Rafts are protein-lipid structural nanodomains involved in efficient signal transduction and the modulation of physiological processes of the cell plasma membrane. Raft disruption in the nervous system has been associated with a wide range of disorders.
DevelopmentWe review the concept of rafts, the nervous system processes in which they are involved, and their role in diseases such as Parkinson’s disease, Alzheimer disease, and Huntington disease.
ConclusionsBased on the available evidence, preservation and/or reconstitution of rafts is a promising treatment strategy for a wide range of neurological disorders.
Los rafts constituyen nanodominios estructurales de naturaleza lipo-proteica que propician la eficiente transducción de señales y la modulación de procesos fisiológicos asociados a la membrana plasmática. En el sistema nervioso, la alteración de estos dominios se ha asociado con el desarrollo de diversos padecimientos.
DesarrolloEn el presente artículo se revisa el concepto de rafts, los procesos del sistema nervioso en los cuales están involucrados y su papel en distintas afectaciones, entre las que se destacan las enfermedades de Parkinson, Alzheimer y Huntington.
ConclusionesDadas las evidencias de su participación en diversas neuropatías, la preservación y/o reconstitución de los rafts se vislumbran como una atractiva estrategia terapéutica.
The current conceptual model of the plasma membrane includes dynamic nanodomains known as membrane rafts. These structures play a relevant role in various cell signalling processes by promoting convergence of the participating molecular elements. In recent years, rafts have received considerable attention due to the fundamental importance of signal transduction in all physiological processes. In the case of the nervous system, it is very interesting to analyse their participation in both physiological and pathological conditions. This article reviews the concept of membrane rafts, their role in different physiological processes in the nervous system, and their relevance to neurological diseases such as Alzheimer disease (AD), Parkinson’s disease, and Huntington disease.
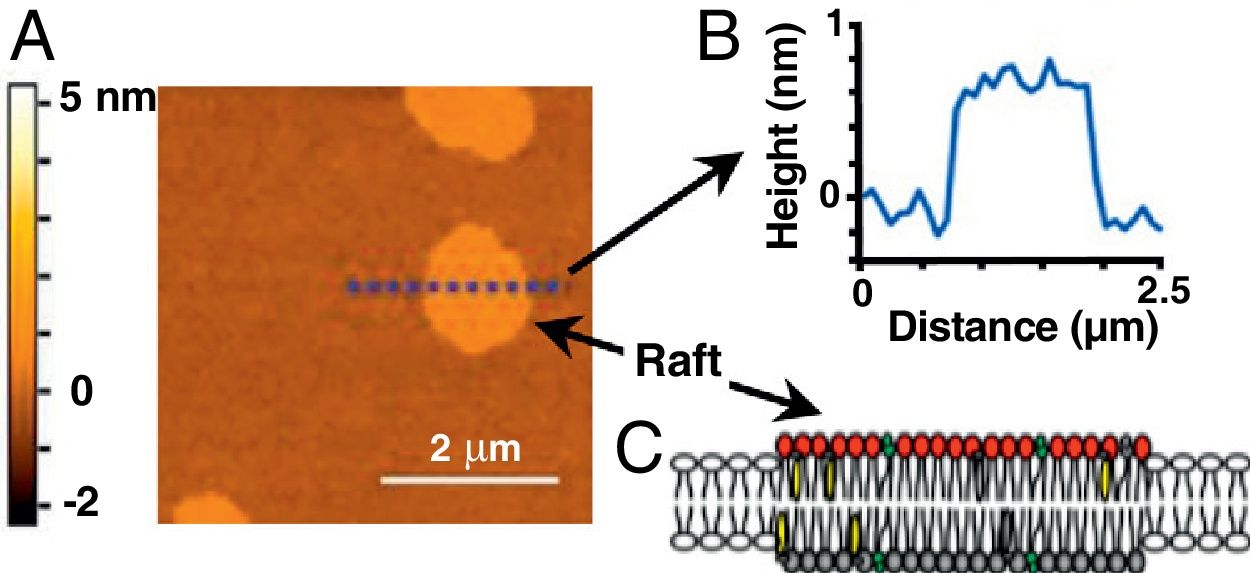
The concept of raftsIn their iconic model, Singer et al.1 defined the plasma membrane as a fluid lipid bilayer containing several protein complexes. They emphasised the random, homogeneous distribution of their elements (due to their free diffusion across the membrane plane) and identified the structural and functional asymmetry between their monolayers. Since its publication, this model has been continuously reviewed and updated.2–5 Chapman,6 for example, incorporated the concept of lateral segregation of lipid elements in discrete domains. This concept was revisited by Simons and van Meer,7 who proposed a model of lipid domains based on the differential distribution of sphingolipids in the apical plasma membrane of epithelial cells. In a subsequent study, Simons and Ikonen8 demonstrated the involvement of cholesterol in the formation and organisation of these domains, which they called lipid rafts, and found that these glycosphingolipid-cholesterol complexes remained tightly packed and behaved as structural units. Thus, the plasma membrane shows the coexistence of at least 2 phases across its surface: a liquid-ordered (Lo) phase and a liquid-disordered (Ld) phase. Rafts correspond to the Lo phase.4,9,10 Regarding the proteins associated with rafts, Simons and Ikonen8 mentioned that, depending on their molecular and thermodynamic properties, they can either be included in the rafts (or anchored to them) or outside them. Lipid rafts were subsequently redefined in favour of the current concept of membrane rafts, which takes into