To estimate the pooled prevalence of cancer in patients with multiple sclerosis (MS) cases who were under treatment with rituximab.
MethodsWe searched PubMed, Scopus, EMBASE, Web of Science, and google scholar along with gray literature up to April 2021.
The search strategy included the MeSH and text words as ((“CD20 Antibody” AND Rituximab) OR “Rituximab CD20 Antibody” OR Mabthera OR “IDEC-C2B8 Antibody” OR “IDEC C2B8 Antibody” OR IDEC-C2B8 OR “IDEC C2B8” OR GP2013 OR Rituxan OR rituximab) AND ((Sclerosis AND multiple) OR (sclerosis AND disseminated) OR "disseminated sclerosis" OR "multiple sclerosis" OR "acute fulminating").
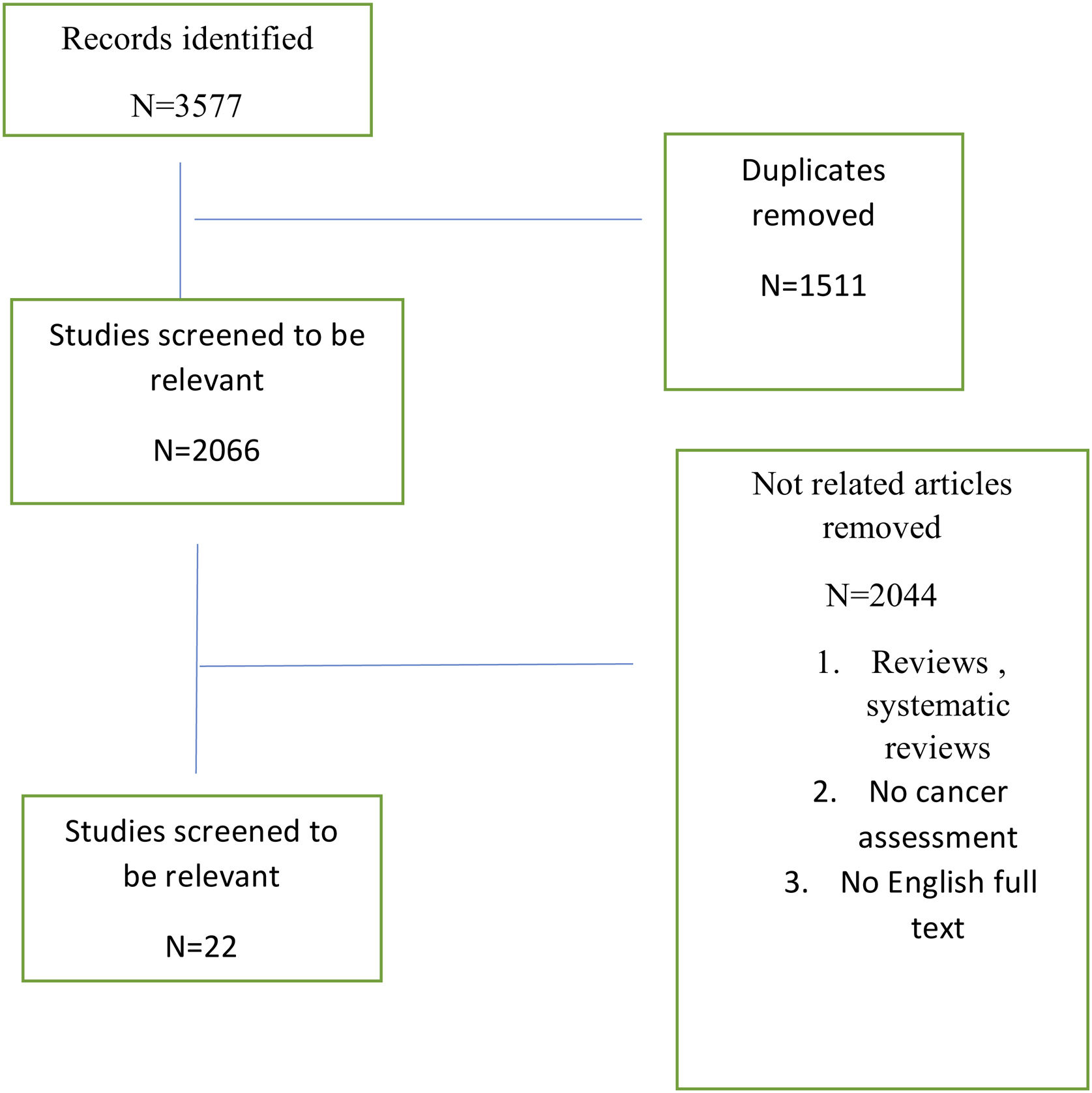
ResultsThe literature search revealed 3577 articles, after deleting duplicates 2066 remained. For the meta-analysis, 22 studies were included. Totally, 15599 patients were enrolled while 133 cancers were detected.
