The effect of SARS-CoV-2 infection in patients with multiple sclerosis (MS) and the influence of disease-modifying therapies (DMT) for MS on COVID-19 are unknown. To date, patients with MS have not been shown to present greater risk of COVID-19 or more severe progression of the disease.
MethodsWe performed a descriptive study of patients with MS presenting SARS-CoV-2 infection diagnosed with PCR. We analysed demographic, clinical, laboratory, and treatment variables in our sample. Presence of antibodies against the virus was also determined.
ResultsRelapsing-remitting MS (RRMS) was the most frequent form of MS in our sample. Prognosis was unfavourable in 10.2% of patients, and was associated with older age and higher scores on the Expanded Disability Status Scale (EDSS). Seroprevalence of antibodies against SARS-CoV-2 was 83.3% in our sample. Development of antibodies was not associated with DMT, lymphocytopaenia, or any of the other variables analysed.
ConclusionsThe incidence of COVID-19 was slightly higher in our sample than in the general population in our province. Unfavourable prognosis was associated with older age and higher EDSS scores. DMT and lymphocytopaenia did not influence the clinical course of COVID-19. Seroprevalence of antibodies against the virus in our sample was similar to that reported for the general population with positive PCR results for the virus; the influence of specific DMTs could not be determined.
El efecto de la infección por SARS-CoV-2 en los pacientes con esclerosis múltiple (EM) y la influencia de los tratamientos modificadores de la enfermedad (TME) es desconocida. Hasta el momento no se ha observado que los pacientes con EM tengan mayor riesgo de infección por COVID-19 ni peor curso evolutivo de la misma.
MétodosEstudio descriptivo de pacientes con EM e infección por SARS-CoV-2 diagnosticada mediante PCR. Hemos analizado variables demográficas, clínicas, de laboratorio y de tratamiento en nuestra muestra. Se ha determinado la presencia de anticuerpos frente SARS-CoV-2 en estos pacientes.
ResultadosLa forma de esclerosis múltiple remitente recurrente (EMRR) fue la más frecuente en los pacientes con EM e infección por COVID-19. El 10.2% presentó una evolución desfavorable, relacionada con una mayor edad y una EDSS más elevada. La seroprevalencia de anticuerpos frente a SARS-CoV-2 en nuestro estudio ha sido del 83,3%. El desarrollo de anticuerpos no está relacionado con el TME, la presencia de linfopenia u otros factores analizados.
ConclusionesLa incidencia de COVID-19 ha sido ligeramente superior a la de la población general de nuestra provincia. La evolución desfavorable se ha relacionado con una mayor edad y una puntuación elevada en la EDSS. El TME y la linfopenia no se han relacionado con el curso de la infección por COVID-19. La seroprevalencia es similar a la encontrada en población general con PCR positiva, sin poder determinar la influencia de los distintos TME.
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is a coronavirus that was first identified in 2019. The associated disease, COVID-19, has rapidly spread across the world, leading the World Health Organization to declare a pandemic in March 2020. During this time, COVID-19 has caused high rates of morbidity and mortality, with a total of 1 928 265 confirmed infections and 50 837 deaths in Spain as of 31 December 2020.1
The impact of SARS-CoV-2 infection on patients with multiple sclerosis (MS) is yet to be determined. Disease-modifying treatments (DMTs) may have an immunosuppressive effect, hindering an adequate immune response to the virus.2 Furthermore, increased risk of infection is a known adverse effect of DMTs, with some causing lymphocytopaenia, which has been identified as a marker of poor prognosis in patients with COVID-19.3,4 Despite this, no relationship has yet been established between DMTs and poorer clinical outcomes.5,6 Furthermore, MS may be exacerbated in association with SARS-CoV-2 infection. In this sense, the rate of MS relapses in patients with COVID-19 is variable, ranging from 4.3% to 21%,7,8 with no available data on long-term disability in these patients.
