Speech disturbances will affect most patients with Parkinson's disease (PD) over the course of the disease. The origin and severity of these symptoms are of clinical and diagnostic interest.
PurposeTo evaluate the clinical pattern of speech impairment in PD patients and identify significant differences in speech rate and articulation compared to control subjects. Speech rate and articulation in a reading task were measured using an automatic analytical method.
PatientsA total of 39 PD patients in the ‘on’ state and 45 age- and sex-matched asymptomatic controls participated in the study. None of the patients experienced dyskinesias or motor fluctuations during the test.
ResultsThe patients with PD displayed a significant reduction in speech and articulation rates; there were no significant correlations between the studied speech parameters and patient characteristics such as L-dopa dose, duration of the disorder, age, and UPDRS III scores and Hoehn & Yahr scales.
ConclusionPatients with PD show a characteristic pattern of declining speech rate. These results suggest that in PD, disfluencies are the result of the movement disorder affecting the physiology of speech production systems.
Las alteraciones en el habla aparecen en la mayoría de los pacientes con la enfermedad de Parkinson (EP) en el curso del trastorno. Su origen y gravedad son de interés clínico y diagnóstico.
ObjetivoEvaluar los patrones de deterioro en el habla en pacientes con la EP, e identificar diferencias en la velocidad de elocución y articulación en comparación con sujetos de control, empleando un método de análisis automático en una tarea de lectura.
PacientesParticiparon 39 pacientes con la EP y 45 controles asintomáticos igualados en sexo y edad. Los pacientes con la EP, en estado on, no presentaban fluctuaciones motoras ni discinesias durante la evaluación del habla.
ResultadosEl grupo de pacientes con la EP muestran una significativa reducción de la velocidad de elocución y articulación. No se encontraron correlaciones significativas entre los parámetros del habla estudiados y las características de los pacientes, tales como la dosis de L-dopa, duración del trastorno, edad, ni en las puntuaciones en las escalas UPDRS III o Hoehn y Yahr.
ConclusionesLos pacientes con la EP muestran un patrón característico de deterioro del ritmo del habla. Estos resultados indican que las disfluencias en la EP son el resultado de la alteración del movimiento que afecta a la fisiología de los sistemas de producción del habla.
Hypokinetic dysarthria is a frequent complication in Parkinson's disease (PD), affecting a sizeable percentage of patients.1 Its prevalence increases with disease progression.
Parkinsonian speech is typically monotonous and aprosodic, with progressive decreases in vocal sonority and intensity at the end of the phonation. It is characterised by delayed voice onset time and long pauses to breathe between words and syllables, resulting in decreased fluency and tempo. Articulation is impaired,2 which inevitably affects speech intelligibility and makes it difficult to recognise a patient's emotional state.3
PD is also associated with other speech and voice alterations: decreased ability to initiate speech, then followed by rapid speech (tachylalia), rapid repetition of words (palilalia), and on occasions the tip of the tongue phenomenon. In addition, voice intensity decreases progressively (hypophonia).1 Many of these alterations are associated with hyperdopaminergic states.4,5
The acoustic characteristics of dysarthric speech in patients with PD reflect the physiological and anatomical changes caused by dopaminergic deficits, including tremor, bradykinesia, muscle rigidity, and postural instability. These changes affect the 3 subsystems linked to motor control of speech: the respiratory, phonatory, and articulatory systems. Rigidity associated with PD affects the respiratory system (which manages the proper airflow and pressure to generate phonation), thereby altering the range of articulatory movements and the ability to modulate vocal intensity. Alterations of the phonatory system mainly affect vocal cord vibration, which results in increased fundamental frequency (F0) and a reduction in speech variability, intonation, and melodic curve. Finally, the articulatory system is also impaired as the disease progresses, leading to imprecise articulation and increased duration of pauses, which are caused by the decreased amplitude of articulatory movements.6
Speech rate (number of sounds a person can produce in a unit of time, including pauses) and articulation rate (number of sounds excluding pauses) are frequently studied in the context of PD. The data from the literature are inconsistent: some authors7–9 reported significant reductions in speech rate when patients were asked to repeat a series of syllables (/pa/, /ta/, /ka/) at a rapid pace, whereas others observed the opposite effect.10,11
Increased speech rate has also been observed during tasks used to evaluate continuous speech (usually reading short texts).12,13 Other studies, however, have found no intergroup differences,14 or else report a decrease in speech rate. Two longitudinal studies illustrate a progressive decline in speech rate over periods ranging from 25 to 32 months.15,16
Speech rate is essentially dependent on the number and duration of pauses. Results regarding pauses are also contradictory: whereas several studies17,18 found no differences in pause number or duration during a reading task, other studies report significant increases in pauses12,13 or the use of fewer yet longer pauses.19
In addition to reducing hypokinesia and rigidity of the systems involved in speech production, levodopa can be expected to increase speech rate. However, results from multiple studies do not support this theory. Whereas some researchers12,20,21 have found no differences in speech rate between ‘on’ and ‘off’ periods, others22 report significant improvements during the ‘on’ period. Likewise, deep brain stimulation of the subthalamic nucleus does not seem to have a significant effect on speech rate22,23; although it may improve speech parameters in some patients,24 this normally does not improve overall intelligibility.25 Many of these inconsistencies may be attributed to small sample sizes (2 to 30 participants), the lack of published assessments of subjects’ cognitive functions, and other aspects associated with the methodology of speech analysis.26
In the present study we analyse speech and articulation rates in a reading task in a representative sample of patients with PD who experienced no motor fluctuations or dyskinesia during the task. Our purpose is to test the hypothesis that speech and articulation rates decrease in PD, and that this decrease is associated with the duration and severity of PD.
