Guillain–Barré syndrome (GBS) is an acute inflammatory polyneuropathy that can lead to respiratory failure. In this study, we evaluate early clinical risk factors for respiratory failure at the time of hospital admission.
MethodsWe studied a retrospective cohort of patients with GBS admitted to a tertiary care center. The potential risk factors studied were sociodemographic characteristics, GBS symptoms, overall and cervical muscle weakness (Medical Research Council [MRC] scores), electromyography findings, and cerebrospinal fluid analysis findings. Unadjusted odds ratios (OR) were calculated and exact logistic regression analysis (adjusted OR) performed to assess the association between baseline risk factors and respiratory failure.
ResultsOverall, 13 of 113 (12%) patients included in the study developed respiratory failure. Unadjusted analyses showed that involvement of any cranial nerve (OR: 14.7; 95% CI, 1.8–117.1), facial palsy (OR: 17.3; 95% CI, 2.2–138.0), and bulbar weakness (OR: 10.7; 95% CI, 2.3–50.0) were associated with increased risk of respiratory failure. Lower MRC sum scores (for scores <30, OR: 14.0; 95% CI, 1.54–127.2) and neck MRC scores (for scores ≤3, OR: 21.0; 95% CI, 3.5–125.2) were associated with higher likelihood of respiratory failure. Adjusted analyses showed that presence of bulbar weakness (OR: 7.6; 95% CI, 1.3–43.0) and low neck MRC scores (scores ≤3, OR: 9.2; 95% CI, 3.5–125.2, vs scores >3) were independently associated with respiratory failure.
ConclusionsBulbar and neck muscle weakness at admission are clinical predictors of increased risk of respiratory failure in patients with GBS. These findings could guide the adequate management of high-risk patients.
El síndrome de Guillain-Barré es una polineuropatía inflamatoria aguda que puede causar insuficiencia respiratoria. Evaluamos los factores de riesgo clínicos en el momento de la hospitalización.
MétodosRealizamos un estudio de una cohorte retrospectiva de pacientes con síndrome de Guillain-Barré hospitalizados en un centro de tercer nivel. Analizamos las características sociodemográficas, síntomas de la enfermedad, fuerza muscular general y cervical (escala del Medical Research Council [MRC]), hallazgos electromiográficos, y resultados del análisis del líquido cefalorraquídeo. Calculamos el odds ratio (OR) sin ajustar y realizamos una regresión logística exacta (OR ajustada) para evaluar la asociación entre los factores de riesgo y la insuficiencia respiratoria.
ResultadosTrece de los 113 pacientes incluidos (12%) presentó insuficiencia respiratoria. Los análisis no ajustados mostraron una asociación entre mayor riesgo de insuficiencia respiratoria y la afectación de cualquier par craneal (OR: 14,7; IC 95%, 1,8-117,1), parálisis facial (OR: 17,3; IC 95%, 2,2-138,0) y debilidad bulbar (OR: 10,7; IC 95%, 2,3-50,0). Unas puntuaciones más bajas en la MRC-total (puntuaciones <30, OR: 14,0; IC 95%, 1,54-127,2) y en la MRC-cervical (puntuaciones <3, OR: 21,0; IC 95%, 3,5-125,2) se asociaron con una mayor probabilidad de presentar insuficiencia respiratoria. En los análisis ajustados, la presencia de debilidad bulbar (OR: 7,6; IC 95%, 1,3-43,0) y una puntuación baja en la MRC-cervical (puntuaciones ≤3, OR: 9,2; IC 95%, 3,5-125,2, frente a puntuaciones >3) se asociaron de forma independiente con la insuficiencia respiratoria.
ConclusionesLa presencia de debilidad bulbar y cervical en el momento de la hospitalización es un factor de riesgo de insuficiencia respiratoria en pacientes con síndrome de Guillain-Barré. Estos hallazgos pueden servir de guía para el manejo de los pacientes con mayor riesgo de presentar dicha complicación.