In the general population, some factors related to higher COVID-19–associated mortality are: age; male sex; comorbidities including chronic kidney disease (CKD), chronic obstructive pulmonary disease (COPD), or cerebrovascular disease; and laboratory findings including leukocytosis or lymphocytopaenia.9 Age, presence of comorbidities, and male sex are markers of poor prognosis in patients with MS who also present COVID-19, as in the general population. Furthermore, higher scores on the Expanded Disability Status Scale (EDSS), progressive forms of MS,5,10 requiring support for walking,11 and obesity are associated with poorer outcomes.
Our study analyses the demographic, clinical, laboratory, and treatment characteristics of patients with MS and SARS-CoV-2 infection. We determined seroprevalence and variables that may be associated with the development of IgG antibodies.
Patients and methodsWe conducted an observational descriptive study of patients with MS (clinically isolated syndrome [CIS], relapsing-remitting MS [RRMS], secondary progressive MS [SPMS], and primary progressive MS [PPMS]) and history of PCR-confirmed SARS-CoV-2 infection. We included patients from the demyelinating disease clinic at Hospital Universitario Clínico San Cecilio (HUCSC) in Granada (Spain) from the beginning of the pandemic to 31 December 2020.
Demographic, clinical, and laboratory data were obtained by reviewing clinical histories and holding telephone interviews. The variables gathered were: age, sex, type of MS, progression time in months, current DMT, baseline EDSS score, lymphocyte count before the infection, comorbidities (diabetes mellitus [DM], arterial hypertension [AHT], obesity, asthma, COPD, ischaemic heart disease, stroke, chronic liver disease, CKD), and institutionalisation. Regarding COVID-19, we recorded symptoms, treatment received, hospitalisation/admission to the ICU, presence of symptoms suggestive of an MS relapse, presence of IgG antibodies against SARS-CoV-2, and date of PCR test.
Data were gathered and handled anonymously. We performed a descriptive statistical analysis of the demographic and clinical variables of patients with MS and COVID-19, as well as serology results. These variables were compared by grouping patients according to MS type and severity of COVID-19 progression. We used the Fisher exact test to compare qualitative variables and the Mann-Whitney U test to compare non-parametric quantitative variables. Statistical analysis was performed using the R Commander software (version 3.6.2, 2019). Our study was approved by our hospital’s ethics committee.
ResultsOf the 475 patients with MS included in our hospital’s database, 28 were diagnosed with PCR-confirmed SARS-CoV-2 infection between March 2020 and December 2020, accounting for 5.89% of patients with MS. Mean age (SD) was 49.1 (16.1) years. Women accounted for 64.3% of the sample, with a mean age of 47.6 (14.9) years; men (35.7%) had a mean age of 51.8 (18.6) years. In our series, 5 patients (17.8%) were institutionalised in assisted living centres due to MS-related disability.
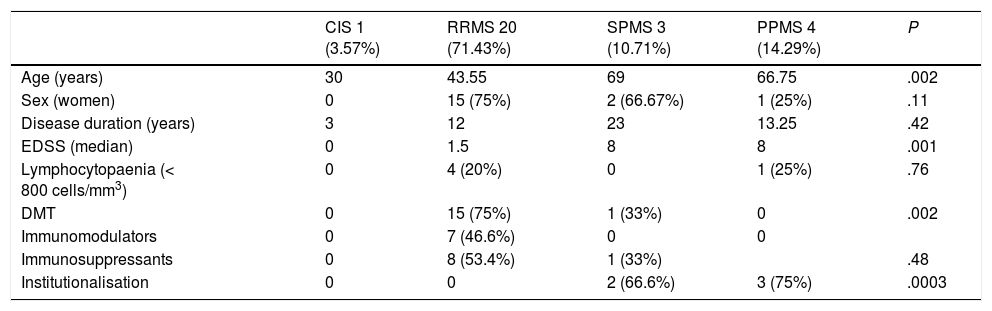
Table 1 includes demographic, clinical, and laboratory data on patients with MS, according to the clinical type of MS. No significant differences were observed between groups, except for higher mean age and EDSS scores in patients with progressive forms. The number of patients receiving DMTs was also different, with a higher percentage among patients with relapsing-remitting forms.
