Epilepsy and sleep are intimately linked; patients with epilepsy frequently present sleep disorders and alterations in sleep quality and architecture. This, in turn, leads to greater prevalence of psychiatric and cognitive comorbidities and poorer quality of life.
DevelopmentSleep is a physiological process regulated by the circadian rhythm. The sleep–wake cycle occurs as a result of the reciprocal activity of numerous regions and circuits in the brain, via neuromediators. The different stages of sleep also influence the propagation of interictal epileptiform discharges and epileptic seizures. Non-rapid eye movement sleep facilitates their propagation, whereas rapid eye movement sleep suppresses it. This connection is made even more evident by the existence of epileptic syndromes that occur only or mainly during specific stages of the sleep–wake cycle. Antiepileptic drugs also influence this cycle. Better sleep quality may have positive effects on seizure control, and vice versa.
ConclusionsNew therapeutic targets involve the sleep–wake cycle, with orexin receptor antagonists having been proposed as a potential antiepileptic treatment. However, more research and controlled clinical trials are needed to corroborate these findings.
La epilepsia y el sueño están íntimamente relacionados y los pacientes con epilepsia (PCE) presentan con frecuencia trastornos de sueño, alteraciones en la calidad de sueño y de su arquitectura. Todo ello conlleva una mayor prevalencia de comorbilidades psiquiátricas, cognitivas y una peor calidad de vida.
DesarrolloEl sueño es un proceso fisiológico regulado por el ritmo circadiano. El ciclo sueño/vigilia tiene lugar mediante la actividad recíproca de diversas regiones y circuitos cerebrales a través de neuromediadores. Por otro lado, las distintas fases del sueño influyen en la propagación de descargas epileptiformes intercríticas (DEI) y de crisis epilépticas (CE). El sueño NREM facilita su propagación y el sueño REM la dificulta. Esta conexión se hace todavía más evidente debido a que hay síndromes epilépticos que ocurren sólo o principalmente durante determinadas fases del ciclo sueño/vigilia. Los diferentes tratamientos empleados en epilepsia también influyen en el sueño. Es posible que una mejora en la calidad de sueño tenga repercusiones positivas en el control de las crisis epilépticas y viceversa.
Conclusionesse han explorado nuevas dianas terapéuticas implicadas en el ciclo sueño/vigilia proponiendo los antagonistas de la de orexina como potenciales agentes antiepilépticos. Sin embargo, se precisan más estudios y ensayos controlados para contrastar estos hallazgos.
The relationship between sleep and epilepsy has been recognised since ancient times. Interest in sleep, epilepsy, and the relationship between them has increased in recent decades.1 Sleep is a physiological state that plays a major role in physical well-being, learning, and memory. Patients with epilepsy present poorer sleep quality, which results in psychosocial, cognitive, and psychiatric comorbidities, and poorer quality of life.2 Understanding and improving sleep in these patients may therefore have a positive impact on epileptic seizures and also on the associated comorbidities.3 Due to the relationship between sleep and epilepsy, antiepileptic treatment may affect the quality and architecture of sleep. Given the wide range of antiepileptic treatments currently available, determining the specific effects of each drug is not straightforward. The neuropeptides involved in the sleep–wake cycle and drugs regulating this cycle have been suggested as potential antiepileptic agents.4 We review the physiology of sleep and the relationship between sleep and epilepsy, and propose new therapeutic targets.