account not only their lipid nature, but also their protein components.11 Rafts were thus defined as small (2-200 nm in diameter), heterogeneous, cholesterol- and sphingolipid-enriched domains, thicker than the surrounding membrane, that compartmentalise a variety of cellular processes. The current plasma membrane model considers the possibility that these rafts may stabilise (as a consequence of the activation of receptors to several agonists) and generate larger platforms through lipid-lipid, lipid-protein, or protein-protein interactions.3,4 These membrane domains or rafts are divided into 2 types: planar lipid rafts and caveolae.3 The first are aligned on the horizontal plane of the membrane, and their experimental characterisation has been very difficult due to their small size (2-20 nm in diameter) and great dynamism (mean lifetime of 1 ms). However, they may be transiently stabilised (mean lifetime < 1 min) by liganded and oligomerised receptor molecules.12 An alternate approach to caracterising them is based on studying artificial membrane models that enable the separation of domains (equivalent to rafts) of larger size and longer duration (Fig. 1).13 In turn, caveolae are plasma membrane invaginations of larger size (50-200 nm in diameter) and much lower dynamism (lifetime > several minutes), which are characterised by the presence of cavin and caveolin proteins.3,8 In this context, Kusumi et al.14 used the powerful single-molecule tracking technique and established that both the proteins and the lipids of the plasma membrane show 2 characteristic patterns of diffusion: short-term confined diffusion (within a compartment) and long-term hop movement between compartments (hop diffusion). From the dynamic analysis of membrane-constituent molecules, these researchers proposed the so-called picket-fence model, in which cytoskeletal elements play an important role. According to this new model, integral proteins that protrude into the cytoplasm act as pickets and interact with actin filaments from the submembrane cytoskeleton, which determines the confinement or corralling of small compartments (∼40-300 nm in diameter) on the inner surface of the plasma membrane. These compartments limit the diffusion of proteins (integral or peripheral) and phospholipids in both monolayers of the membrane. The current model of the plasma membrane assumes that rafts are housed within these compartments2,3,14 and represent between 1% and 25% of the total membrane surface area, depending on the cell type.14,15 The functional relevance of both compartments and rafts is supported by the subcellular compartmentalisation of the processes to which they give rise, promoting greater specificity and efficiency. In particular, rafts play an important role in the spatial and temporal organisation of the different molecular elements involved in the transduction of extracellular signals, apoptosis, viral infection, cell adhesion and migration, protein targeting during endocytosis and exocytosis, the cytoskeleton, and synaptic transmission and plasticity.3
Raft-type domain image obtained with atomic force microscopy. A) Topographic image of a model lipid bilayer (60/20/20 mol% of dioleoyl phosphatidylcholine/sphingomyelin/cholesterol), showing domains equivalent to rafts (without proteins). The colour scale to the left corresponds to the height of the sample in nanometres. B) Graphical analysis of the heights of the sample shown in (A) at the dotted line. The zero value corresponds to the level of the phospholipid head in the liquid-disordered or outside rafts phase. C) Schematic representation of a raft in a lipid bilayer.
Adapted from Mensch et al.13 (with authorisation). Copyright ©2018, American Chemical Society.