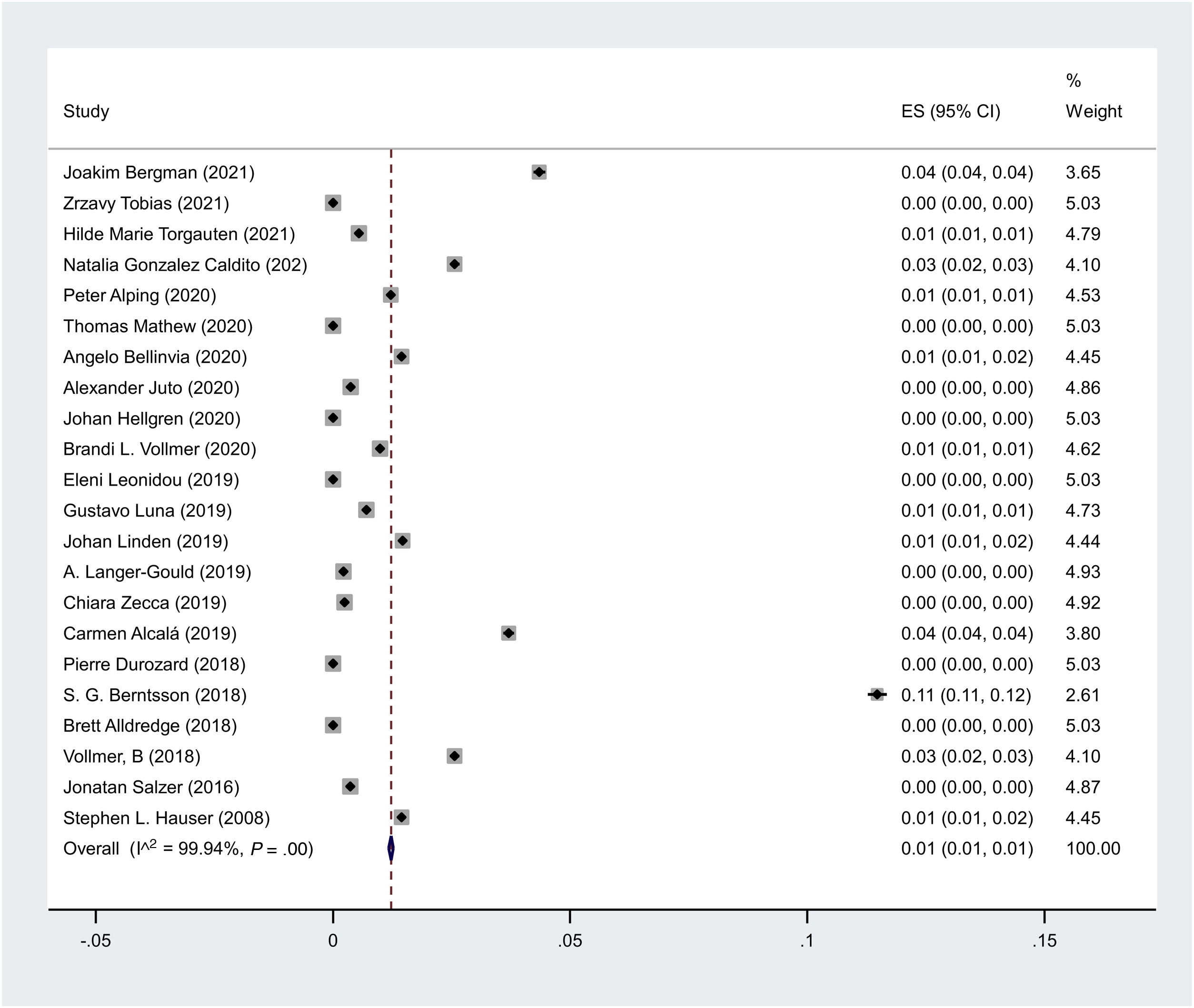
The pooled prevalence of cancer in MS patients under treatment with rituximab is 1in 100,000 (I2 = 99.9%, p < 0.001).