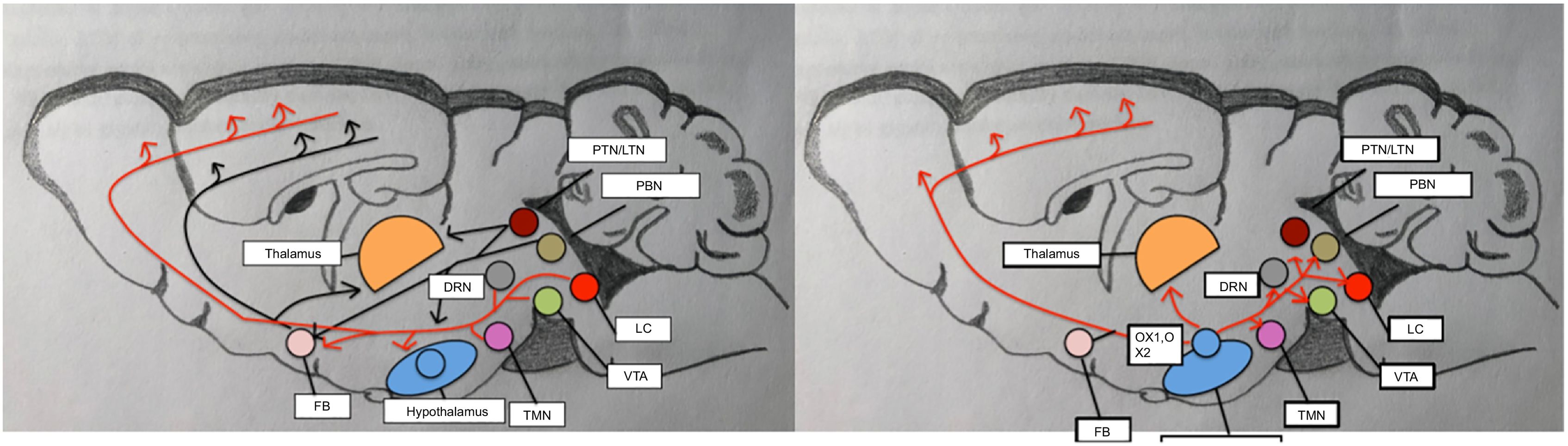
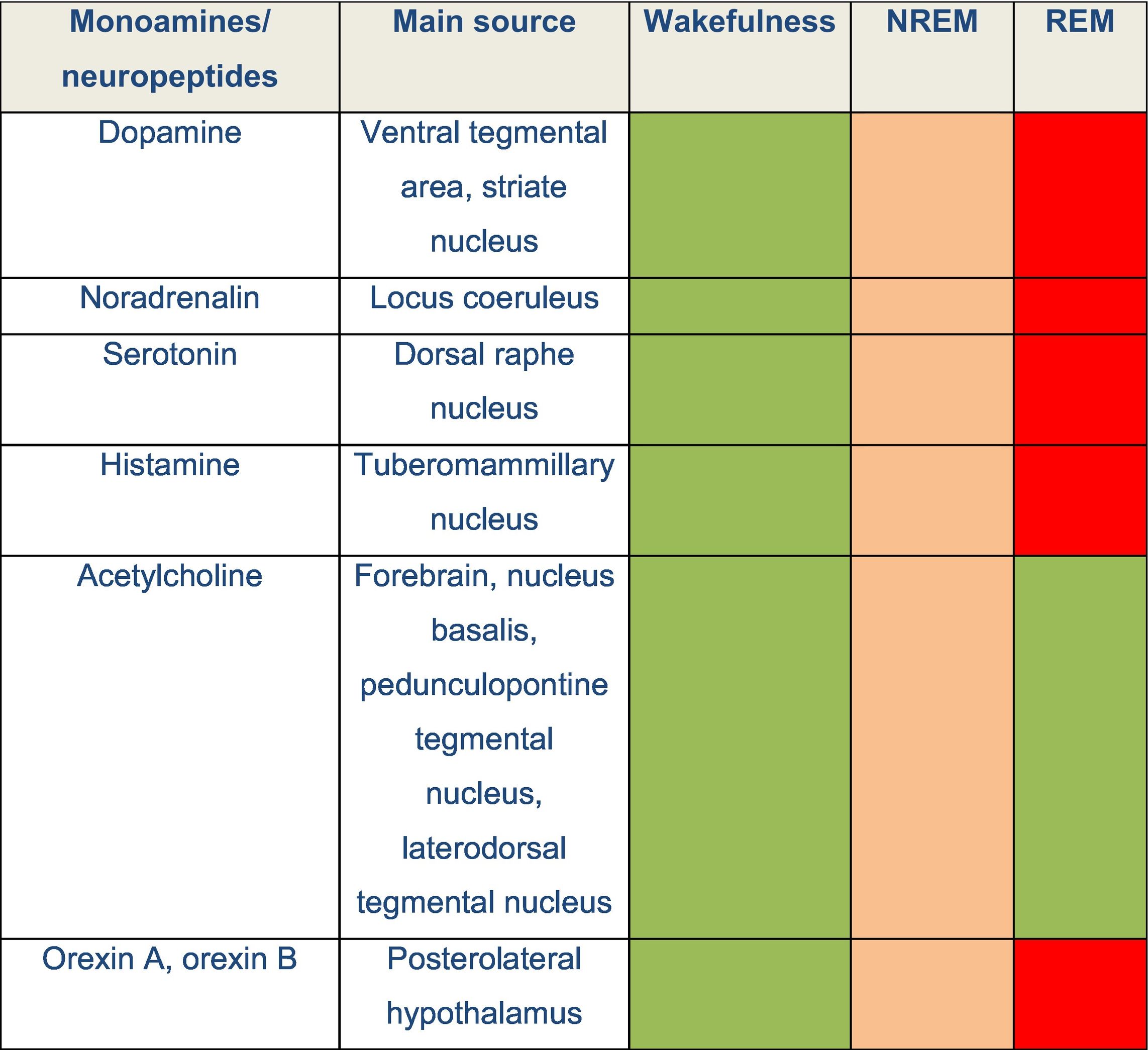
DevelopmentThe physiology of sleepThe sleep–wake cycle is regulated by a complex neural network that activates and inhibits different areas of the central nervous system. Wakefulness is the state in which living beings perform motor activities and respond to internal and external stimuli. During wakefulness, electroencephalography (EEG) shows high-frequency, low-amplitude activity and electromyography reveals varying degrees of muscle activity. Pathways promoting wakefulness ascend from the paramedian region of the midbrain and split into a dorsal pathway toward the thalamus and a ventral pathway that innervates the hypothalamus, forebrain, and cortex. Other related structures that play a significant role in maintaining wakefulness are the pedunculopontine nucleus, the laterodorsal tegmental nucleus, the parabrachial nucleus, and the posterolateral hypothalamus. Table 1 and Fig. 1 summarise the main monoamines and neuropeptides involved, as well as the nuclei that form the neural system regulating wakefulness.4,5,6 The neurotransmitters gamma-aminobutyric acid (GABA) and glutamate also play a relevant role in different phases of the sleep–wake cycle, with different functions according to their localisation.
Circuits that regulate wakefulness. Adapted from Scammell et al.5