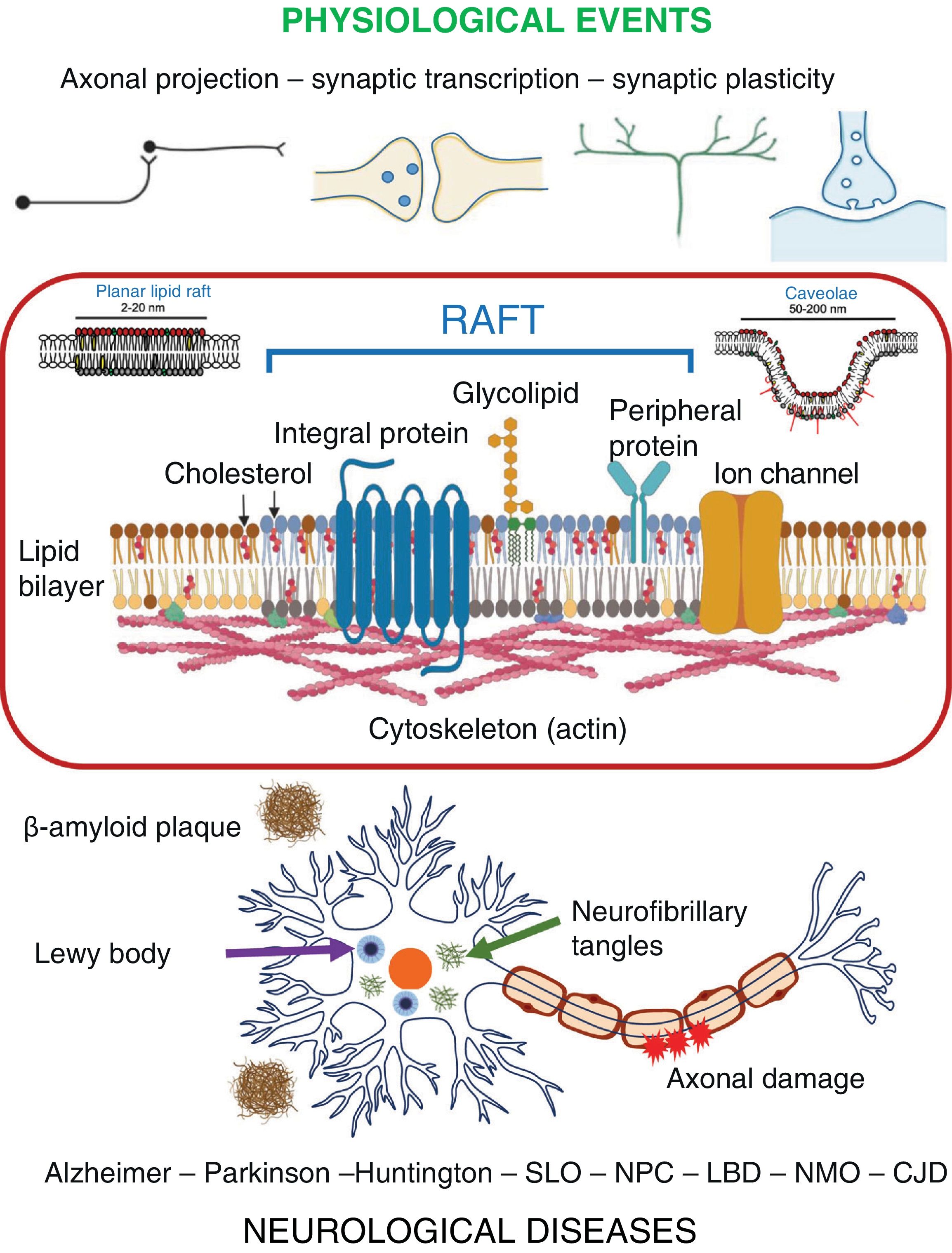
In the nervous system, rafts have been identified not only in neurons but also in astrocytes, oligodendrocytes, and microglia.16,17 Many reports associated with the nervous system assume the structural and functional equivalence of rafts and non-ionic detergent-resistant membrane domains (DRM), the fraction into which both planar lipid rafts and caveolae are partitioned during plasma membrane isolation procedures, although this assumption is not strictly accurate.9 Despite this, participation of rafts in signalling mechanisms and physiological processes associated with the plasma membrane of nervous system cells is widely acknowledged.16,17 These processes include axonal growth and projection, neurotransmission, synaptic plasticity, learning, and memory (Fig. 2).17
Participation of rafts in axonal growth and projectionAxonal growth cones are the sensory and mobile structures used by developing neurons to localise and identify targets during the development of neural circuits. Different molecule and receptor families are involved in this process.18 Recent studies suggest that some of these elements are characteristically located in DRM-rafts.19,20 The term DRM-rafts used here emphasises the biochemical properties of the experimental approaches described. Netrin-1 is a laminin-associated protein, which attracts or repels growth cones through its interaction with DCC receptors.21 These receptors are palmitoylated at one of their transmembrane domains, which favours their association with rafts, an event that is also promoted by binding to their ligand netrin-1. It should be noted that a point mutation at this palmitoylation site (substitution of cysteine 1121 with valine) attenuates their signalling coupled to mitogen-activated protein kinase (MAPK).22 Similarly, functional destabilisation or alteration of DRM-rafts secondary to treatment with methyl-beta-cyclodextrin (MBCD, a drug that preferentially removes cholesterol from the plasma membrane) or addition of exogenous GM1 ganglioside (a lipid component of rafts) blocks the activation of the MAPK pathway and growth cone guidance induced by netrin-1.19 Brain-derived neurotrophic factor (BDNF) and semaforin 3A also promote growth cone guidance by binding to TrkB and neuropilin-1 receptors, respectively, through signalling pathways that are attenuated by treatment with MBCD or by the application of ganglioside GM1.19 These studies suggest that rafts have a significant role in the processes of axonal growth and projection.
Relevance of rafts in synaptic transmissionThe role of rafts in synaptic transmission is particularly important in two essential events: the release of neurotransmitters from presynaptic terminals, and the signalling of their respective postsynaptic receptors. The arrival of an action potential at the presynaptic membrane sequentially causes depolarisation of the membrane, opening of voltage-gated Ca2+ channels, influx of Ca2+ to the nerve terminal, exocytosis of synaptic vesicles, and release of neurotransmitters into the synaptic cleft. The released neurotransmitters target their respective postsynaptic receptors, triggering signalling cascades. Several studies have shown the relevance of cholesterol and rafts in this process.23,24 For example, rafts have been reported to contain proteins involved in exocytosis, such as SNARE proteins (SNAP25, syntaxin 1, and VAMP2), Munc18, synaptophysin, and