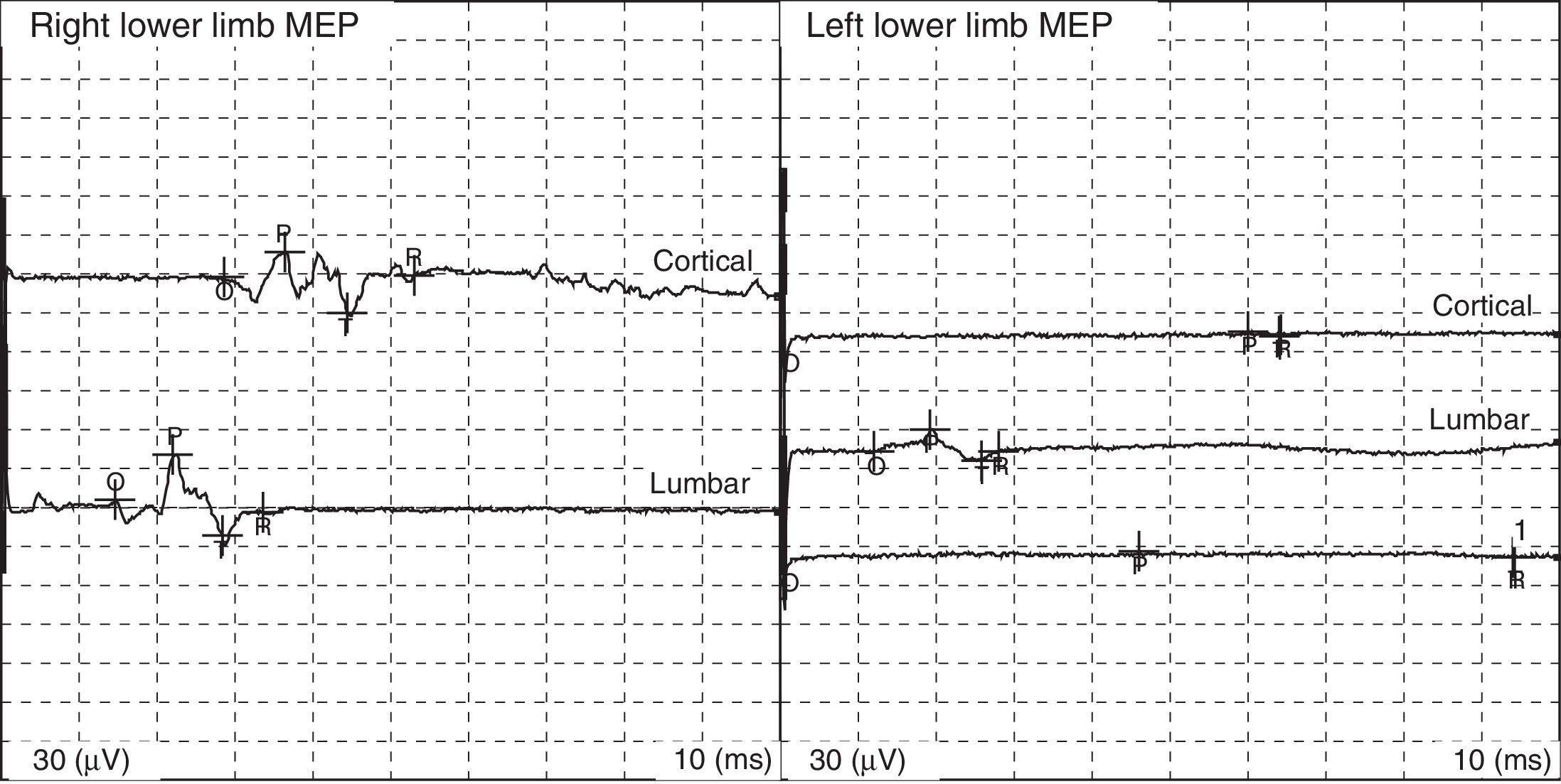
Fig. 1 shows some examples of MEPs within the normal range; Table 1 displays normal values for adults.
MEPs are considered abnormal in the following cases:
- •
Lack of response.
- •
Increase in cortical MEP latency.
- •
Increase in CMCT.