Several neurochemical mechanisms promote arousal and fast cortical activity. Monoaminergic neurons located in the rostral brainstem and caudal hypothalamus directly innervate the cortex and such subcortical regions as the hypothalamus and thalamus. Wake-promoting signals also arise from the parabrachial nucleus, the pedunculopontine and laterodorsal tegmental nuclei, and forebrain. Neuropeptides orexin A and B are produced in the lateral hypothalamus and excite neurons in the cortex, thalamus, and wake-promoting regions of the brainstem.
DRN: dorsal raphe nucleus; FB: forebrain; LC: locus coeruleus; LTN: laterodorsal tegmental nucleus; OX-A: orexin A; OX-B: orexin B; PBN: parabrachial nucleus; PTN: pedunculopontine tegmental nucleus; TMN: tuberomammillary nucleus; VTA: ventral tegmental area.
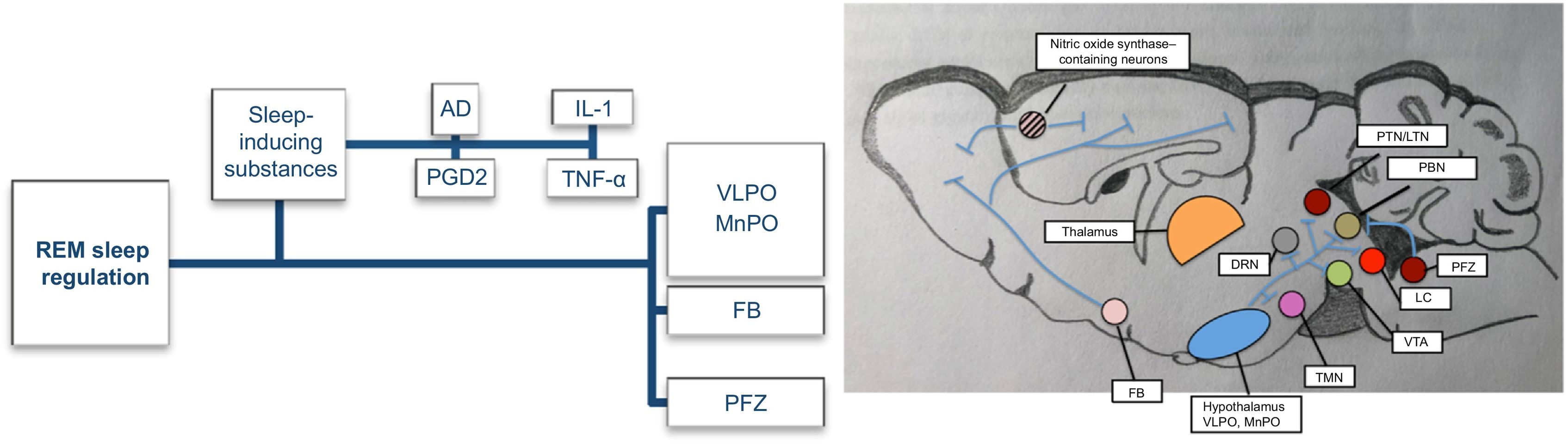
Prolonged periods of wakefulness are followed by periods of non-rapid eye movement (NREM) sleep. During this phase, EEG typically reveals slower frequencies in the delta (0–4 Hz) and theta ranges (4–7 Hz). The first stage, N1, is a short period of somnolence or falling asleep characterised by slower background activity intermixed with alpha rhythm, reduced muscle activity, and slow horizontal eye movements. Vertex waves are typical of this stage. Stage N2 presents slower, more organised activity than N1 and is characterised by the appearance of two biomarkers, sleep spindles and κ-complexes. Sleep spindles are rhythmic activity with bursts of 7–15 Hz, generated by the interaction between the thalamic reticular nucleus and thalamocortical neurons. κ-complexes are generated in the frontal cortex and propagate to the thalamus; they preserve sleep by suppressing