synaptotagmins.25 Furthermore, studies in cultures of rat hippocampal neurons showed that the use of MBCD or cholesterol synthesis inhibitors (mevastatin or zaragozic acid) significantly reduces the rate of exocytosis.25 In rat presynaptic terminals (synaptosomes), it has been reported that voltage-gated CaV2.1 or P/Q type calcium channels (predominant in central nervous system synapses) are located in rafts and that destabilisation of these rafts by application of saponin or MBCD reduces Ca2+ influx through these channels.26 Other studies support the relevance of DRM-rafts in glutamatergic, GABAergic, dopaminergic, cholinergic, serotonergic, and purinergic signalling.27–30 In most of these cases, DRM-rafts contain postsynaptic neurotransmitter receptors, proteins associated with their signalling cascades, and transporters that recapture neurotransmitters, and the function of these proteins is affected when the rafts are destabilised.
Rafts and synaptic plasticitySynaptic plasticity refers to a change in the efficiency of synaptic transmission, and involves modifications to the neurotransmitter release mechanism and/or changes to postsynaptic elements.31 The efficiency of synaptic transmission may be modulated through existing synapses or by the generation of new ones. These modifications, in turn, may affect the functioning of neural circuits or networks, and processes such as neurodevelopment, learning, and memory.17,31,32 Consistent with their relevance in synaptic transmission, rafts are also known to participate in synaptic plasticity.30,33 A study in rat hippocampal slices showed that a decrease in plasma membrane cholesterol levels (induced by MBCD) interfered with the long-term potentiation mechanism typically observed in this preparation.33,34 The same model was used to show that the removal of cholesterol significantly attenuates the response of postsynaptic N-methyl D-aspartate (NMDA) and α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors.29 Another study on rats undergoing spatial memory training found that NMDA receptors in the insular cortex and hippocampus relocate to DRM-rafts, without a change in their level of expression.27 Another important aspect is the action of neurotrophic factors or neurotrophins (NGF, BDNF, neurotrophin-3, and neurotrophin-4), which are essential to neuronal survival, differentiation, and synaptic plasticity.35 Repetitive neuronal stimulation is known to promote the expression and activity of these proteins, which in turn results in more efficient neurotransmission and synaptic plasticity through a mechanism regulated by the presence of their receptors in DRM-rafts.36,37 The use of BDNF in cultures of cortical and hippocampal neurons from rats has been reported to promote the synthesis and incorporation of cholesterol into rafts, as well as the expression of caveolin-2 and the presynaptic proteins synaptophysin, SNAP-25, and syntaxin.38 All these effects are mediated by the activation and relocation of TrkB and p75 BDNF receptors in DRM-rafts.39 We may therefore expect that alterations in the synthesis of cholesterol and/or constituent proteins of rafts, promoted by BDNF, would modify their composition and organisation and affect the synaptic plasticity processes in which they participate. Finally, the presence of receptor tyrosine kinases (ErbB-type receptors for neuregulins) associated with synaptic plasticity mechanisms has also been reported in rafts.