Demographic, clinical, and analytical characteristics of patients with multiple sclerosis and SARS-CoV-2 infection.
| CIS 1 (3.57%) | RRMS 20 (71.43%) | SPMS 3 (10.71%) | PPMS 4 (14.29%) | P | |
|---|---|---|---|---|---|
| Age (years) | 30 | 43.55 | 69 | 66.75 | .002 |
| Sex (women) | 0 | 15 (75%) | 2 (66.67%) | 1 (25%) | .11 |
| Disease duration (years) | 3 | 12 | 23 | 13.25 | .42 |
| EDSS (median) | 0 | 1.5 | 8 | 8 | .001 |
| Lymphocytopaenia (< 800 cells/mm3) | 0 | 4 (20%) | 0 | 1 (25%) | .76 |
| DMT | 0 | 15 (75%) | 1 (33%) | 0 | .002 |
| Immunomodulators | 0 | 7 (46.6%) | 0 | 0 | |
| Immunosuppressants | 0 | 8 (53.4%) | 1 (33%) | .48 | |
| Institutionalisation | 0 | 0 | 2 (66.6%) | 3 (75%) | .0003 |
CIS: clinically isolated syndrome; DMT: disease-modifying treatment; EDSS: Expanded Disability Status Scale; PPMS: primary progressive multiple sclerosis; RRMS: relapsing-remitting multiple sclerosis; SPMS: secondary progressive multiple sclerosis.
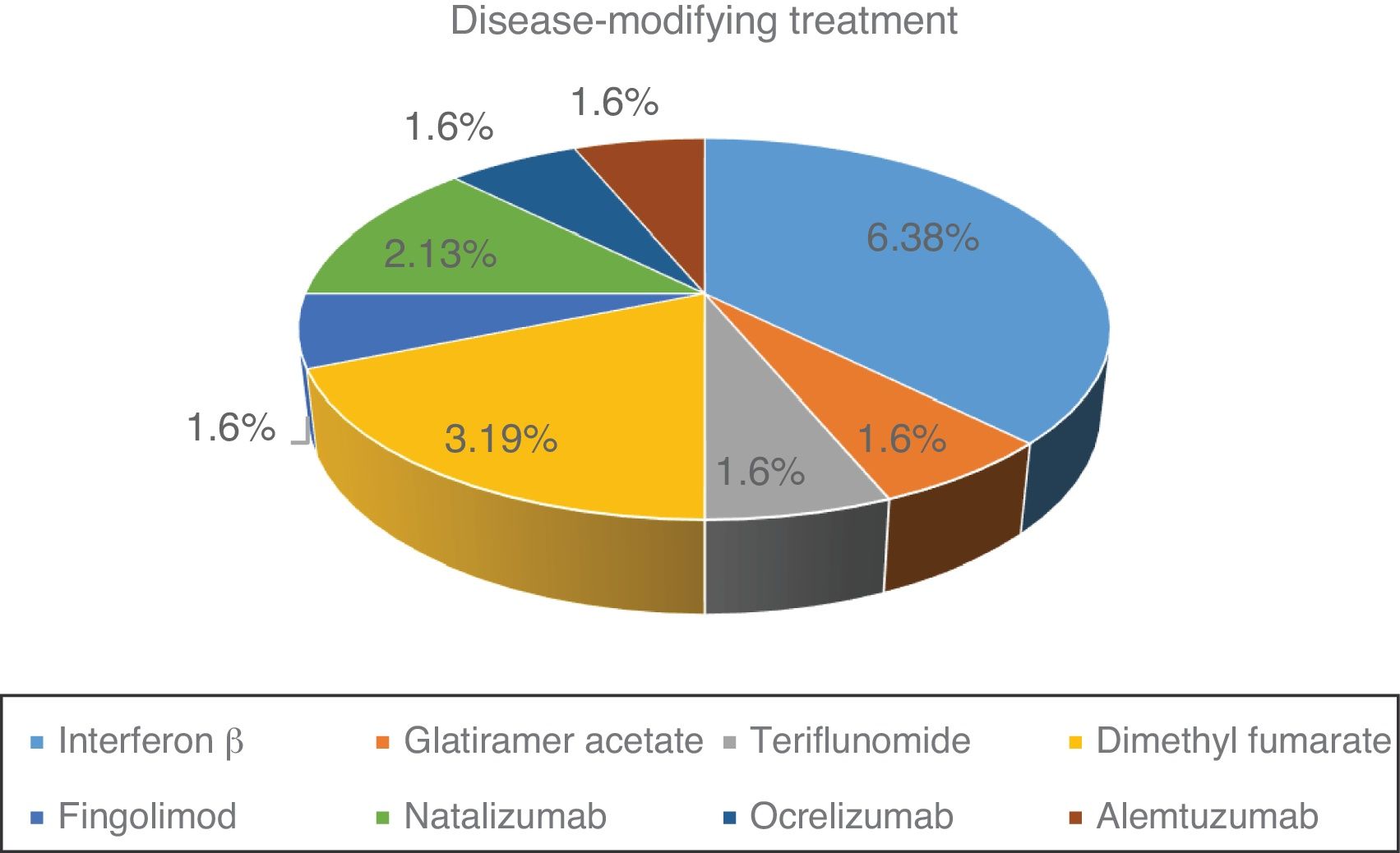
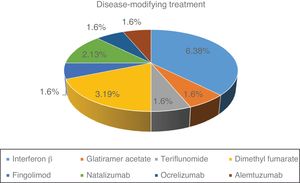
Fig. 1 shows the distribution of the different DMTs in our series. At the time of SARS-CoV-2 infection, 57.1% of patients were under treatment. Of the 16 patients treated, 11 (68.7%) were receiving first-line treatments and 5 (31.3%) second-line treatments.
The most frequent comorbidity in our series was obesity, in 8 patients (28.6%), followed by active smoking, in 7 patients (25%), AHT in 6 (21.43%), DM in 3 (10%), asthma in 2 (7.1%), and cardiovascular disease in one (3.6%). No significant association was observed between comorbidity and the type of MS, with the exception of DM, which was less frequent in the group of patients with RRMS (P > .017).