Guillain–Barré syndrome (GBS) is an acute inflammatory polyneuropathy due to an autoimmune response directed against peripheral nerve antigens.1 The incidence of GBS have found rates to be between 0.16 and 4.0/100,000/year in individuals of all ages, with the highest rates been reported in adults, especially those aged over 75 years. While more than half of cases are clinically “mild,” many patients develop complications which can led to substantial morbidity and mortality.2 Respiratory failure remains the most serious complication of GBS and occurs in up to one third of the patients.3 Unfortunately, the onset of respiratory insufficiency is often insidious and late or urgent intubations are associated with increased risk of complications and prolonged hospitalization.4 Thus, early identification of GBS subjects at high risk of respiratory failure is important to implement adequate monitoring and rapidly provide respiratory support when clinically indicated.
Prior studies had identified risk factors for invasive mechanical ventilation, such as presenting to the hospital <7 days from the time of onset of symptoms, inability to lift the elbow, presence of bulbar involvement, grade of disability on admission, or descending weakness.5–8 However, these studies did not included patients requiring non-invasive support, an increasingly common ventilatory mode. Pulmonary function test findings, such as a reduction in the vital capacity and maximal inspiratory or expiratory pressures are also recognized risk factors for impending respiratory failure.6 Nevertheless, spirometry, or pressure transducers, are not routinely available, the presence of facial weakness may influence the results, and the procedures may lead to delays in initiating ventilatory support.
In this study, we analyzed data from a cohort of subjects hospitalized due to GBS to identify early clinical risk factors, present at the time of admission, for respiratory failure requiring invasive or non-invasive ventilatory support.
MethodsStudy design and settingThis study used data from a retrospective cohort of subjects with GBS admitted between March 31st, 2008 and December 24th, 2017 to a tertiary neurological care center. Eligible subjects were >18 years of age with diagnosis of GBS based on Asbury and Cornblath's criteria.9 Patients having a diagnosis of chronic inflammatory demyelinating polyradiculoneuropathy or critical illness polyneuropathy were non-eligible.
The study outcome was the development of acute respiratory failure, defined as the need of invasive or non-invasive mechanical ventilation within the first week of admission.
Data collectionData were collected within 24h of admission to the hospital. Demographic and clinical variables included age, gender, and presence of comorbidities. GBS-related data included information about the preceding triggering event (upper respiratory infection, diarrhea, mononucleosis like syndrome, or vaccination) and time from onset of muscular weakness to hospital admission.
All participants underwent a standardized neurological examination conducted by the treating physician to obtain information on muscular weakness, pattern of motor involvement (proximal, distal, cranial or general), sensory abnormalities, distal reflexes, or presence of pain. Overall muscular strength (abduction of the arm, flexion of the forearm, extension of the wrist, flexion of the leg, extension of the knee, dorsal flexion of the foot) was assessed using the Medical Research Council (MRC) scale. The MRC is a validated tool where 6 muscles are tested bilaterally (individual score from 0 to 5), with a MRC sum score ranging from 0 (tetraparalytic) to 60 (normal strength)10; based on preestablished cut-offs, patients were categorized into the following groups: scores >50, 50–41, 40–31, ≤30. The MRC scale was also applied to evaluate neck muscular strength and subjects were classified into 3 groups: score 5, 4, ≤3. We also collected data on any cranial nerve involvement, oculomotor or facial palsy, and bulbar weakness (impaired gag reflex, dysarthria, or dysphagia).
Cerebrospinal fluid (CSF) was collected and analyzed for cell count, glucose and protein concentration. Cytoalbuminological dissociation was defined as a CSF cell count <5cells/ml combined with a CSF protein level >0.45g/L. GBS electrophysiological subtype (acute inflammatory demyelinating polyneuropathy, acute motor axonal neuropathy, or acute motor-sensory axonal polyneuropathy) was determined using data from the first nerve conduction study (NCS) and classified according to the Hadden criteria.11
Statistical analysisThe baseline characteristics of the study population were summarized using descriptive statistics. The Kaplan–Meier method was used to estimate mean time to respiratory failure; patients who did not developed respiratory failure were censored at 7 days of admission. We used a T-test, chi-square test, or Fisher's exact test to compare the baseline characteristics of GBS patients that developed vs. did not develop respiratory failure. Two-sided tests were used and p values <0.05 were considered statistically significant. We calculated unadjusted odds ratios (OR) with 95% confidence intervals (CI) to assess the association between potential risk factors at the time of admission and risk of respiratory failure.