MethodsStatistical designThe study was framed with a cross-sectional, analytical, observational, and retrospective design.
PatientsBetween 2011 and 2012, we recruited 84 participants with no history of drug use, alcohol abuse, or depression (Geriatric Depression Scale score<10). Participants were divided into 2 groups: individuals older than 60 with no known neurological diseases (n=45) and patients with PD (n=39).
The control group (mean age±SD: 71.95±10.22 years) was comprised of right-handed individuals whose native language was Spanish and who had no history of brain damage, neurological or psychiatric disorders, or dementia (MMSE score>25). All participants were enrolled in a course taught in Salamanca as a part of an inter-university programme for adults older than 55. None of them were taking anticholinergics, cholinesterase inhibitors, or neuroleptics, all of which were exclusion criteria in our study.
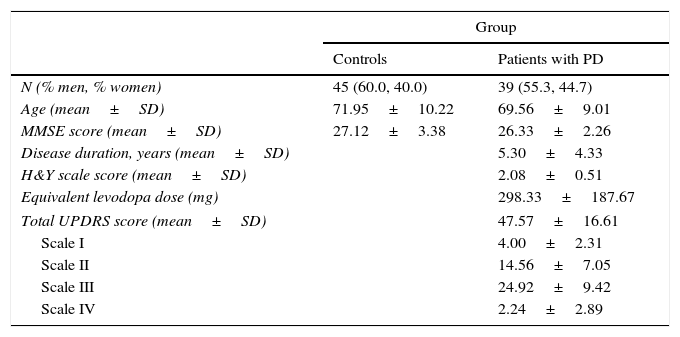
The PD group (mean age: 69.56±9.01 years) included patients from the neurology department at Hospital Clínico de Salamanca who were diagnosed with and treated for idiopathic PD, according to the UK Parkinson's Disease Society Brain Bank criteria.27 Participants’ characteristics are summarised in Table 1.
Description of the study groups.
| Group | ||
|---|---|---|
| Controls | Patients with PD | |
| N (% men, % women) | 45 (60.0, 40.0) | 39 (55.3, 44.7) |
| Age (mean±SD) | 71.95±10.22 | 69.56±9.01 |
| MMSE score (mean±SD) | 27.12±3.38 | 26.33±2.26 |
| Disease duration, years (mean±SD) | 5.30±4.33 | |
| H&Y scale score (mean±SD) | 2.08±0.51 | |
| Equivalent levodopa dose (mg) | 298.33±187.67 | |
| Total UPDRS score (mean±SD) | 47.57±16.61 | |
| Scale I | 4.00±2.31 | |
| Scale II | 14.56±7.05 | |
| Scale III | 24.92±9.42 | |
| Scale IV | 2.24±2.89 | |
SD: standard deviation; PD: Parkinson's disease; MMSE: Mini-Mental State Examination; UPDRS: Unified Parkinson's Disease Rating Scale; H&Y: Hoehn and Yahr scale.
Mini-Mental State Examination (MMSE).28 The MMSE, the most widespread screening test, offers the most commonly used severity index for dementia. The maximum total score is 30 points.
Unified Parkinson's Disease Rating Scale (UPDRS).29 The UPDRS is a classification system used to monitor the longitudinal course of PD. The maximum total score is 159 points.