ConclusionThe results of this systematic review and meta-analysis show that the pooled prevalence of cancer in MS patients who received rituximab is 1 in 100,000 cases.
estimar la prevalencia combinada de cáncer en pacientes con casos de esclerosis múltiple (EM) que estaban en tratamiento con rituximab.
MétodosRealizamos búsquedas en PubMed, Scopus, EMBASE, Web of Science y Google Scholar junto con literatura gris hasta abril de 2021.
La estrategia de búsqueda incluyó el MeSH y palabras de texto como (("CD20 Antibody” AND Rituximab) OR “Rituximab CD20 Antibody” OR Mabthera OR “IDEC-C2B8 Antibody” OR “IDEC C2B8 Antibody” OR IDEC-C2B8 OR “IDEC C2B8” OR GP2013 O Rituxan O rituximab) Y ((Esclerosis Y múltiple) O (esclerosis Y diseminada) O "esclerosis diseminada" O "esclerosis múltiple" O "fulminante aguda").
ResultadosLa búsqueda bibliográfica reveló 3577 artículos, después de eliminar los duplicados quedaron 2066. Para el meta-análisis se incluyeron 22 estudios. En total, se inscribieron 15599 pacientes y se detectaron 133 cánceres. La prevalencia combinada de cáncer en pacientes con EM en tratamiento con rituximab es de 1 en 100.000 (I2 = 99,9 %, p < 0,001).
ConclusiónLos resultados de esta revisión sistemática y meta-análisis muestran que la prevalencia combinada de cáncer en pacientes con EM que recibieron rituximab es de 1 en 100 000 casos.
Multiple sclerosis (MS) is an autoimmune disease of central nervous system that affects women more than men all over the world.1 Different medications are administered in subjects with MS while their efficacy and safety vary.
It was assumed that MS is a T-cell mediated disease while, nowadays B-cells along with T-cells are considered in pathogenesis of MS.2 So, new disease modifying therapies (DMTs) are considered to have superior effects on disease progression and disability status.
CD20-positive B-cells play an important role in the pathogenesis of MS and therapies targeting these cells such as Rituximab (RTX) (immunoglobulin G1, mouse chimeric) are used widely nowadays.3 Rituximab is a chimeric monoclonal antibody which reduces the number of CD20 presenting B-cells by targeting plasma cell precursors.4 It acts peripherally and its level is not high in CSF.5 The B-cells depletion will result in antibody production decrease as well as inactivation of T-cells and macrophages.6 It is not associated with increased risk of cancer in MS.
Rituximab is approved for patients with rheumatoid arthritis (RA) and lymphatic cancers which is used in MS cases (off-label).7 A recent systematic review demonstrated that rituximab has acceptable efficacy and safety in patients with MS.3
Cancers and autoimmune diseases share common etiologies such as smoking, genetics, and vitamin D deficiency and administration of some medications.8,9
Previous published studies show that the prevalence of cancers in MS patients receiving rituximab is diverse. So, we designed this systematic review and meta-analysis to estimate the pooled prevalence of cancer in patients with MS cases who were under treatment with rituximab.
MethodsWe searched PubMed, Scopus, EMBASE, Web of Science, and google scholar along with gray literature up to April 2021.
The search strategy included the MeSH and text words as (("CD20 Antibody” AND Rituximab) OR “Rituximab CD20 Antibody” OR Mabthera OR “IDEC-C2B8 Antibody” OR “IDEC C2B8 Antibody” OR IDEC-C2B8 OR “IDEC C2B8” OR GP2013 OR Rituxan OR rituximab) AND ((Sclerosis AND multiple) OR (sclerosis AND disseminated) OR "disseminated sclerosis" OR "multiple sclerosis" OR "acute fulminating").