We used exact logistic regression to assess the adjusted association between clinical predictors at admission and respiratory failure. The decision to include predictors in the adjusted model were based on prior knowledge, simplicity of assessment (i.e., we gave preference to risk factors that could be easily ascertained in clinical practice), and strength of the association on univariate analysis. Given the relatively low number of events, we favored parsimonious models with a limited number of covariates (e.g., we combined levels of covariates that showed a similar strength of association with respiratory failure on univariate analysis). All analyses were done in SPSS statistical software version 23.0.0.0 (IBM, Chicago, USA). The study was exempt by the Institutional Review Board.
ResultsDuring the study period, 113 potentially eligible participants were admitted to the neurological center. No patient met any of the exclusion criteria. The mean (range) age of the study population was 47 (25–76) years and 55% were male. A trigger event was reported by 76 (67%) subjects, most commonly upper respiratory tract infections (35%) and diarrhea (25%). The median (range) time from onset of weakness to hospital admission was 8 (0–31) days. (Table 1).
Baseline characteristics of study subjects according to the presence or absence of respiratory failure.
| Characteristics | All patients (n=113) | Respiratory failure (n=13) | No respiratory failure (n=100) | p-value |
|---|---|---|---|---|
| Age, years, mean (SD) | 47 (21–83) | 45 (25–83) | 47 (21–76) | .61 |
| Female, No. (%) | 51 (45.1) | 7 (53.8) | 44 (44.0) | .50 |
| Comorbidities, no. (%) | ||||
| Diabetes | 8 (7.1) | 2 (15.4) | 6 (6.0) | .22 |
| Pre-existing neuropathy | 7 (6.2) | 1 (7.7) | 6 (6.0) | .81 |
| Pre-existing facial paralysis | 8 (7.1) | 0 | 8 (8.0) | .29 |
| Cancer | 8 (7.1) | 1 (7.7) | 7 (7.0) | .93 |
| HIV | 3 (2.7) | 0 | 3 (3.0) | .53 |
| Autoimmune disease | 22 (19.5) | 4 (30.8) | 18 (18.0) | .27 |
| GBS medical risk factors, no. (%) | .42 | |||
| Onco-hematological disease | 2 (1.8) | 1 (7.7) | 1 (1.0) | |
| Hepatitis | 6 (5.3) | 1 (7.7) | 5 (5.0) | |
| Previous HSV-CMV | 6 (5.3) | 1 (7.7) | 5 (5.0) | |
| Previous GBS | 4 (3.5) | 2 (15.4) | 2 (2.0) | |
| GBS trigger, no. (%) | .02 | |||
| Upper airway infection | 40 (35.4) | 1 (7.7) | 39 (39.0) | |
| Diarrhea | 28 (24.8) | 5 (38.5) | 23 (23.0) | |
| Mononucleosis like | 5 (4.4) | 2 (15.4) | 3 (3.0) | |
