The premonitory phase comprises a wide range of symptoms that precede the onset of pain in a migraine episode by up to 48hours. Premonitory symptoms are often not recognised by patients but do have a significant impact on their quality of life. As these symptoms represent the first stage of a migraine attack, they are crucial in improving our understanding of the key points of the origin of migraine.
DevelopmentThis paper uses a question-answer format to review the main clinical studies analysing premonitory symptoms, their predictive capacity, the relationship between these symptoms and the biology of migraine, and the role of neuroimaging in the premonitory phase. Finally, we discuss the relevance of these symptoms as potential therapeutic targets in the future.
ConclusionsThis study of the premonitory phase has demonstrated that the hypothalamus plays an essential role in the origin of migraine attacks. We should consider the search for new targets in acute migraine treatment in order to act before the onset of the pain. This would imply a radical change in the lives of patients with migraine.
La fase premonitoria comprende una amplia esfera de síntomas que anteceden hasta en 48horas al dolor en una crisis de migraña. Este periodo a menudo no es reconocido por el propio paciente y, sin embargo, también merma de forma significativa su calidad de vida.
Al ser el estadio más precoz de una crisis de migraña es fundamental para ayudarnos a comprender puntos clave del origen de la misma.
DesarrolloA lo largo de esta revisión, en forma de pregunta-respuesta, se repasan los principales estudios clínicos que analizan de forma dirigida los síntomas premonitorios, se valora la capacidad predictiva de los mismos, se relacionan estos síntomas con la biología de la migraña, se revisa el papel de la neuroimagen en esta fase y, por último, la relevancia como potencial diana terapéutica que pueda tener en un futuro.
ConclusionesEl estudio de la fase premonitoria nos ha mostrado que el hipotálamo tiene un papel esencial en el origen de una crisis de migraña, y nos hace plantearnos la búsqueda de nuevas dianas en el tratamiento de una crisis de migraña dirigidas a actuar antes del inicio del dolor, un hecho que implicaría un antes y un después en la vida del paciente migrañoso.
Migraine attacks are often identified with headache, as it is typically the most disabling phase; however, headache is by no means the only symptom of migraine. Attacks encompass a far broader range of symptoms that significantly increase patients’ disability and can manifest hours before the onset of pain: this affects their quality of life on numerous days per month.
The premonitory phase of migraine is that which precedes pain, when patients present premonitory symptoms (PS), defined in the third edition of the International Classification of Headache Disorders, beta version (ICHD-3 beta), as preceding and forewarning of a migraine attack by 2-48hours, occurring before the aura in migraine with aura and before the onset of pain in migraine without aura.1 Examples of PS include fatigue, euphoria, depression, increased appetite, or cravings for a particular type of food. The use of such terms as “prodrome” or “warning symptoms” is not recommended as they are ambiguous and are used for other purposes.
According to Maniyar et al.,2 2 elements must be considered with regard to this definition of PS. Firstly, symptoms do not precede or forewarn of migraine attacks; rather, they are a part of the attack itself. Secondly, these symptoms may present during the interval between aura and/or pain onset, and in the subsequent 2hours. The purpose of this time interval is to clearly differentiate PS from aura; however, the gap does not truly exist, as has been shown by various prospective studies3; moreover, aura is clearly distinct in pathophysiological terms. It is therefore necessary to review the definition before arriving at a final version.
Gowers4 was the first to describe a PS of migraine (drowsiness) in 1899. However, it was not until 1980, with the work of Blau,5 that the phase was clearly acknowledged and the term “complete migraine” was coined. At that time the first mention was made of the potential role of the hypothalamus in PS, and therefore in migraine causation; this hypothesis stood in opposition to the then prevailing vascular theory of migraine aetiology.
In addition to the PS mentioned above, a number of rarer but very striking symptoms have been reported: in his popular book Migraine, Oliver Sacks6 describes unusual cases such as a usually phlegmatic patient who developed a nearly uncontrollable tendency to laugh, dance, or sing for several hours before pain onset. Other examples include a feeling of narrowness in the head, a slightly bent forward position, and changes in facial expression.7,8
As it is the earliest stage of migraine, studying the premonitory phase may shed light on which brain areas are involved in the onset of a migraine attack and may in the future lead to the design of treatment strategies pre-empting pain.