Inclusion criteria: Cross sectional studies, cohort studies. Articles published in the English language.
Exclusion criteria: case-reports, RCT studies.
We collected data regarding first author, country of origin, number of enrolled patients, number of possible/confirmed cases, mean age, F/M ratio, mean Expanded Disability Status Scale (EDSS), mean duration of the disease, and number of patients with cancer.
Risk of bias assessmentWe evaluated the risk of potential bias by the NEWCASTLE - OTTAWA QUALITY ASSESSMENT SCALE (adapted for cohort studies).10 The value ≥ 4 was considered acceptable for us.
Statistical analysisAll statistical analyses were performed using STATA (Version 14.0; Stata Corp LP, College Station, TX, USA). We used random effects model.
To determine heterogeneity, inconsistency (I2) was calculated.
We estimated the prevalence in 100 000 cases.
ResultsThe literature search revealed 3577 articles, after deleting duplicates 2066 remained. For the meta-analysis, 22 studies were included (Fig. 1).
Totally, 15599 patients were enrolled while 133 patients with cancers were detected (Table 1).
Basic characteristics of the included studies.
| Author | Year | Country | Design | T. MSF. MSM. MS | MS type | Age | EDSS | Disease duration (mean (sd)) | Total Malignancy type | Duration of treatment (mean) (month) | NOS |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Joakim Bergman11 | 2021 | Sweden | Trial | 23 | SP 15 | 46 (9) | Median (IQR) | 14 (8) | Basalioma 1 | 12 | 4 out of 6 |
| 16 | PP 8 | 6.5 (1.0) | |||||||||
| 7 | |||||||||||
| Zrzavy Tobias12 | 2021 | Austria | Cohort | 119 | RR 87 | 36.8 (10.2) | 3.0 (3.0) | 8.7 (6.5) | 0 | - | 7 Out of 9 |
| 89 | SP 32 | ||||||||||
| 30 | |||||||||||
| Hilde Marie Torgauten13 | 2021 | Norway | Cohort | 365 | RR 320 | 42.3 (12.2) | 2 (0-8) | 5.3 (7) | Malignant disease 2 (colon cancer, melanoma) | 19.2 | 8 Out of 9 |
| 255 | SP 23 | ||||||||||
| 110 | PP 22 | ||||||||||
| Natalia Gonzalez Caldito14 | 2020 | USA | Cohort | 623 | RR 37 | 43.89 (12.66) | NR | NR | 16 | - | 7 Out of 9 |
| 361 | Progressive 35 | Breast cancer 11 | |||||||||
| 145 | NR 551 | Malignant melanoma 5 | |||||||||
| NR 117 | |||||||||||
| Peter Alping7 | 2020 | Sweden | Cohort | 4187 | PP 168 | 42.9 (11.3) | 2.8 (2.0) | 10.3 (8.7) | 51 | 26.4 | 7 Out of 9 |
| 2968 | RR 3215 | Basal cell 20 | |||||||||
| 1219 | PR 71 | CIN3 (females) 15 | |||||||||
| SP 732 | Breast (females) | ||||||||||
| 6 | |||||||||||
| Prostate (males) | |||||||||||
| 2 | |||||||||||
| Melanoma 4 | |||||||||||
| Non-Melanoma Skin Cancer 3 | |||||||||||
| Lymphoma 1 | |||||||||||
| Thomas Mathew15 | 2020 | India | Cohort | 80 | RR 58 | NR | NR | NR | 0 | Between 12 and 36 | 5 Out of 9 |
| 54 | SP 15 | ||||||||||
| 26 | PP 7 | ||||||||||
| Angelo Bellinvia16 | 2020 | Italy | Cohort | 69 | RR 53 | 30.2 (10.8) | 3.0 (2–6) | 7.8 (5.7) | pancreatic carcinoma 1 | 16 | 5 Out of 9 |
| 51 | Progressive 16 | ||||||||||
| 18 | |||||||||||
| Alexander Juto17 | 2020 | Sweden | Cohort | 808 | RR 808 | 38.5 (10.3) N = 502 | 2.0 (0–8) N = 502 | 7.9 (SD 7.2) N = 502 | Cholangiocarcinoma 2 | - | 6 Out of 9 |