Hoehn and Yahr scale (H&Y).30 The H&Y scale is a simple descriptive scale which defines PD stages based on disability and signs of deterioration.
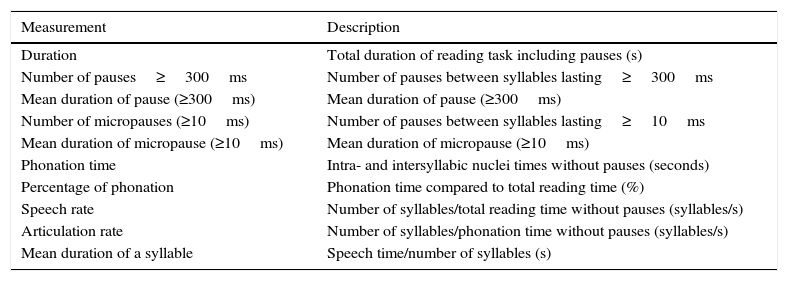
Speech was recorded using a professional Fostex FR-2LE recorder with a resolution of 24 bits, a 48kHz sampling rate, and an AKG D3700S cardioid microphone. All samples were edited using Praat voice analysis software, version 5.1.42.31 This programme enables acoustic analysis, articulatory synthesis, and edition and manipulation of audio recordings. Appendix A lists the definitions of the fluency parameters analysed in this study.
ProcedureAll patients signed an informed consent form according to the protocols approved by the ethics committees at the participating institutions. We conducted 2 sessions. In the first session, we registered the participants’ personal and clinical information and administered the MMSE, UPDRS, and H&Y scale. Recordings were made in the second session, held 3 to 5 days later. During speech recording, all patients in the PD group were in the ‘on’ stage and showed no motor fluctuations or dyskinesia.
The reading task, which was conducted under controlled conditions, consisted of reading the first sentence of Don Quixote by Miguel de Cervantes on a screen at a font size of 48 points. The paragraph comprised 126 syllables. While this sentence is not phonetically balanced, we chose it because all participants were likely to be familiar with it.
The same examiner recorded the participants’ attempts in a quiet but not soundproof room. The microphone was placed 8cm from the speaker's mouth and at an angle of approximately 45° in order to minimise breath noise.
Assessing speech rate implies detecting syllable nuclei. Syllables and pauses were detected automatically using de Jong and Wempe's algorithm,32 which analyses peaks in intensity preceded and followed by drops of at least 2dB. Fluency studies have demonstrated the validity and reliability of this method.33
After identifying the syllable nuclei automatically, we verified each one using a wide-band spectrogram and sonogram; syllable nuclei were corrected when necessary. Pauses were also identified automatically. Periods of silence lasting≥300ms were considered pauses, and periods of silence lasting≥10ms were considered micropauses. These criteria for pause identification are frequently used in fluency studies in PD.16
Data analysisData were analysed using IBM SPSS statistical software for Apple (version 21). We tested for the normality of the scores for the variables ‘speech rate’ and ‘articulation rate’. According to the Kolmogorov–Smirnov test, these variables followed a normal distribution (z=0.046; P=.20). We used the t test for independent samples to compare the means of both groups and establish different fluency profiles. We also used Pearson correlation to assess the relationship between variables.
ResultsStatistical contrasts were performed to ensure there were no statistically significant differences between the groups with regard to clinical and sociodemographic variables. We found no intergroup differences in age (t82=1.07; P=.265) or in sex distribution (χ2=3.11; P=.078). Likewise, no differences were seen in cognitive abilities measured with the MMSE (t82=1.33; P=.227). Lastly, we found no differences in verbal fluency between the 2 groups tested for both semantic verbal fluency (t82=0.41; P=.639) and phonemic verbal fluency (t82=0.47; P=.655).