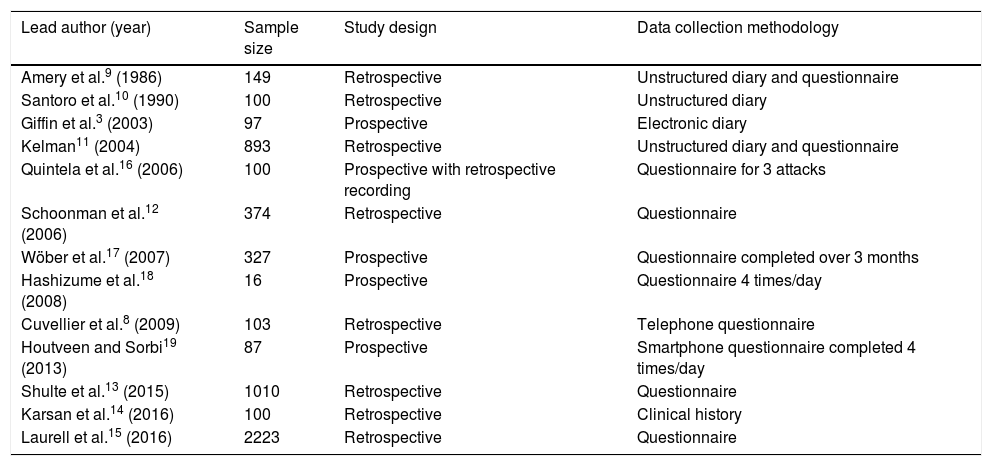
What can be learned from clinical studies?Table 1 summarises the clinical studies performed to date on PS.
Clinical studies assessing premonitory symptoms.
| Lead author (year) | Sample size | Study design | Data collection methodology |
|---|---|---|---|
| Amery et al.9 (1986) | 149 | Retrospective | Unstructured diary and questionnaire |
| Santoro et al.10 (1990) | 100 | Retrospective | Unstructured diary |
| Giffin et al.3 (2003) | 97 | Prospective | Electronic diary |
| Kelman11 (2004) | 893 | Retrospective | Unstructured diary and questionnaire |
| Quintela et al.16 (2006) | 100 | Prospective with retrospective recording | Questionnaire for 3 attacks |
| Schoonman et al.12 (2006) | 374 | Retrospective | Questionnaire |
| Wöber et al.17 (2007) | 327 | Prospective | Questionnaire completed over 3 months |
| Hashizume et al.18 (2008) | 16 | Prospective | Questionnaire 4 times/day |
| Cuvellier et al.8 (2009) | 103 | Retrospective | Telephone questionnaire |
| Houtveen and Sorbi19 (2013) | 87 | Prospective | Smartphone questionnaire completed 4 times/day |
| Shulte et al.13 (2015) | 1010 | Retrospective | Questionnaire |
| Karsan et al.14 (2016) | 100 | Retrospective | Clinical history |
| Laurell et al.15 (2016) | 2223 | Retrospective | Questionnaire |
The majority of studies are retrospective8–15; patients therefore may not recall all PS, and independently associating symptoms with each attack is not straightforward. While prospective studies resolve these limitations, the patient samples are much smaller.3,16–19
It is also important to note the data collection methodology followed in each study.20 Data may be collected in free-form or unstructured diaries, which can result in bias as patients may not remember to record their symptoms daily, in addition to the excessive burden of having to complete the diary. Checklists and questionnaires are restricted to the items listed, potentially not including relevant variables; they may also lead patients to record symptoms that they may otherwise have forgotten or that are not linked to migraine. While these disadvantages also affect studies using smartphones and other electronic devices, such techniques do enable real-time data collection, which is valuable in analysing the predictive capacity of these symptoms.
Taking these considerations into account, the main conclusions of such studies are described below.
The premonitory phase of migraine may present with a broad range of manifestations, including such mood alterations as euphoria, hyperactivity, sadness, and apathy; cognitive alterations; gastrointestinal and appetite disorders; neck stiffness; yawning; sensory alterations (photo, phono-, or osmophobia); skin alterations; dizziness; blurred vision; and changes in body temperature or sweating. Various articles3,8,11–17 report yawning, irritability, apathy, neck stiffness, photophobia, nausea, fatigue, and difficulty concentrating among the most frequent symptoms.