cortical arousal from external stimuli.4 Slow waves are generated by the cortex and thalamus, which synchronise with different cortical areas. Lastly, stage N3 is characterised by generalised synchronous activity of slow delta waves (<4 Hz); muscle tone is still preserved in this stage.4,5 This homeostatic response is probably mediated by sleep-inducing substances, whose levels increase during wakefulness and which act as paracrine mediators. The circuits involved in NREM sleep generation are presented in Fig. 2.5
NREM sleep-promoting pathways. Adapted from Scammell et al.5
The image presents the main substances and brain regions that promote NREM sleep. The accumulation of sleep-inducing substances during wakefulness activates the ventrolateral preoptic area and median preoptic nucleus. GABAergic neurons in the latter two regions promote sleep by inhibiting wake-promoting neurons in the hypothalamus and brainstem through GABA and galanin. The forebrain also contains neurons that promote sleep via direct projections to the cortex. GABAergic neurons of the parafacial zone promote sleep by inhibiting the parabrachial nucleus.
AD: adenosine; DRN: dorsal raphe nucleus; FB: forebrain; IL-1: interleukin 1; LC: locus coeruleus; LTN: laterodorsal tegmental nucleus; MnPO: median preoptic nucleus; PBN: parabrachial nucleus; PFZ: parafacial zone; PGD2: prostaglandin D2; PTN: pedunculopontine tegmental nucleus; TMN: tuberomammillary nucleus; TNF-α: tumour necrosis factor α; VLPO: ventrolateral preoptic area; VTA: ventral tegmental area.