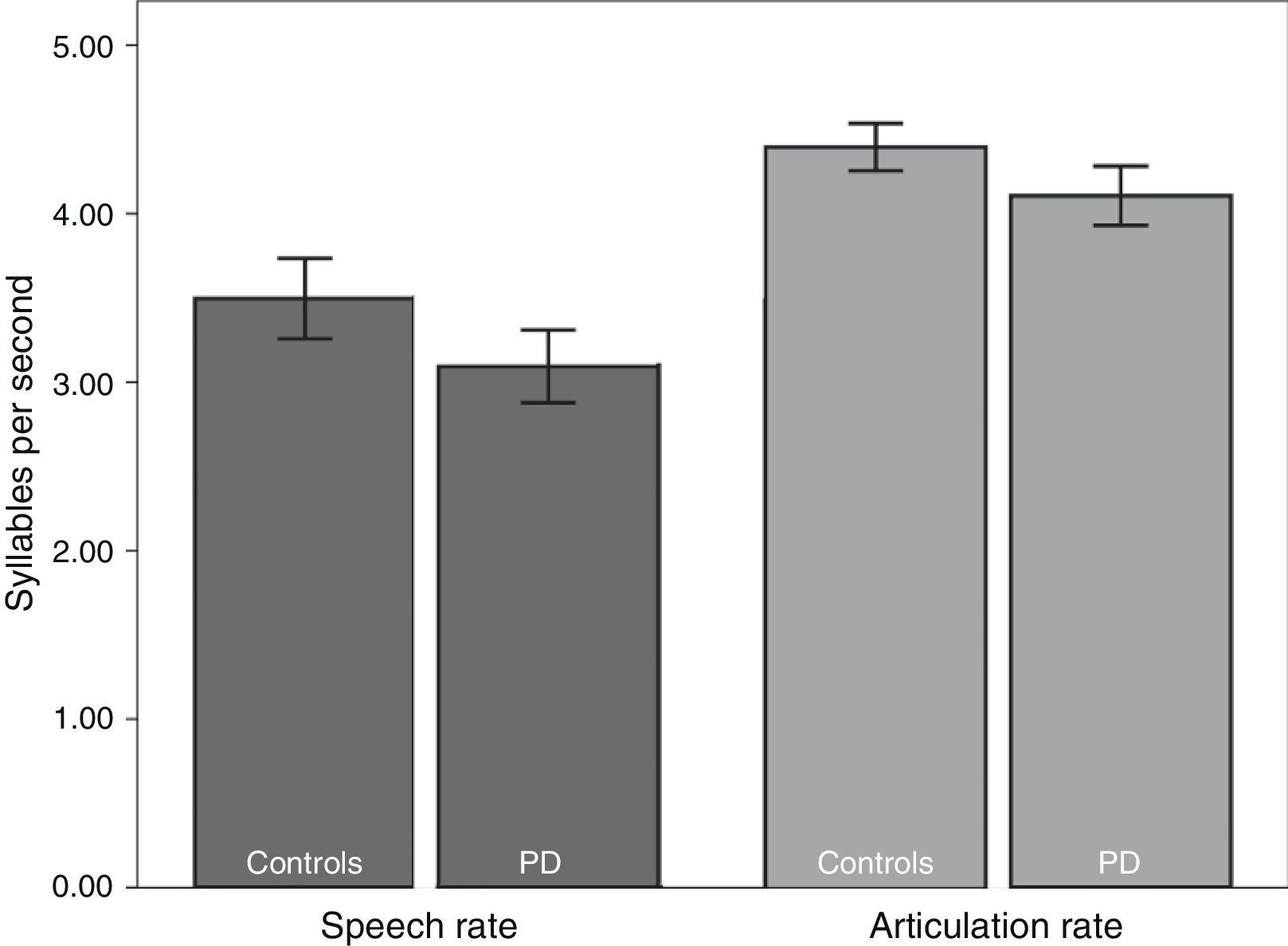
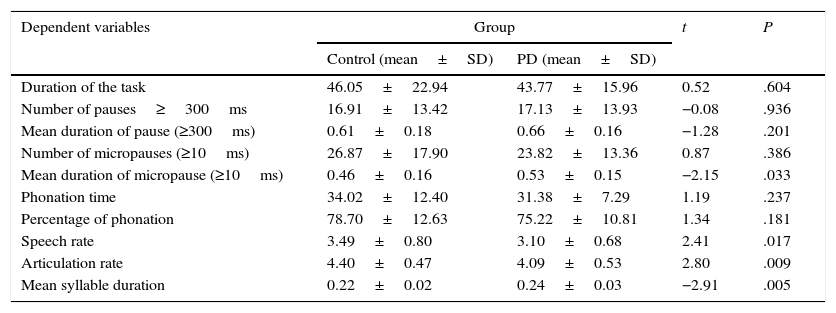
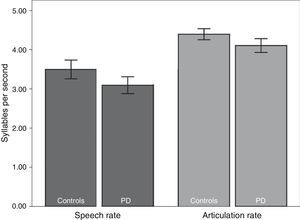
We subsequently tested for any possible intergroup differences in the study variables. According to the results (Table 2), the PD group displayed slower rates of speech (t82=2.41; P<.05) and articulation (t82=2.80; P<.01) than the control group (Fig. 1). In addition, mean syllable duration was longer in the PD group (t82=−2.81; P<.01). The number of pauses and micropauses was similar in the 2 groups; however, mean duration of micropauses was longer in the group with PD (t82=−2.15; P<.05) than in the control group.