Lymphocyte count before SARS-CoV-2 infection was normal in 23 patients (82.1%). The remaining patients (27.9%) presented lymphocytopaenia, which was grade 1 in one patient (3.6%), grade 2 in one patient (3.6%), and grade 3 in 3 patients (10.7%). There was no significant association between type of MS and previous history of lymphocytopaenia. Mean lymphocyte count was 1540 cells/mm3 in patients treated with beta interferon, 1930 cells/mm3 in those treated with glatiramer acetate, 2350 cells/mm3 for teriflunomide, 1340 cells/mm3 for dimethyl fumarate, 450 cells/mm3 for fingolimod, 4030 cells/mm3 for natalizumab, 1190 cells/mm3 for ocrelizumab, and 43 cells/mm3 for alemtuzumab.
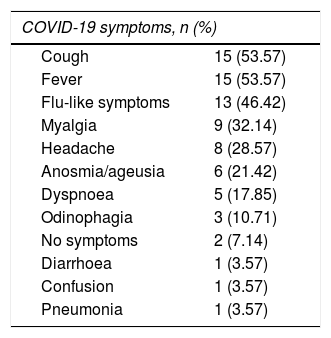
These patients’ symptoms were similar to those reported in other series. Infection was asymptomatic in 2 patients (7.1%). One patient was an institutionalised 65-year-old man with untreated progressive MS, who underwent a PCR test due to close contact with a PCR-positive patient. The other was a 21-year-old man with internuclear ophthalmoplegia, who required hospital admission and underwent a PCR test. Table 2 lists the symptoms associated with COVID-19. We found no significant association between symptoms of SARS-CoV-2 infection and type of MS. Patients with anosmia/ageusia presented a lower mean age: 35.8 vs 52.7 years (P = .02). Progression was good in 89.3% of patients. Only 3 (10.7%) presented unfavourable progression, requiring hospital admission due to cytokine release syndrome and the need for oxygen therapy. Regarding treatment for COVID-19, 5 patients (17.85%) received corticotherapy, either with methylprednisolone boluses or with oral dexamethasone. None had to be admitted to the ICU, and one (3.6%) died due to COVID-19–associated complications. Only one patient (3.6%) presented symptoms compatible with a relapse, although he showed no symptoms of COVID-19. His symptoms improved after administration of methylprednisolone and subsequent decreasing doses of oral prednisone.