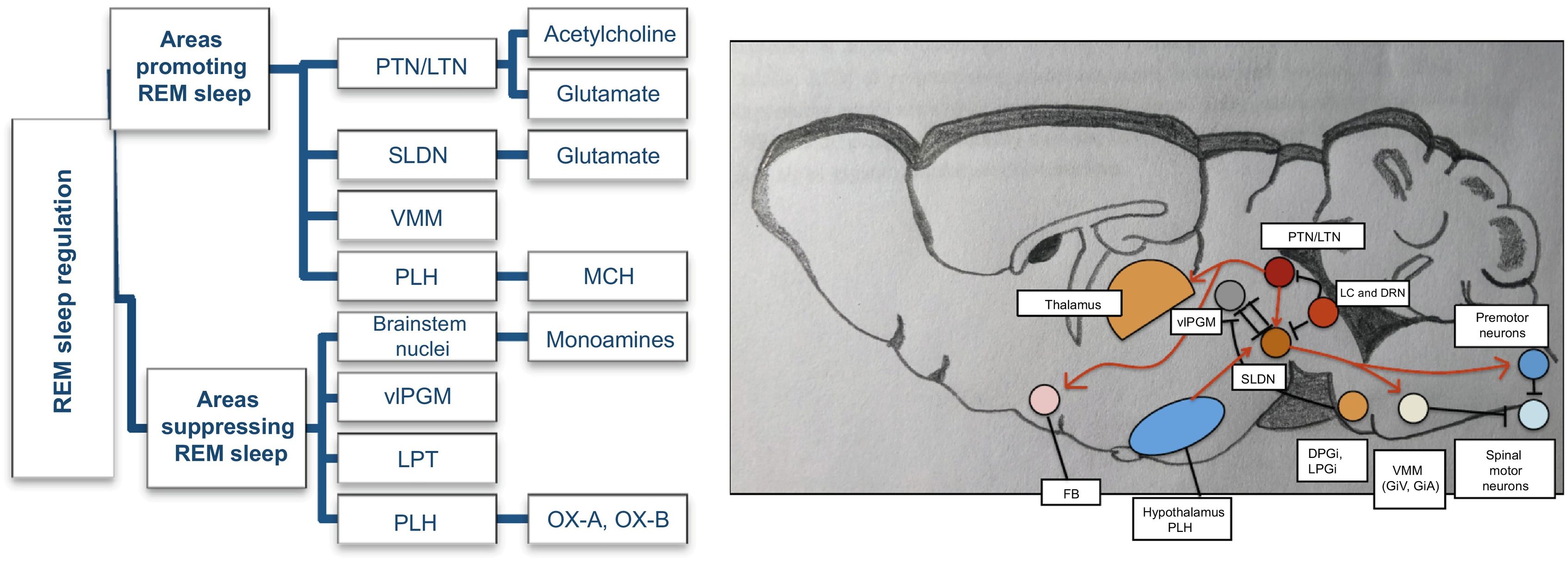
During rapid eye movement (REM) sleep, EEG presents low-amplitude, high-frequency activity, similar to that observed during wakefulness, as well as some theta activity. This phase is characterised by generalised asynchronous activity, loss of muscle tone, and vivid dreams. Fig. 3 presents the circuit that regulates REM sleep, and the interactions between the different brain regions participating in REM sleep regulation.
REM sleep-promoting pathways. Adapted from Scammell et al.5
Main areas promoting and suppressing REM sleep, and the monoamines/neuropeptides involved. The sublaterodorsal nucleus plays a crucial role in REM sleep regulation. Glutamatergic neurons of the sublaterodorsal nucleus cause muscle paralysis during REM sleep by exciting GABAergic/glycinergic neurons in the ventromedial medulla and spinal cord, hyperpolarising motor neurons. Cholinergic neurons of the pedunculopontine and laterodorsal tegmental nuclei also promote REM sleep and help drive the fast EEG activity typical of REM sleep. During wake and NREM sleep, the sublaterodorsal nucleus is inhibited by GABAergic neurons of the ventrolateral periaqueductal grey and lateral pontine tegmentum and monoaminergic neurons of the locus coeruleus and raphe nucleus. During REM sleep, the ventrolateral periaqueductal grey is inhibited by GABAergic neurons of the sublaterodorsal nucleus and medulla. The posterolateral hypothalamus contains neurons that produce melanin-concentrating hormone, which also promotes REM sleep by acting on the sublaterodorsal nucleus.
BS: brainstem; DPGi: dorsal paragigantocellular reticular nucleus; DRN: dorsal raphe nucleus; GiA: alpha gigantocellular reticular nucleus; GiV: ventral gigantocellular reticular nucleus; LC: locus coerulleus; LPGi: lateral paragigantocellular nucleus; LPT: lateral pontine tegmentum; LTN: laterodorsal tegmental nucleus; MCH: melanin-concentrating hormone; OX-A: orexin A; OX-B: orexin B; PLH: posterolateral hypothalamus; PTN: pedunculopontine tegmental nucleus; SLDN: sublaterodorsal nucleus; vlPGM: ventrolateral region of the periaqueductal grey matter; VMM: ventromedial medulla.