Descriptive statistics and comparison of means of the study variables in both groups.
| Dependent variables | Group | t | P | |
|---|---|---|---|---|
| Control (mean±SD) | PD (mean±SD) | |||
| Duration of the task | 46.05±22.94 | 43.77±15.96 | 0.52 | .604 |
| Number of pauses≥300ms | 16.91±13.42 | 17.13±13.93 | −0.08 | .936 |
| Mean duration of pause (≥300ms) | 0.61±0.18 | 0.66±0.16 | −1.28 | .201 |
| Number of micropauses (≥10ms) | 26.87±17.90 | 23.82±13.36 | 0.87 | .386 |
| Mean duration of micropause (≥10ms) | 0.46±0.16 | 0.53±0.15 | −2.15 | .033 |
| Phonation time | 34.02±12.40 | 31.38±7.29 | 1.19 | .237 |
| Percentage of phonation | 78.70±12.63 | 75.22±10.81 | 1.34 | .181 |
| Speech rate | 3.49±0.80 | 3.10±0.68 | 2.41 | .017 |
| Articulation rate | 4.40±0.47 | 4.09±0.53 | 2.80 | .009 |
| Mean syllable duration | 0.22±0.02 | 0.24±0.03 | −2.91 | .005 |
SD: standard deviation.
We also analysed the relationship between the variables linked to speech and age, progression of PD, levodopa dose, and scores on the different questionnaires (MMSE, UPDRS, and H&Y scale). As could be expected, we found a positive correlation between levodopa dose and disease progression (number of years from diagnosis) (r=0.405; P<.05). However, we found a statistically non-significant correlation between disease progression and fluency variables, and between levodopa dose and fluency variables, except for articulation rate (r=−0.432; P<.05). This shows that articulation rate slows as the dose of levodopa increases. H&Y scores showed a weak correlation with fluency variables, except for the percentage of phonation (r=−0.398; P<.05). Likewise, global UPDRS scores were not significantly correlated with speech rate (r=−0.090; P>.05) or articulation rate (r=0.030; P>.05).
DiscussionThe main purpose of our study was to analyse speech and articulation rates in a group of patients with PD. Our results show that PD is associated with decreased speech and articulation rates, which reflects results from other studies.15,16,19
Speech is the result of complex, coordinated actions requiring a greater number of muscle fibres than those in any other human motor system. Speech tempo is the result of continuous time adjustments of speech-related motor movements to generate sounds at a very high speed. Motor disorders in PD and their impact on the respiratory, phonatory, and articulatory systems are likely to affect speech tempo in patients with PD8 as a result of alterations in motor control circuits in the basal ganglia.34
Our results support the hypothesis that speech and articulation rates are slower in patients with PD, in contrast with other studies reporting the opposite effect.12,13 Increases in speech rate may be attributed to a compensatory mechanism that makes patients with PD speak at a faster rate, involuntarily and under certain circumstances. Various studies of lip and jaw movements during speech in patients with PD may explain this phenomenon. Caligiuri35 reported normal lip movements at normal speech rates (3 to 5 syllables per second); however, lip movements decreased when patients were asked to speak faster (5 to 7 syllables per second). This suggests that patients may try to speak more slowly to control their tendency to articulate poorly when speaking at faster rates. Likewise, Ackermann et al.36 showed that articulation rate was more accelerated when patients are asked to produce syllable repetitions at frequencies of 2.5-6Hz than when they had to do so at frequencies of 8-9Hz. Task demands in studies of fluency37 (or of speech in real-life situations) may explain why speech rate varies. For example, speech rate increases during spontaneously produced conversational speech whereas intelligibility decreases: the lack of an external temporal model to facilitate the synchronisation of motor speech (for example, syllable repetition or reading a text) makes motor control alterations more evident, and speech less fluent.
Decreases in the articulation rate may be explained by the high number of patients with PD who also exhibit articulatory dysfunction. In a study by Logemann et al.,8 45% of 200 patients with PD had this type of dysfunction, attributed to inadequate closing of the vocal tract as a result of hypokinesia. In addition, Sapir et al.38 reported articulatory dysfunctions in 50% of a group of 42 patients with PD who were receiving pharmacological treatment. Kleinow et al.39 reported normal lower lip and jaw movements during production of a syntactically simple sentence according to the spatiotemporal index. However, when they were asked to change speed and volume, the results varied significantly.