Symptoms reported during the acute phase of COVID-19.
| COVID-19 symptoms, n (%) | |
|---|---|
| Cough | 15 (53.57) |
| Fever | 15 (53.57) |
| Flu-like symptoms | 13 (46.42) |
| Myalgia | 9 (32.14) |
| Headache | 8 (28.57) |
| Anosmia/ageusia | 6 (21.42) |
| Dyspnoea | 5 (17.85) |
| Odinophagia | 3 (10.71) |
| No symptoms | 2 (7.14) |
| Diarrhoea | 1 (3.57) |
| Confusion | 1 (3.57) |
| Pneumonia | 1 (3.57) |
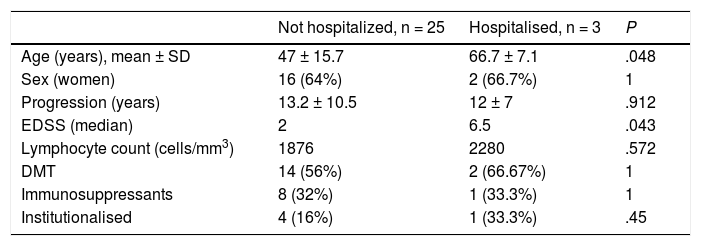
Table 3 presents the demographic and clinical characteristics of patients with severe COVID-19, requiring hospital admission, and those who were not admitted. There were no significant differences between these 2 groups, with the exception that hospitalised patients were older and presented higher EDSS scores (median of 6.5). There was no significant association between disease severity and initial COVID-19 symptoms and disease severity, or with the DMTs used at the time of infection. We also observed no association between progression and presence of comorbidities.
Demographic and clinical characteristics of patients with severe COVID-19.
| Not hospitalized, n = 25 | Hospitalised, n = 3 | P | |
|---|---|---|---|
| Age (years), mean ± SD | 47 ± 15.7 | 66.7 ± 7.1 | .048 |
| Sex (women) | 16 (64%) | 2 (66.7%) | 1 |
| Progression (years) | 13.2 ± 10.5 | 12 ± 7 | .912 |
| EDSS (median) | 2 | 6.5 | .043 |
| Lymphocyte count (cells/mm3) | 1876 | 2280 | .572 |
| DMT | 14 (56%) | 2 (66.67%) | 1 |
| Immunosuppressants | 8 (32%) | 1 (33.3%) | 1 |
| Institutionalised | 4 (16%) | 1 (33.3%) | .45 |
EDSS: Expanded Disability Status Scale; DMT: disease-modifying treatment.
Data are presented as mean ± standard deviation or absolute frequencies and percentages.
Seroprevalence was analysed in 24 patients (85.7% of the series). Twenty patients (83.3%) tested positive for IgG antibodies against SARS-CoV-2, with negative results obtained in 4 patients (16.7%). Antibody determination was performed between 1-8 months after the first positive PCR test result, after a median time of 2 months. Latency between positive PCR test results and antibody determination was 1-2 months in 11 patients; 3-6 months in 7 patients, and more than 6 months in 3 patients. Of the patients with negative PCR test results, one was receiving no DMT and the other 3 were receiving ocrelizumab, fingolimod, and dimethyl fumarate. Seropositivity in our sample showed no association with the use or type of DMT, or lymphocyte levels. There was no association between IgG antibodies against SARS-CoV-2 and the demographic, clinical, and treatment variables analysed.
DiscussionIncidence of COVID-19 among patients with MS amounted to 5.9%, which is slightly lower than the incidence of 6.3% reported for the general population of Granada.13 This figure is also lower than that reported in the series of patients with MS and COVID-19 from the Multiple Sclerosis Centre of Catalonia (Cemcat).7 This may be related to the fact that we only included patients with a PCR-confirmed diagnosis and excluded cases suspected due to compatible clinical and/or radiological signs.