Participation of rafts in learning and memoryIt is generally acepted that learning and memory processes are based on synaptic plasticity mechanisms, which, as mentioned above, are related to rafts.30,33 Spatial memory formation in rats, for example, involves the translocation of NMDA receptors into DRM-rafts through a mechanism dependent on the scaffolding protein PSD-95.27 Another interesting example is the growth and arborisation of dendrites in cortical neurons from primary cultures of the mouse hippocampus, which is promoted by the overexpression of caveolin-1 and by the concomitant translocation of the protein PSD-95 and the NMDA and TrkB receptors in DRM-rafts.40 Another study found that adult rats fed on a cholesterol-enriched diet for 2 to 4 months exhibited better spatial memory than those receiving a regular diet.41 However, another study with mice treated with statins (cholesterol synthesis inhibitors) showed better performance in learning and memory tests.42 These apparently contradictory results may be explained by the need for an optimum level of cholesterol to achieve raft stability and the development of these processes, as has been shown in other cases.43 Finally, in experiments with rat hippocampal slices treated with statins for 2 to 4 hours, long-term potentiation was favoured without altering baseline levels of synaptic transmission,34 suggesting a specific effect on synaptic plasticity mechanisms. These results suggest that changes in cholesterol levels affect raft formation and related processes (synaptic plasticity, learning, and memory).
The role of rafts in neurological diseasesIn recent years, alterations in the composition and organisation of the DRM-raft have been shown to promote the dysregulation of signalling pathways and neural circuits, which in turn has been associated with several neurological diseases, including AD, Parkinson’s disease, and Huntington disease (Fig. 2).17,32
Alzheimer diseaseAD is the most common neurodegenerative disease and the leading cause of progressive dementia in old age. It is characterised by the intracellular presence of neurofibrillary tangles of Tau protein and the extracellular accumulation of β-amyloid peptide (Aβ), derived from the processing of β-amyloid precursor protein (APP) by the enzymes β- and γ-secretase.44 Recently, there has been increasing interest in the neurotoxicity of Aβ oligomers.45–47 APP is located in DRM-rafts and, consequently, the synthesis, accumulation, and subsequent aggregation of Aβ peptide preferentially occurs in these domains.48–52 Furthermore, the whole group of enzymes participating in the generation of APP is located in DRM-rafts.50,51 This evidence supports the hypothesis that changes in the structure and composition of membrane rafts may be associated with the onset of AD. In this line, epidemiological studies show lower prevalence of AD in patients receiving long-term treatment with statins, which cross the blood-brain barrier.53 Furthermore, post mortem analysis of the temporal cortex of patients with the disease has shown a decrease in DRM-rafts,54 whereas the hippocampus showed lower cholesterol content in these patients than in individuals without the disease.55 Other groups have also reported alterations in the composition of DRM-rafts in samples of the frontal cortex of patients with AD.56 In rat models, long-term application of cholesterol synthesis inhibitors has been shown to reduce the degree of Aβ aggregation.57 Furthermore, a unilamellar vesicle model showed that the GM1 ganglioside regulates the interaction of Aβ monomers, as well as their oligomerisation and neurofibrillary tangle formation, through a cholesterol-dependent mechanism.58,59 These results support the proposal that AD may be considered as a plasma membrane disorder, and that changes in raft composition and structure represent a potential target for its treatment.46,48,53,60