Considering the methodological biases described above, the considerable discrepancies between different authors’ findings regarding prevalence of PS are unsurprising. Different studies report prevalence rates of 9%,21 33% to 39%,10,11,13 and as high as 77% to 92%.9,12,15,16,26 We believe that prevalence is probably nearer the higher published figures, when patients are properly instructed to recognise symptoms and clinical history is taken in a targeted way.
Patients typically display a mean of 3 PS per attack12,15; however, rates of 7 and even 12 symptoms per migraine have been reported, depending on the method of data collection.13,16
Few studies have addressed whether patients present the same PS in all attacks. Quintela et al.16 analysed 3 attacks per patient, reporting PS consistency at 63% for at least 2 attacks and 30% for all 3. The most consistent PS were difficulty concentrating, sadness, anxiety, and yawning.
While the ICHD-3 beta establishes that PS can appear up to 48hours before pain onset,1 they are reported to appear a mean time of 6-10hours before headache.11,13,19 Another interesting observation is that some PS, such as hyperactivity and euphoria, are more common in the earlier part of the premonitory phase (25-36hours before pain onset), with others appearing nearer pain onset (<12hours, including neck stiffness, fatigue, and irritability).19 According to these variables, PS can be classified as evolutive or non-evolutive, depending on their individual characteristics.22 Non-evolutive symptoms appear at the earliest point of the premonitory phase, present a constant level of intensity, and disappear hours before pain onset. Evolutive symptoms appear nearer the time of pain onset and intensify over time. This phenomenon is consistent with pathophysiological and neuroimaging findings, which are addressed later in the article.
Another common observation is the existence of migraine profiles with greater levels of PS.9–12,15,16 These patients describe more intense pain with more accompanying symptoms, trigger factors, postdromal symptoms, and family history of migraine; pain is unilateral, pulsatile, and long-lasting, with delayed response to triptans. Patients who often experience PS are therefore thought to present a stronger migraine profile, which may entail early failure of their systems of antinociceptive adaptation to the environment and to stressful events. There is controversy in the literature as to whether the frequency of PS is age-related. Several studies identified no age-related differences,12,15,16 whereas others suggest that PS are more common in young patients.10 Similarly, some studies report no sex-related differences in PS frequency,11,16 whereas others find higher rates among women9,12; however, analysis of other factors would probably reveal that this difference is due to the greater intensity of attacks in women. Migraine with aura has been found not to present a greater prevalence of PS,12 although the mean number of PS per episode is higher and these symptoms are more intense; curiously, patients with scintillating scotoma present fewer symptoms.15
Some patient profiles more frequently present specific types of PS.13 For example, increased appetite and nausea are more frequent in women; patients experiencing aura more frequently present osmophobia, yawning, difficulty concentrating, dizziness, and nausea; and patients with symptoms accompanying pain usually also experience these prior to pain onset.
Some authors16 report that patients receiving preventive treatment (specifically with amitriptyline, beta blockers, flunarizine, topiramate, or fluoxetine) present fewer PS, which may indicate that this treatment has a protective effect, treating the entire range of migraine symptoms rather than pain only.
Finally, PS are not specific to migraine; they have also been observed in other processes, such as cluster headache.23 This is unsurprising, as both conditions involve common anatomical and pathophysiological mechanisms. PS are not specific to adult patients: studies by Cuvillier et al.8 and Karsan et al.14 describe PS in children and adolescents, including in a boy of 18 months of age. These studies report high rates of PS prevalence (67%-85%) and a similar mean number of symptoms to that observed in adult patients, as well as a more characteristic symptom of this age group: changes in facial expression, which are easy to detect in children.
How predictive are premonitory symptoms of pain onset?The main study into patients’ ability to predict pain in migraine attacks based on their PS was conducted by Giffin et al.3 in 2003. Patients considered good predictors (n=97) were able to predict at least one attack in 72% of cases, and 82% of patients predicted over half of their attacks; 68% of migraines were predicted more than 6hours before pain onset. The most predictive PS were difficulty speaking (92% likelihood of correct prediction), difficulty reading and writing (90%), and yawning (84%). The least predictive symptoms were tiredness, neck stiffness, nausea, and vomiting. It is also striking that patients who were almost certain that they would experience an attack were correct on nearly 95% of occasions.