| Vaccination | 3 (2.7) | 0 (0) | 3 (3.0) | |
| None | 37 (32.7) | 4 (30.8) | 33 (33.0) | |
| Time weakness-admission, days, mean (SD) | 8 (0–31) | 7 (1–21) | 8 (0–31) | .07 |
| >7 | 48 (42.5) | 5 (38.6) | 43 (43.0) | |
| 4–7 | 32 (28.3) | 1 (7.7) | 31 (31.0) | |
| <3 | 33 (29.2) | 7 (53.7) | 26 (26.0) | |
| Motor weakness distribution, no (%) | .92 | |||
| No weakness | 22 (19.5) | 2 (15.4) | 20 (20.0) | |
| Proximal | 20 (17.7) | 2 (15.4) | 18 (18.0) | |
| Distal | 67 (59.3) | 9 (69.3) | 58 (58.0) | |
| General | 3 (2.7) | 0 | 3 (3.0) | |
| Isolated cranial nerve | 1 (0.9) | 0 | 1 (1.0) | |
| Symptoms, no. (%) | ||||
| Pain | 68 (60.2) | 10 (76.9) | 58 (58.0) | .19 |
| Any cranial nerve involvement | 57 (50.4) | 12 (92.3) | 45 (45.0) | .002 |
| Involvement of cranial nerve VII | 53 (46.9) | 12 (92.3) | 41 (41.0) | .001 |
| Bulbar involvement | 8 (7.1) | 4 (30.8) | 4 (4.0) | <.001 |
| Ocular movement involvement | 10 (8.8) | 1 (7.7) | 9 (9.0) | .88 |
| Paresthesia | 87 (77.0) | 10 (76.9) | 77 (77.0) | .99 |
| Sensitive deficit | 100 (88.5) | 13 (100) | 87 (87.0) | .18 |
| Normal reflexes | 9 (8.0) | 1 (7.7) | 8 (8.0) | .10 |
| Local areflexia | 38 (33.6) | 1 (7.7) | 37 (37.0) | |
| General areflexia | 66 (58.4) | 11 (84.6) | 55 (55.0) | |
| Ataxia | 20 (17.7) | 2 (15.4) | 18 (18.0) | .82 |
| MRC sum score, no. (%) | .05 | |||
| 51–60 | 60 (53.1) | 4 (30.8) | 56 (56.0) | |
| 41–50 | 38 (33.6) | 5 (38.6) | 33 (33.0) | |
| 31–40 | 11 (9.7) | 2 (15.4) | 9 (9.0) | |
| <30 | 4 (3.5) | 2 (15.4) | 2 (2.0) | |
| Neck MRC score, no. (%) | <.001 | |||
| 5 | 51 (45.1) | 2 (15.4) | 49 (49.0) | |
| 4 | 49 (43.4) | 5 (38.5) | 40 (40.0) | |
| <3 | 13 (11.5) | 6 (46.1) | 7 (7.0) | |
| CSF results, mean (SD) | ||||
| Proteins (mg%) | 95 (12–605) | 139 (17–428) | 89 (12–605) | .06 |
| CA dissociation | 78 (69.0) | 10 (76.9) | 68 (68) | .75 |
| GBS classification, no. (%) | .07 | |||
| AMAN | 4 (3.5) | 2 (15.4) | 2 (2.0) | |
| AMSAN | 10 (8.8) | 2 (15.4) | 8 (8.0) | |
| AIDP | 82 (72.6) | 9 (69.2) | 73 (73.0) | |
| Unclassified | 11 (9.8) | 0 (0) | 11 (11.0) | |
| Miller–Fisher | 6 (5.3) | 0 (0) | 6 (6.0) | |
Abbreviations: AIDP: acute inflammatory demyelinating polyneuropathy, AMAN: acute motor axonal neuropathy, AMSAN: acute motor-sensory axonal neuropathy, CA: cytoalbuminological, CSF: cerebrospinal fluid, CMV: citomegalovirus, GBS: Guillain–Barre syndrome, HIV: human immunodeficiency virus, HSV: herpes simplex virus, MRC: Medical Research Council.