Parkinson’s diseaseThe phospholipid/cholesterol ratio in the brain is increased in patients with Parkinson’s disease.61 Although no direct relationship has been established to date between this change and the characteristic nigrostriatal neurodegeneration observed in the disease,62 it has been suggested that alterations in the lipid composition and, therefore, in the biophysical properties of the plasma membrane may be associated with Parkinson’s disease.63,64 Such modifications may affect the function of proteins such as α-synuclein, parkin, PINK1, and DJ-1, known molecular markers of Parkinson’s disease.62,65–68 In the case of α-synuclein, it has been observed that the interaction of this protein with gangliosides and cholesterol promotes its internalisation and subsequent effect on the plasma membrane of cultured microglial cells.69 A study using atomic force microscopy observed that the presence of α-synuclein hinders raft formation in an artificial membrane model.70 Another study combining atomic force microscopy and neutron scattering techniques reported that the presence of GM1 ganglioside favours the binding of α-synuclein to a lipid bilayer model.71 Post mortem analysis of the frontal cortex of patients with Parkinson’s disease revealed significant alterations in the composition of DRM-rafts, including a higher proportion of saturated fatty acids and a significant decrease in the levels of cerebrosides, compared with samples from patients without Parkinson’s disease.61 Decreased expression of different gangliosides (GD1a, Gd1b, and Gt1b) and other lipid components (phosphatidylethanolamine, phosphatidylcholine, and cerebrosides) has been reported in the substantia nigra of male patients with Parkinson’s disease.72 Although the mentioned studies support the relevance of DRM-rafts in Parkinson’s disease, this hypothesis must be strengthened, without overlooking the above-mentioned debate on the supposed equivalence of rafts and DRM.
Huntington diseaseHuntington disease is a progressive neurodegenerative disease characterised by motor, cognitive, and behavioural alterations.73 It is caused by the abnormal incorporation of glutamine residues at the N-terminal of the huntingtin protein. In mouse models, this disease is associated with a decrease in cholesterol synthesis in the cerebral cortex and striatum, and with neuronal death.74 Post mortem studies of the striatum of patients with Huntington disease revealed a significantly decreased concentration of gangliosides,75 whereas striatum samples from humans and mouse models showed attenuated expression of the genes encoding glycosyltransferases specifically involved in ganglioside synthesis.76 Another group reported the accumulation of mutant huntingtin protein in DRM-rafts, also in mouse models.77 Finally, the addition of exogenous cholesterol to cultured striatal neurons from mouse models reduced their mortality rate,74 whereas intracerebral infusion of the GM1 ganglioside in the same model improved motor dysfunction and reduced the degree of neurodegeneration.78 This suggests that increasing the biosynthesis and/or availability of cholesterol and/or gangliosides may alleviate some aspects associated with Huntington disease.
However, other studies with mouse models have reported the accumulation of cholesterol and mutant huntingtin protein in DRM-rafts.77,79 Furthermore, in cells expressing the mutant huntingtin, it was observed that in addition to huntingtin, caveolin-1,80 GM1 ganglioside, and NMDA receptors are also preferentially located in DRM-rafts. Interestingly, treatment of these cells with MBCD or simvastatin decreased the presence of DRM-rafts and attenuated NMDA-mediated excitotoxicity.80 Consistent with these results, the administration of drugs that counteract the accumulation of cholesterol (simvastatin) may be beneficial in the treatment of Huntington disease. Similarly, levels of cholesterol 24-hydroxylase (CYP46A1), an enzyme that catalyses the conversion of cholesterol to 24-hydroxycholesterol in the central nervous system, are reduced in the putamen of patients with Huntington disease and in the striatum of Huntington disease mouse models.81 Notably, replacement of CYP46A1 in the striatum of mouse models alleviated motor symptoms, increased the average size of medium spiny neurons, and restored cholesterol homeostasis.81 These results suggest that the enzyme CYP46A1 may be a therapeutic target in Huntington disease.
Further studies are clearly needed to solve the apparent contradiction regarding the role of cholesterol and the GM1 ganglioside in Huntington disease, and to establish the molecular and functional principles regulating the delicate balance between stability and instability and between function and dysfunction of rafts, and their corresponding implications in this disease.