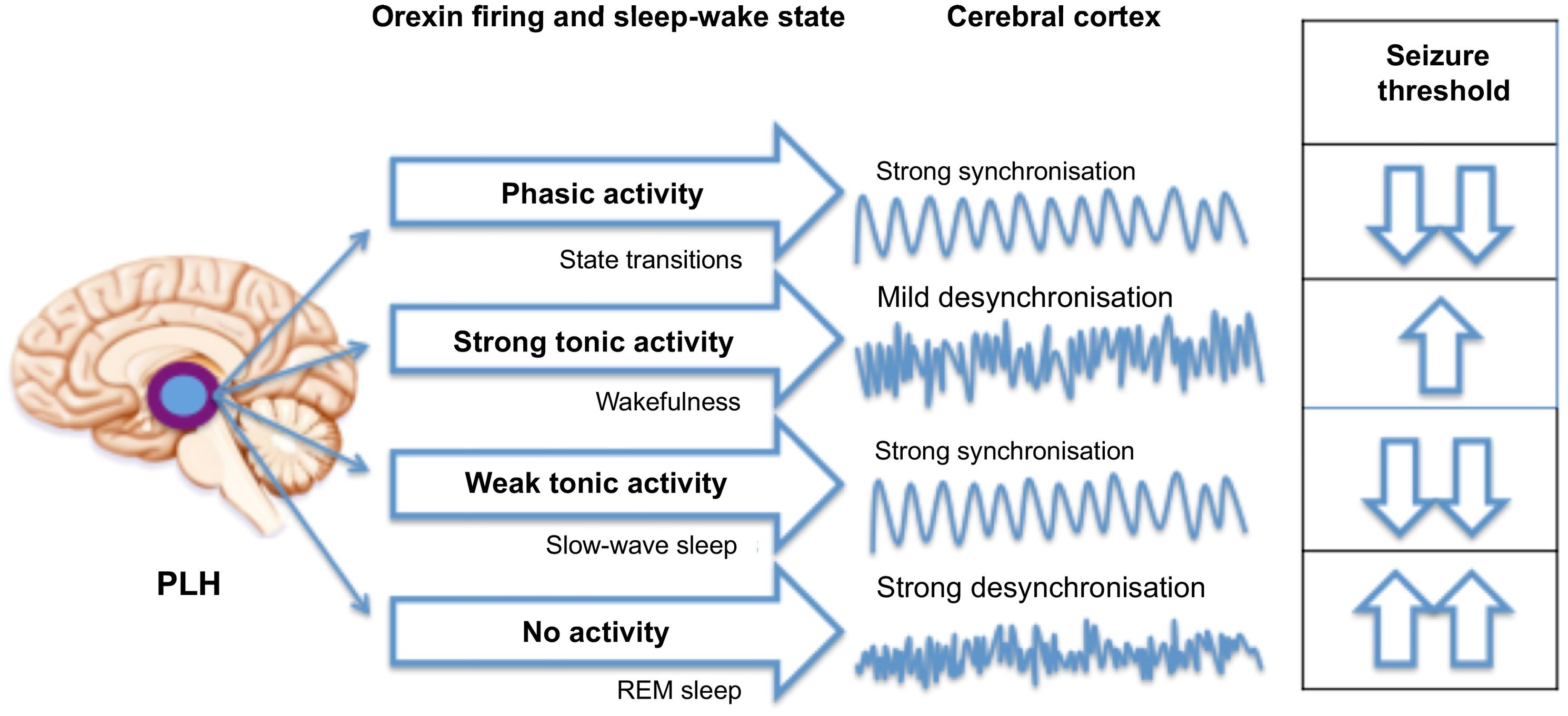
The average length of the NREM/REM sleep cycle is 90 min, and the cycle repeats 4–6 times per night. Over the course of sleep, NREM sleep becomes shorter and REM sleep stages get longer. Transitions between wakefulness and sleep are regulated by a circadian mechanism that responds to environmental changes in light and temperature. In mammals, this mechanism is located in the suprachiasmatic nucleus of the hypothalamus, which receives light input from the retinohypothalamic tract and secretes melatonin. In addition to regulating the sleep–wake cycle, the suprachiasmatic nucleus regulates other metabolic and physiological functions.5 Other molecular mechanisms are also involved in this process; among these, the transcription and translation of core clock genes deserve special attention. Early in the morning, the aryl hydrocarbon receptor nuclear translocator-like protein 1 (BMAL1) and circadian locomotor output cycles kaput (CLOCK) genes heterodimerise and activate the transcription of the period (PER1, PER2, PER3) and cryptochrome genes (CRY1, CRY2). At night, PER and CRY genes block the transcription of BMAL and CLOCK and subsequently degrade, and the cycle begins again.7,8Fig. 4 presents the main mechanisms involved in the sleep–wake cycle.