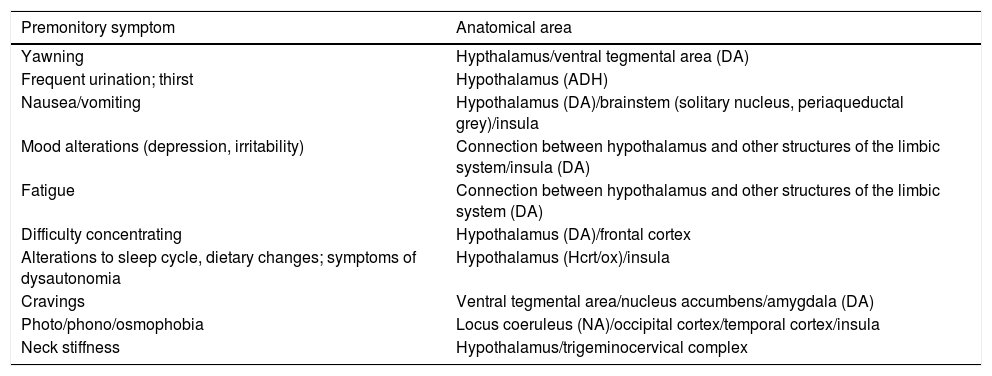
How are premonitory symptoms related to the biology of migraine?PS offer insight into the anatomical regions and neurochemical mechanisms involved in the onset of a migraine attack (Table 2). From a practical viewpoint, these areas and mechanisms represent new targets and may lead to new treatment approaches to be applied before pain onset.
Relationship between premonitory symptoms and neuroanatomical areas.
| Premonitory symptom | Anatomical area |
|---|---|
| Yawning | Hypthalamus/ventral tegmental area (DA) |
| Frequent urination; thirst | Hypothalamus (ADH) |
| Nausea/vomiting | Hypothalamus (DA)/brainstem (solitary nucleus, periaqueductal grey)/insula |
| Mood alterations (depression, irritability) | Connection between hypothalamus and other structures of the limbic system/insula (DA) |
| Fatigue | Connection between hypothalamus and other structures of the limbic system (DA) |
| Difficulty concentrating | Hypothalamus (DA)/frontal cortex |
| Alterations to sleep cycle, dietary changes; symptoms of dysautonomia | Hypothalamus (Hcrt/ox)/insula |
| Cravings | Ventral tegmental area/nucleus accumbens/amygdala (DA) |
| Photo/phono/osmophobia | Locus coeruleus (NA)/occipital cortex/temporal cortex/insula |
| Neck stiffness | Hypothalamus/trigeminocervical complex |
ADH: antidiuretic hormone; DA: dopamine; Hcrt/ox: hypocretin/orexin; NA: noradrenaline.
PS are mainly associated with the hypothalamus, the dopaminergic system, and the insular cortex.
HypothalamusGiven the central role of the hypothalamus in PS, one therapeutic approach targets such neuropeptides as orexin/hypocretin,24,25 which are involved in hypothalamic functions including the regulation of sleep/arousal, appetite, and stress. Such treatment may be critical to maintaining allostatic load and consequently in preventing the next migraine episode. Allostatic load is defined as the amount of brain activity needed for proper management of physiological and emotional stress.26
With relation to this, an interesting study by Burstein et al.26 proposes that homeostatic changes, such as those occurring in situations of anxiety, overload, and dietary or sleep alterations, may determine whether pain develops, according to the level of brainstem tone or allostatic load when the stimulus is received. Thus, nociceptive signals are inhibited in cases of high load and patients do not develop pain. The contrary case would be migraine state, with low cyclical activity, where pain does not subside. This explains the fact that the same triggers (e.g., fatigue or mood changes) do not always lead to a migraine attack in a patient.
The hypothalamus may explain many PS; its role in migraine pathogenesis is also supported by the role of sleep alterations and hormonal fluctuations as trigger factors and the circadian rhythm observed in migraine occurrence. The activation of the hypothalamus and the brainstem before pain onset suggests that they play an important role in the aetiopathogenesis of migraine.