The decrease in speech rate observed in our study may be attributed to physiological alterations resulting from speech motor control impairments associated with PD. The antagonistic muscle pairs display the muscle tone necessary for speech production when innervation is normal, but they contract simultaneously in patients with PD due to dysfunction of the basal ganglia. This phenomenon provokes poor control over muscle contraction, making normal speech articulation impossible.40 Likewise, reduced lip and jaw movement41 results in imprecise articulation and therefore in lower speech and articulation rates.15
In our sample, the number of micropauses was smaller in the group with PD than in the control group, although the difference was not statistically significant. This finding agrees with results reported by Skodda et al.19 Pronounced increases in the number of pauses have been reported by several researchers. For example, Logemann et al.8 found that 89% of 200 patients with PD made an excessive number of pauses while speaking; this finding suggests that laryngeal muscle innervation is impaired, leading to difficulties in initiating phonation with articulatory movements. Fräile and Cohen42 reported a significant decrease in voiceless speech periods in patients with PD, which was likely due to their difficulty inhibiting laryngeal activity. This alteration in the ability to inhibit motor movements explains both delayed response initiation and delayed inhibition of an already initiated response.43 This may act as a compensatory strategy to maintain normal speech rate by reducing the duration and number of pauses.42 Kegl et al.44 reported similar results in a syllable repetition task and concluded that incomplete closing of the vocal tract may be employed to avoid or minimise the difficulties of initiating phonation by maintaining a continuous level of activation in the vocal cords.
In addition, absence of a significant association between the evaluated fluency variables and the scores on the UPDRS and H&Y scale has previously been described.16,19 The UPDRS has been reported to have low reliability45 and insufficient assessment of speech components: it includes only 2 items for speech (items 5 and 18), both of which have a low kappa index (0.660 and 0.602, respectively).45
Our results also show no association between the dose of levodopa and fluency variables, in contrast with other studies.12,20,21 Our study does show that speech rate decreases as the dose of levodopa increases; however, this effect may be attributed to deterioration secondary to disease progression, since the dose of levodopa will be increased gradually as PD progresses over the years.
Overall, our results show that patients with PD have markedly impaired verbal fluency which, together with the listed vocal alterations,15,46 significantly affect patients’ communication skills and quality of life. In addition, these alterations barely improve with pharmacological treatment20,21,47 or deep brain stimulation.23,25,48
Our study has a number of limitations. The task used to assess speech fluency is one of its limitations, since the patients’ familiarity with the sentence may have altered the results. However, the relatively large sample size minimises bias. Using methods for analysing spontaneous speech is an interesting alternative. In addition, we evaluated speech in subjects who were experiencing no motor fluctuations or dyskinesias. Further studies should aim to compare speech during ‘on’ and ‘off’ stages, include larger sample sizes, and longitudinally assess the associations between different parameters affecting speech.
Conflicts of interestThe authors have no conflicts of interest to declare.
The present study received funding from the Spanish Ministry of Science and Innovation (BFU 2010-17754, D.E.L.) and the regional government of Castile-Leon (#GRS 857/A/, T.L.A.; BIO/SA84/, J.J.G.M.).
| Measurement | Description |
|---|---|
| Duration | Total duration of reading task including pauses (s) |
| Number of pauses≥300ms | Number of pauses between syllables lasting≥300ms |
| Mean duration of pause (≥300ms) | Mean duration of pause (≥300ms) |
| Number of micropauses (≥10ms) | Number of pauses between syllables lasting≥10ms |
| Mean duration of micropause (≥10ms) | Mean duration of micropause (≥10ms) |
| Phonation time | Intra- and intersyllabic nuclei times without pauses (seconds) |
| Percentage of phonation | Phonation time compared to total reading time (%) |
| Speech rate | Number of syllables/total reading time without pauses (syllables/s) |
| Articulation rate | Number of syllables/phonation time without pauses (syllables/s) |
| Mean duration of a syllable | Speech time/number of syllables (s) |
Please cite this article as: Martínez-Sánchez F, Meilán JJG, Carro J, Gómez Íñiguez C, Millian-Morell L, Pujante Valverde IM, et al. Estudio controlado del ritmo del habla en la enfermedad de Parkinson. Neurología. 2016;31:466–472.