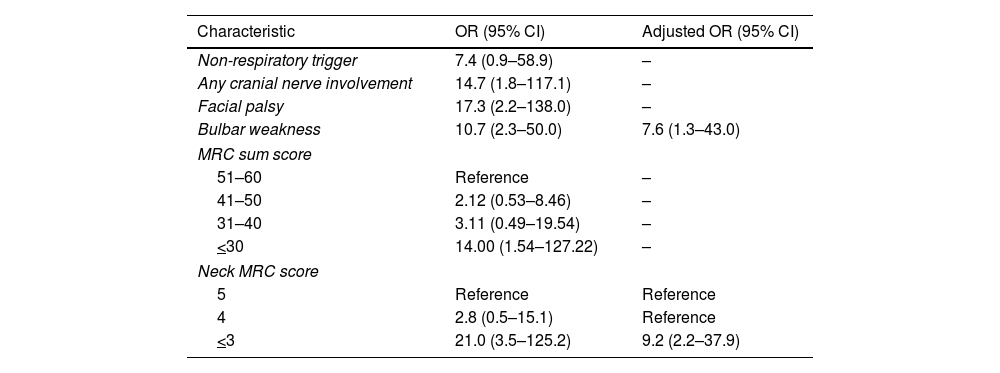
Overall, 12% (13/113) subjects developed respiratory failure (69% required invasive ventilation), at a mean (range) time of 21–7 days from hospital admission (Fig. 1). No other patient developed respiratory failure in the first month. In unadjusted analyses, there were no differences between the groups in the distribution of age, sex, presence of comorbidities, sensory abnormalities, or complementary studies (p>0.05 for all comparisons; Table 1). Presence of any cranial nerve involvement (OR: 14.7, 95% CI: 1.8–117.1), facial palsy (OR: 17.3, 95% CI: 2.2–138.0), and bulbar weakness (OR: 10.7, 95% CI: 2.3–50.0) were significantly associated with increased risk of respiratory failure. Lower MRC sum scores (41–50 score OR: 2.12, 95% CI: 0.53–8.46; 31–40 score OR: 3.11, 95% CI: 0.49–19.54; and <30 score OR: 14.0, 95% CI: 1.54–127.2 compare with a score >50) were also associated with increased risk of respiratory failure. Similarly, subjects with lower neck MRC scores (4 score OR: 2.8, 95% CI: 0.5–15.1 and <3 score OR: 21.0, 95% CI: 3.5–125.2 compare to a score of 5) had higher odds of respiratory failure (Table 2).
Early clinical risk factors for acute respiratory failure in patients with Guillain–Barre syndrome.
| Characteristic | OR (95% CI) | Adjusted OR (95% CI) |
|---|---|---|
| Non-respiratory trigger | 7.4 (0.9–58.9) | – |
| Any cranial nerve involvement | 14.7 (1.8–117.1) | – |
| Facial palsy | 17.3 (2.2–138.0) | – |
| Bulbar weakness | 10.7 (2.3–50.0) | 7.6 (1.3–43.0) |
| MRC sum score | ||
| 51–60 | Reference | – |
| 41–50 | 2.12 (0.53–8.46) | – |
| 31–40 | 3.11 (0.49–19.54) | – |
| <30 | 14.00 (1.54–127.22) | – |
| Neck MRC score | ||
| 5 | Reference | Reference |
| 4 | 2.8 (0.5–15.1) | Reference |
| <3 | 21.0 (3.5–125.2) | 9.2 (2.2–37.9) |
Abbreviations: GBS: Guillain–Barre syndrome, MRC: Medical Research Council, OR: odds ratio.
Adjusted analyses showed that presence of bulbar weakness (OR: 7.6, 95% CI: 1.3–43.0) and low-neck MRC scores (<3 score OR: 9.2, 95% CI: 3.5–125.2 vs. score >3) were independently associated with increased risk of respiratory failure.
DiscussionOur study shows that, in patients with GBS, respiratory failure may be predicted by simple clinical markers. In our patients, bulbar or neck weakness at admission, two clinical predictors that are evaluated as part of the routine physical exam, were independently and strongly associated with subsequent need for ventilatory support. These physical exam findings could be used to guide decisions about early ICU admission and/or respiratory monitoring.