Two theories address the involvement of the hypothalamus in pain origin. According to the first, which is more general, an alteration in the hypothalamus-brainstem axis affects the activation threshold for transmission of nociceptive signals from the thalamus, which selects, amplifies, and prioritises transmission to the cortex (if allostatic load is sufficient). The second hypothesis is more specific, postulating that the hypothalamus is connected to the preganglionic parasympathetic neurons of the superior salivatory nucleus, which are in turn connected to the postganglionic parasympathetic neurons of the sphenopalatine ganglion, causing vasodilation with the release of proinflammatory agents, resulting in activation of the trigeminovascular system.27
Hypothalamic activation was first demonstrated by Denuelle et al.,28 who performed H215O PET scans on 7 patients in the first hours after pain onset and after administration of sumatriptan. Hypothalamic activation is observed at the time of pain onset and persists despite pain being relieved through treatment; this may explain the recurrence of pain when the effect of the triptan ceases, and raises the prospect of searching for new therapeutic targets.
Dopaminergic systemThere is currently a certain controversy in the literature as to the relationship between migraine and dopamine; however, there is consensus that patients with migraine present chronic dopaminergic hypofunction. This dopaminergic dysfunction leads to overexpression of dopamine receptors, resulting in increased sensitivity to dopaminergic stimulation and reduced inhibitory control in the trigeminal neurons. This increases these neurons’ susceptibility to activation in response to migraine trigger stimuli.25,26
Migraine attacks begin when dopamine concentration is low, which stimulates presynaptic receptors (causing such PS as yawning or drowsiness); subsequently, dopamine levels increase, leading to activation of postsynaptic receptors (which presents as nausea or hypotension); this reaches a point at which it is too late to prevent activation of the trigeminovascular system, resulting in pain. Dopamine plays an important part in this system's modulation of nociception, particularly through dopamine D2 receptors (DRD2).
When dopamine returns to the baseline level, patients show signs of drowsiness and fatigue; in some patients, dopamine levels remain elevated, causing euphoria and frequent urination.29
Dopaminergic involvement in migraine is further supported by patients’ response to various treatments: the DRD2 antagonist domperidone prevents migraine onset7; this drug is effective at the peripheral level, as it does not cross the blood–brain barrier (hence the absence of any extrapyramidal effects). Metoclopramide is a DRD2 antagonist that does cross the blood–brain barrier; this drug prevents nausea in addition to pain. The dopaminergic agonist apomorphine, which particularly acts on DRD4 receptors, causes yawning, nausea, and drowsiness, although it does not trigger pain, even in more sensitive patients. Continuous administration, reducing chronic dopaminergic hypofunction, inverts the cycle and reduces migraine frequency and intensity. Flunarizine is a calcium channel blocker used as a preventive treatment for migraine. However, it also acts as a dopamine receptor antagonist. Its effect on the dopaminergic system appears not to be mediated by DRD2.30
Another significant point supporting the involvement of dopamine in the premonitory phase of migraine is that in patients with Parkinson's disease (the paradigm of dopaminergic deficit), migraine frequency decreases as the disease progresses; these patients also respond better to dopaminergic treatments.25,29
InsulaMigraine should not be considered a disease of the insula; however, this region does play an important role in the integration of many dynamic processes involved in migraine, as demonstrated by Borsook et al.31 The insula is involved in cognitive changes, autonomic response, sensory alterations, osmophobia, nausea and vomiting, migraine-associated vertigo, and sleep and mood alterations occurring in different phases of migraine, including the premonitory phase. Exercise, proper hydration, reducing stress, good sleep/wake rhythm, and treatment with triptans reduce activation of the insular cortex. However, patients with medication overuse or chronic migraine show increased insular activity. Another point to be considered is whether functional changes to the insula over time may be attributed to age-related changes in patients with migraine.