- •
Decrease in amplitude when comparing the two sides and each side with CMAP.
Criteria signalling abnormal values of latencies, intervals, and amplitudes are differences exceeding 2.5 to 3 standard deviations (SD) compared to mean values for an age- and sex-matched control group.
The most typical anomaly in MS is the lengthening of CMCT (Figs. 2 and 3).
Over the last few years, researchers have taken significant steps towards developing and applying new MEP techniques providing specific, simple, and repeatable means of detecting demyelinating lesions that correlate to the level of clinical disability, and also to severe demyelination and axonal destruction.
- •
Electrical nerve root stimulation. Electrical nerve root stimulation may be used to measure PMCT or verify the presence of proximal muscle blockade. The electrical stimulator is typically placed lengthwise over the spinal column with the cathode caudal to the anode. Proximal conduction block can be assessed since supramaximal stimulation can be achieved. The main drawback is that stimulation may be painful since it causes contraction of the paravertebral muscles.
- •
Paired-pulse stimulation.24 This technique uses 2 independent magnetic stimulators discharging to the same coil (BIStim module). The intensity of each stimulus and the time between stimuli may be adjusted independently. This method is employed to observe how the first stimulus affects the response to the second stimulus. Low-intensity stimuli typically inhibit the MEPs that should have been generated by stimuli above the intensity threshold applied with a frequency between 1 and 5ms (intracortical inhibition). Longer intervals cause facilitation.
- •
Bihemispheric stimulation. This technique uses 2 independent magnetic stimulators which, in this case, discharge to different focal coils. The first stimulus is applied to the motor area of one hemisphere, and the second to the motor area of the contralateral hemisphere. Stimulus to one hemisphere generally inhibits the MEP that would be produced by a suprathreshold stimulus applied to the contralateral hemisphere with an interval of 7 to 9ms (interhemispheric inhibition).
- •
Triple stimulation technique.25,26 The triple stimulation technique (TST) is a more accurate and less variable way of estimating cortical excitability than measuring the motor threshold. However, this technique requires a supramaximal stimulus at the Erb point and may cause discomfort in some patients.
- •
Repeated transcranial magnetic stimulation.27,28 Repeated transcranial magnetic stimulation (rTMS) of the cortex may change its excitability. This technique was first developed to examine cortical physiology, but it soon began to be used to study cognitive processes. It entails applying repeated stimuli to the cerebral cortex in the form of a series of magnetic pulses. Frequencies lower than 1Hz decrease cortical excitability, whereas frequencies higher than 1Hz will increase it.
Repetitive transcranial magnetic stimulation may have a therapeutic effect for certain neurological and psychiatric diseases. This is because it stimulates neuronal plasticity, which promotes acquisition of new skills and functional recovery of damaged areas.
ConclusionsMagnetic stimulation is a useful tool for analysing CNS dysfunctions. Measuring the different parameters related to producing MEPs in patients and healthy subjects may deliver further information about dysfunction in MS patients. Some MEP parameters are highly sensitive and specific for identifying demyelinating lesions in the corticospinal tract of the CNS. Rigorous analysis of the results offers quantitative information which may be used to determine the diagnosis and prognosis. The information we are able to obtain from the lesion itself is still limited, even when non-conventional MEP techniques are used. MEP studies in patients with MS must be carried out under appropriate technical conditions, and following correctly established indications. MEPs should be considered the first choice in patients with symptoms that are compatible with a first episode of demyelinating disease, especially if symptoms include medullary syndrome. MEPs should also be performed in patients with a clinical diagnosis of MS and normal or inconclusive results from a brain MRI. Implementing this consensus will aid in rationalising healthcare resources and optimising the effectiveness of complementary tests ordered to study MS.
Conflicts of interestThe authors have no conflicts of interest to declare.
This study was performed with the support of the RETICS programme at Instituto de Salud Carlos III: Red Española de Esclerosis Múltiple (RD07-0060).
Please cite this article as: Fernández V, Valls-Sole J, Relova JL, Raguer N, Miralles F, Dinca L, et al. Recomendaciones para la utilización clínica del estudio de potenciales evocados motores en la esclerosis múltiple. Neurología. 2013;28:408–416.