Assessment of ventilatory status and making decisions regarding the need for ventilatory support are major aspects of the management of hospitalized GBS patients. Prior studies have evaluated potential risk factors for respiratory failure in this population. Muscular strength, including head, elbow, and foot is a frequently identified risk for the need of ventilatory support.5,8,12 These strength test support ours results, and we provide a numerical cutoff (MRC scale) to be as objective as possible. Similarly, Lawn et al., in a study including 114 patients with GBS, showed that involvement of cranial nerves, particularly the facial nerve, is more common among patients who later develop respiratory failure.6 In our study cranial nerve involvement was associated with increased risk of respiratory failure. However, when it was adjusted for cofounders, it was not an independent risk. Other studies reported that a shorter time between onset of weakness to hospitalization was associated with respiratory failure,13 particularly among patients with more extensive muscular weakness.12,14,15 We did not find any association with the time of weakness.
Baseline spirometry (at time of admission) may also help identify high risk patients with serial measures used to monitor progression and make decisions regarding the need for invasive or non-invasive ventilation.6 However, these studies defined respiratory failure as the need for invasive ventilation. Our study extends these results by showing that two clinical parameters (bulbar and neck weakness) easily obtained during baseline examination are strongly associated with the risk of respiratory failure, including both invasive and non-invasive ventilatory support. Moreover, we used a validated measure with clearly pre-established cutoffs, which could facilitate implementation of these findings into clinical practice.
Several mechanisms may explain the association between bulbar weakness and respiratory failure in hospitalized patients with GBS. First, bulbar weakness leads to inability of protecting the airway and difficulties clearing secretions. Second, muscular bulbar involvement induces upper airway collapse, rising airway resistance, work of breathing and respiratory muscle load, promoting fatigue, and ultimately respiratory failure. A functional and undamaged cervical plexus is needed to counterbalance gravity and maintain the head upright. Neck muscles are controlled by nerves arising from the deep cervical plexus (C2–C5) while the phrenic nerve supplying the diaphragm originates from the deep cervical roots C3–C5. Thus, neck weakness is likely a marker for diaphragmatic muscle compromise providing physiologic plausibility to our observed associations. Additionally, neck weakness may also contribute to swallowing impairment and increased risk of aspiration because proper neck posture promotes successful swallowing.
The study has strengths and limitations that are worth discussing. Unlike previous studies that focused on more severe patients, this study uses a broader study population (regardless of their severity), that allowed us to identify a more conservative 12% incidence of respiratory failure, compared to the 30–50% reported in other series, that were limited to patients with more severe disease. This may better represent the overall population of GBS patients.
Our study has clear limitations, which are linked mainly to its retrospective and monocentric design, consequently the results may not be generalizable to other settings. We retrospectively analyzed data collected as part of a GBS registry. However, predictors were captured at admission and thus, reviewers were not aware of the study outcomes (i.e., subsequent respiratory failure). Moreover, data capture was standardized to avoid biases in the assessment of risk factors for respiratory failure. Our study had a relatively small sample size which limited the number of predictors that we were able to evaluate in adjusted analyses and generated relatively high uncertainty (i.e., large CI) in the estimation of the ORs. However, GBS is a relatively rare disease and our sample size is amongst the largest reported in studies of this condition.
In summary, our study showed that bulbar and neck weakness at the time of admission can be used to identify GBS subjects at high risk of respiratory failure. These are relatively easy to measure clinical variables that could guide decisions about the level of monitoring and/or placement of GBS patients into the ICU or similar settings.
FundingAuthors received no financial support for this study. Dr. Wisnivesky received consulting honorarium from Sanofi, Glaxosmithkline and Bannok and research grants from Sanofi and Quorum.
Authors contributionAuthorship requirements have been met: LPM conceived and designed the study, collected, interpreted, and analyzed data, searched literature, and wrote the manuscript; JPW designed the study, interpreted, and analyzed data, and wrote the manuscript; MW and FRL conceived the study, collected data, searched literature, and critically revised the manuscript; FAV and NAW conceived the study and critically revised the manuscript.
Conflict of interestAll other authors have no conflict of interests to declare.
The authors acknowledge and thank the Methods in Epidemiologic, Clinical and Operations Research (MECOR) Program, the American Thoracic Society (ATS) and Asociación Latinomericana de Tórax (ALAT), for their support and dedication in building research capacity in Latin America and other countries around the globe.