Are premonitory symptoms associated with neuroimaging findings?A study by Maniyar et al.32 marked a turning point in the field of neuroimaging studies of the premonitory phase of migraine. Migraine was induced with nitroglycerin infusion in 8 patients with frequent PS. H215O PET imaging was used to observe the brain areas activated at the beginning of the attack; the main areas activated were the hypothalamus, ventral tegmental area, periaqueductal grey, dorsal pons, putamen, pulvinar nucleus, and various cortical regions, according to the PS reported by each patient. It should be noted that activation was different during the early and late premonitory phase. The activation of areas including the hypothalamus, ventral tegmentum, periaqueductal grey, and putamen diminished nearer pain onset, whereas the dorsal pons remained active in all phases and the insula was activated nearer the time of pain onset. These differences are probably associated with the distinction between evolutive and non-evolutive symptoms, as discussed earlier. This represents a further contribution to our understanding of PS, and means that future treatments may target the areas activated in the early premonitory phase.
Another study by the same group analysed a subgroup of patients displaying photophobia in the premonitory phase; the visual cortex was activated independently of the trigeminal system, unlike in the pain phase, in which both are activated.33 These findings show that pain is perceived as being more intense in the presence of light.
In another study, the authors selected a subgroup of patients presenting nausea34; in this case, the trigeminal system was activated from the early premonitory phase, suggesting a more complex relationship. This may help address such questions as why some patients show an improvement in pain upon vomiting.
In another important study on neuroimaging and migraine, by Shulte and May,35 a single patient underwent functional MRI studies on 30 consecutive days. The main findings were hypothalamic activation and an anomalous connection with the spinal trigeminal nucleus in the period preceding each migraine (24hours), and with the pons in the pain phase. This supports the hypothesis that the hypothalamus-brainstem axis is the true origin of migraine attacks; the functional change observed in hypothalamic-brainstem connectivity rules out that the dorsal pons is responsible for attacks, the previously accepted theory.
A study published several years earlier by Stankewitz et al.36 follows the same line. The researchers used MRI to demonstrate that patients with migraine have lower levels of spinal trigeminal nucleus activation at baseline than healthy controls; this increases in the preictal stage (shortly before the attack), reaching the same level as that of controls, and subsequently decreases in the pain phase. The authors conclude that migraine attacks could be predicted through observation of hypothalamus-mediated activation of the spinal trigeminal nucleus.
What is the therapeutic relevance of premonitory symptoms?Several studies suggest administering treatment during the premonitory phase in order to prevent pain onset. Waelkens7 uses domperidone for acute treatment in response to the onset of PS, while Luciani et al.37 use naratriptan. These authors reach similar conclusions, with both studies using samples of approximately 20 patients. Pain was entirely prevented in approximately two-thirds of patients; in the remaining patients, pain did occur but was less intense. Both studies stress the existence of a “point of no return” after which the pain cycle begins, which is established at a minimum of 2hours; treatment must be administered before this moment. However, these studies selected patients considered good predictors in order to determine when to administer medication. This approach involves certain limitations: not all patients are able to predict attacks, and false positives may have been included, with patients incorrectly predicting migraine attacks.
A very interesting study by Cady et al.38 divided patients (n=76) into 2 groups: group A received daily treatment with topiramate and took frovatriptan to treat migraine headache; group B took frovatriptan in the premonitory phase. No significant difference was observed between groups for the number of pain days; this indicates that pain may be prevented by treating PS. Nonetheless, patient satisfaction was greater in group A. The authors give 2 explanations for this result: firstly, this group had a higher number of dropouts due to adverse reactions to topiramate, with these patients being excluded from the final analysis; secondly, daily topiramate reduces the prevalence of PS, as mentioned above, resulting in improved quality of life.
ConclusionsThe premonitory phase is the period preceding pain onset, and is often not recognised by patients. Despite the limited evidence available, its role in migraine is very significant, as suggested in many recent studies.
Analysis of the premonitory phase has led to the discovery of the “true origin” of migraine attacks, located on the hypothalamus-brainstem axis; this theory is supported both by patients’ descriptions of symptoms and by neuroimaging findings. This also offers a new perspective for future treatment strategies. An interesting approach is to act on these targets during the premonitory phase to prevent trigeminal activation, thereby targeting pain onset and improving patients’ quality of life.
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: Gago-Veiga AB, Vivancos J, Sobrado M. Fase premonitoria, una etapa clave en la migraña. Neurología. 2021;36:298–304.