| 579 | 38.6 (10.0) | 2.0 (0–6) N = 77 | 8.0 (SD 7.0) N = 77 | sigmoid cancer 1 | |||||||
| 229 | N = 77 | ||||||||||
| Johan Hellgren18 | 2020 | Sweden | Cohort | 83 | RR 66 | 44 (17–69) | NR | 6.4 (0.0–26.3) | 0 | 23.8 | 5 Out of 9 |
| 55 | SP 13 | ||||||||||
| 28 | PP 4 | ||||||||||
| Brandi L. Vollmer19 | 2020 | USA | Cohort | 907 | RR 574 | 43(12.5) | NR | 9.1(8.3) | 9 | 40.6 | 6 Out of 9 |
| 608 | SP 215 | Breast cancer 4 | |||||||||
| 299 | PP 118 | Papillary thyroid cancer 2 | |||||||||
| Glioblastoma 1 | |||||||||||
| Bladder cancer 1 | |||||||||||
| Metastaticsquamous cell carcinoma 1 | |||||||||||
| Eleni Leonidou20 | 2019 | Turkey | Cohort | 30 | RR 10 | mean (SD) | 4.5 (2.5–7.5) N = 10 | 14 (7) N = 10 | 0 | 18 | 5 Out of 9 |
| 14 | SP 17 | 37.9 (7.3) N = 10 | 6 (3.5–8) N = 17 | 15 (6.5) N = 17 | |||||||
| 16 | PP 3 | 47.3 (9) N = 17 | 6 (5–8) N = 3 | 11 (12.8) N = 3 | |||||||
| 52 (20) N = 3 | |||||||||||
| Gustavo Luna21 | 2019 | Sweden | Cohort | 3260 | RR 3260 | 40.4 (10.6) | 2.1 (1.5) | 8.7 (7.6) | 23 | 36 | 7 Out of 9 |
| 2358 | |||||||||||
| 902 | |||||||||||
| Johan Linden22 | 2019 | Sweden | Cohort | 272 | RR 207 | 40.7 (11.7) | 2.0 (0–9) | 9.6 (8.0) | 4 | 43 | 6 Out of 9 |
| 187 | SP 45 | Cervical cancer 2 | |||||||||
| 85 | PP 20 | Basalioma 2 | |||||||||
| A. Langer-Gould23 | 2019 | USA | Cohort | 3165 | NR | NR | NR | NR | Breast cancer 7 | 24 | NA |
| *ABS* | 3057 | ||||||||||
| 108 | |||||||||||
| Chiara Zecca24 | 2019 | Switzerland | Cohort | 414 | NR | NR | NR | NR | mediastinal neoplasm 1 | 22.8 | 5 Out of 9 |
| Carmen Alcalá25 | 2019 | Spain | Cohort | 27 | RR 27 | 34.6 (8.3) | 3.1 (1.3) | NR | Cancer 1 | 32 | 5 Out of 9 |
| 21 | |||||||||||
| 6 | |||||||||||
| Pierre Durozard26 | 2018 | France | Cohort | 50 | RR 50 | 37.5 (19–63) | 4.5 (0–7) | NR | Neoplastic 0 | 13.2 | 5 Out of 9 |
| 38 | |||||||||||
| 12 | |||||||||||
| S.G. Berntsson27 | 2018 | Sweden | Cohort | 61 | RR 43 | Mean 41.5 | NR | Mean years 6.6 | 7 | - | 5 Out of 9 |
| 39 | SP 13 | Neoplasms malignant (rectal squamous cancer) 2 | |||||||||
| 22 | PP 5 | Colorectal carcinoma 5 | |||||||||
| Brett Alldredge28 | 2018 | USA | Cohort | 40 | RR 23 | NR | NR | NR | 0 | 34.8 | 4 Out of 9 |
| 28 | PP 17 | ||||||||||
| 12 | |||||||||||
| Vollmer, B29 | 2018 | USA | Cohort | 125 | RR 78 | mean 44.1 years | NR | Mean 10.2 years | malignant cancer 2 | 31.1 | NA |
| *ABS* | 90 | SP 30 | |||||||||
| 35 | PP 17 | ||||||||||
| Jonatan Salzer30 | 2016 | Sweden | Cohort | 822 | RR 557 | 42.6 (11.1) | 3 (0–9) | 11.3 (8.5) | 3 | 21.8 | 6 Out of 9 |
| 545 | SP 198 | Basalioma 2 | |||||||||
| 277 | PP 67 | Pyoderma gangrenosum 1 | |||||||||
| Stephen L. Hauser31 | 2008 | USA | Trial | 69 | RR 69 | 39.6 ± 8.7 | Median (range) | 9.6(6.4) | malignant thyroid neoplasm 1 | 12 | 5 Yes out of 6 |
| 52 | 2.5 (0–5) | ||||||||||
| 17 |
EDSS: Expanded Disability Status Scale; F.MS: female MS population; M.MS: male MS population; NOS: NEWCASTLE - OTTAWA QUALITY ASSESSMENT SCALE; NR: not reported; PP: primary progressive; PR: progressive-relapsing; RR: relapsing-remitting; SP: secondary progressive; T.MS: total MS population.