Smith-Lemli-Opitz syndromeThe Smith-Lemli-Opitz syndrome is characterised by developmental abnormalities, incomplete myelination, and intellectual disability.82 In this syndrome, the cholesterol content of DRM-rafts is significantly reduced due to the lack of the enzyme 3 β-hydroxysterol-Δ 7-reductase.83,84 Consequently, higher levels of 7-dehydrocholesterol promote changes in the organisation and dynamics of the plasma membrane that may be associated with manifestations of this syndrome.85,86
Niemann-Pick disease type CNiemann-Pick disease type C (NPC) mainly affects adults. It causes progressive dementia, psychiatric symptoms, and abnormalities in the central nervous system (clinical symptoms shared with AD). It is caused by mutations in the NPC1 and NPC2 proteins, and is characterised by the accumulation of cholesterol in endolysosomal vesicles.87 This disease affects vital cellular processes, including vesicular fusion and autophagy. It has been reported that cultured mouse striatal neurons in which the expression of NPC proteins was knocked out do not respond to the application of BDNF, even when they express the TrkB receptor for this neurotrophin.88 These studies suggest that in the absence of functional NPC proteins, cholesterol remains sequestered in endolysosomes, the cholesterol content of the plasma membrane (and the endoplasmic reticulum) decreases, and NPC affects the signalling systems located in the rafts (activation of TrkB receptors by BDNF).89
Lewy body dementiaThis neurodegenerative disease manifests with neuropsychiatric symptoms, cognitive impairment, dementia, and moderate parkinsonian symptoms. It is characterised by the aggregation of α-synuclein molecules, forming Lewy bodies in several structures of the central nervous system, including the brainstem, limbic system, and cerebral cortex.90 Post mortem studies of patients with Lewy body dementia (LBD) have shown that DRM-rafts in frontal cortex cells present significantly lower levels of cholesterol and higher levels of sterol esters than in tissues from individuals without this disease.64 In addition, these patients present significantly higher concentrations of lysophosphatidylcholine, which is undetectable in lipid extracts from the brains of individuals without Lewy body dementia. These lipid species are partly generated by free radical–induced oxidation of polyunsaturated phosphatidylcholines (fatty acids), and their presence indicates oxidative damage to membrane phospholipids. This results in a significant increase in the phospholipid/cholesterol ratio. These changes affect the distribution of proteins associated with DRM-rafts, such as the voltage-dependent anion channel 1 and the cellular prion protein (PrPc), which are displaced from these domains, whereas the level of APP in these domains is increased.63
Neuromyelitis opticaNeuromyelitis optica is an inflammatory demyelinating disease of the central nervous system caused by binding of the NMO-IgG antibody to the aquaporin-4 protein (AQP4) in astrocytes, triggering a cytotoxic process.91 AQP4 is a transmembrane protein mainly located in the DRM-rafts. In OS3 cells (a mouse astrocyte line), application of MBCD or simvastatin causes the translocation of AQP4 to regions outside DRM-rafts, which decreases the cytotoxicity promoted by NMO-IgG obtained from patients with the disease.92
Creutzfeldt-Jakob diseaseTransmissible spongiform encephalopathies, such as Creutzfeldt-Jakob disease, are caused by PrP.93 This degenerative disease is due to a conformational change of PrPc that gives rise to the infectious form PrPSc.94 Rafts appear to play a critical role in this conversion, as administration of statins or filipin to hamster brain cells, neuroblastoma (ScN2a) cells, or PrPc-expressing CHO cells removes PrPc from DRM-rafts and prevents the formation of PrPSc.95
ConclusionsMembrane rafts are lipoproteic structural domains that promote the efficient modulation of physiological processes associated with the plasma membrane. The principles underlying the assembly-dissociation-signalling dynamics of these molecular complexes in different cellular scenarios and contexts are currently a highly active area of research. In the nervous system, alterations in these domains have been implicated in the development of several neurological diseases (Fig. 2). The pathophysiological relevance of rafts may lead to new therapeutic strategies for these diseases.
FundingThis study was partially funded by the Mexican National Council of Science and Technology (CONACYT) through agreements IFC-2016-1955 (U.M., S.S.-A., A.A.R.-M.) and CB-284443 (A.A.R.-M.) and by the Universidad Autónoma de San Luis Potosí through agreements C19-FAI-05-59.59 (U.M.) and C18-FRC-08-03.03 (A.A.R.-M.). These institutions did not participate in the design, development, analysis, drafting, or decision to submit this study for publication.
Conflicts of interestThe authors have no conflicts of interest to declare.