The most frequent type of MS was RRMS. Patients with progressive forms were older and presented higher EDSS scores. The most frequent comorbidities were obesity, AHT, and active smoking. The prevalence of comorbidities was lower in the French series,12 whereas the frequency of obesity, DM, and AHT was similar to that reported by the New York University Multiple Sclerosis Comprehensive Care Center.8
With severe COVID-19 defined as the need for hospitalisation or ICU admission, and the remaining cases considered as mild or moderate, only 3 patients presented severe COVID-19. This represents a hospitalisation rate of 10.7%, which is half of that reported in other series but higher than the 8.3% rate of hospitalisation in patients with COVID-19 in the general population of the province of Granada.13 As in other registries, we observed that patients with severe forms were older and presented higher EDSS scores.7,8,10,14 We found no relationship between presence of comorbidities and severity of COVID-19, probably due to the small sample size and the low percentage of hospitalised patients.
Despite the influence of DMTs on immunity, these drugs seem not to increase the risk of SARS-CoV-2 infection or poorer progression of the disease,15–17 even in patients with lymphocytopaenia.7 The data obtained in our series are similar to those from other studies, with no relationship between more severe COVID-19 and the use of DMTs18 or lymphocytopaenia.7 Of our patients, 57% were receiving DMTs. Of the patients under treatment, only 2 had to be hospitalised, and none presented lymphocytopaenia. We only recorded one death, a patient receiving no DMT and whose lymphocyte count was normal. The fact that DMTs do not negatively impact the course of COVID-19 may partly be explained by their action mechanism.19 More severe cases of COVID-19 are those that develop respiratory distress syndrome partly mediated by a hyperimmune response. This response causes an excessive release of proinflammatory components, or cytokine storm, leading to T-cell depletion, inflammation, and lung damage.20 Considering the effects of the different treatments on the immune system, it has been suggested that some DMTs may be able to prevent or mitigate these proinflammatory responses to COVID-19.5,21,22
This issue is especially relevant due to the epidemiological implications and the influence on vaccination schedules of patients with MS, as DMTs may affect the development of antibodies against SARS-CoV-2. The seroprevalence described in asymptomatic patients with MS is comparable to that of the general population with a similar degree of exposure.23 To date, little evidence is available on the percentage of patients with MS who are able to develop antibodies against SARS-CoV-2 after the infection, and whether this ability is particularly conditioned by DMTs. In the Cemcat registry, 45.6% of patients presented positive serology results for SARS-CoV-2. Patients treated with anti-CD20 antibodies less frequently developed antibodies.7 In our series, 83.3% of patients presented positive results for antibodies against SARS-CoV-2. This percentage is significantly higher than that observed in the Cemcat registry, but similar to the 88.6%-–91% obtained in the general population with positive PCR findings for the virus.24 Seropositivity showed no significant association with DMTs, lymphocytopaenia, or such other variables as age. The difference between our results and other studies may be justified by the inclusion of patients with positive PCR tests only, and by the variable latency between positive PCR test and antibody determination.
The main limitation of our study is its cross-sectional, observational design, which only included PCR-confirmed cases. IgG antibodies against SARS-CoV-2 were determined at different times, and no medium-term follow-up is available. As only 57% of our patients were receiving treatment at the time of SARS-CoV-2 infection, we cannot draw definitive conclusions on the influence of treatment and seroprevalence.
The impact of different DMTs on the development of antibodies and whether antibodies persist is yet to be determined. Larger seroprevalence studies in patients with MS and COVID-19 are necessary.
FundingThis study received no funding of any kind.
Conflicts of interestRaquel Piñar Morales has received lecture honoraria from Almirall, Biogen, Merck, Novartis, Roche, and Sanofi-Aventis. Francisco J. Barrero Hernández has received consulting fees and lecture honoraria from Almirall, Biogen, Merck, Novartis, Roche, Sanofi, and TEVA.
Please cite this article as: Piñar Morales R, Ramírez Rivas MA, Barrero Hernández FJ, Infección por SARS-CoV-2 y seroprevalencia en pacientes con esclerosis múltiple. Neurología. 2021;36:698–703.