All studies did not report the type of the carcinomas, while among reported types, breast cancer and basal cell carcinoma were the most frequent types.
The pooled prevalence of cancer in MS patients under treatment with rituximab is 1 in 100 000 (I2 = 99.9%, P < .001) (Fig. 2).
DiscussionTo our knowledge this is the first systematic review and meta-analysis evaluating the pooled prevalence of cancer in MS cases who were under treatment with rituximab. The results show that the pooled prevalence is 1 in 100 000 cases, which is ignorable. A recent systematic review and meta-analysis showed that the relative risk of cancer in MS patients comparing with controls is 0.83 (95% CI: 0.73–0.96),32 showing lower risk of any type of cancers in MS population. A previously done systematic review, showed that the pooled incidence of cancer in MS population was 4.3% (95% CI: 2.6–6.1%), and the pooled prevalence reported as 2.2% (95% CI: 1.1–3.2%). Their results showed that cervical, breast, and digestive cancers had higher incidence than other types of cancer.33
Literature shows that the incidence of cancer in MS population is lower than general population without considering any specific DMTs.34,35
The relationship between cancer and MS is complex. Persistent inflammation leads to promotion of tumorigenesis. Prolonged administration of immunosuppressive treatments increases the risk of malignancies.33
Rituximab is administered as an off-label medication for MS for years which was used for cancer therapy then extended to systemic vasculitis and rheumatoid arthritis.36 In 2004, for the first time, rituximab was used for relapsing-remitting patients with MS which followed by a multi-centric randomized control trial (RCT).37,38
Alping et al. enrolled 4187 MS patients who were under treatment with rituximab, 1620 under treatment with fingolimod, and 1670 with natalizumab, and found that 33 had cancer in rituximab group. The most frequent carcinoma in MS cases was basal cell carcinoma followed by cervical intraepithelial neoplasia (CIN), which were higher in rituximab group.7 Their results demonstrated that prevalence of malignancies in their MS population was 26.6% vs 28.9% in general population (the difference was not significant).
In an Italian study, Zecca et al. enrolled 414 MS patients and reported only one mediastinal cancer among them.39
A study by van Vollenhoven et al. enrolling patients with RA treated with rituximab demonstrated that the risk of cancer in these patients is not higher than general population.40
This systematic review and meta-analysis has some strength. First, we included all studies evaluating safety of rituximab in MS patients. Second, we recorded all types of cancers.
The study had some limitations, too. The duration of follow up and treatment was not same in all studies. Also, all studies did not report the type of malignancies in patients who were enrolled in their study.
ConclusionThe results of this systematic review and meta-analysis show that the pooled prevalence of cancer in MS patients who received rituximab is 1 in 100 000 cases.
Conflict of interestThe authors declare no conflict of interest.