Hypothesised mechanisms of the “orexi-cortical” axis. Adapted from Marcus.25
Originating in the posterolateral nucleus of the hypothalamus, orexinergic neuronal firing characteristics determine sleep–wake state to modulate the degree of cortical synchronisation, which influences seizure threshold.
PLH: posterolateral hypothalamus.
The timing and severity of epileptic seizures are influenced by the circadian rhythm. Core clock genes regulate the circadian rhythm, but also contribute to epileptic susceptibility. These genes may alter the rhythm of the excitatory–inhibitory balance, which confers a periodicity to seizures. Although the exact role of these genes in seizure pathogenesis is unknown, disruptions in their function may play an important role. Some types of epilepsy occur most frequently during sleep; EEG hypersynchronisation and the circadian rhythm are believed to be involved.1,7,8 Interictal epileptiform discharges (IEDs) and different types of seizures tend to follow temporal patterns. For instance, temporal lobe epilepsy follows a bimodal trend (morning and evening), whereas frontal lobe seizures mostly occur at night, occipital lobe seizures peak in the early evening, and parietal lobe seizures present a variable temporal pattern.1,4,7,8 Likewise, tonic, tonic–clonic, and hypermotor seizures may be more frequent during sleep, whereas clonic, myoclonic, and hypomotor seizures are more frequent during the day. Daily and weekly oscillations in epileptic activity have also been described; therefore, epilepsy may be regarded as a cyclic disorder.7
In the late nineteenth century, Gowers observed that 20% of patients with epilepsy presented seizures exclusively at night, 21% only during the day, and 37% at any time of the day. With the development of EEG, Gibbs demonstrated that epileptiform activity increased during sleep; EEG monitoring has improved our understanding of the circadian rhythm in epilepsy. IEDs may be inhibited or activated by specific sleep stages. They occur more frequently and propagate more easily during NREM sleep.1,7 This is because of enhanced slow wave synchrony; as mentioned previously, slow waves originate in the thalamic–cortical circuit and contribute to the formation of sleep spindles during stage N2.1,2,7 During REM sleep, on the other hand, asynchronous neuronal discharge patterns hinder the propagation of epileptiform discharges; however, when they appear, they have a high localisation value.1,7
Sleep-related epilepsyPaediatric patients may present some idiopathic epileptic syndromes of childhood that typically manifest during sleep. Benign epilepsy with centrotemporal spikes or benign rolandic epilepsy is the most characteristic form. Spike-wave discharges in the centrotemporal region are activated during NREM sleep.1,2,7,9 In patients with self-limiting focal epilepsy of childhood with occipital paroxysms or Panayiotopoulos syndrome, nearly two-thirds of seizures start during NREM sleep.9 Landau-Kleffner syndrome is a malignant variant of idiopathic focal epilepsies of childhood that involves perisylvian opercular structures and is characterised by language regression.1,9 Epileptic encephalopathy with continuous spikes and waves during slow-wave sleep is another malignant variant associated with different seizure types and neurocognitive regression.1,2,7 In adults, the most frequent type of sleep-related epilepsy is focal epilepsy, particularly temporal lobe epilepsy and frontal lobe epilepsy. Nocturnal frontal lobe epilepsy is characterised by salvos of paroxysmal arousals associated with clusters of hypermotor seizures lasting several seconds. Differential diagnosis includes NREM sleep parasomnias, and frequently requires video-EEG or polysomnography. Patients with temporal lobe epilepsy wake up with aura and subsequently develop typical focal seizures, presenting amnesia after the episode.1,2
Wake-up epilepsyIdiopathic generalised epilepsy is particularly sensitive to sleep deprivation, alcohol use, and photostimulation. Most generalised seizures (myoclonic and tonic–clonic) occur within 2 hours of awakening.1,2
Epilepsy, sleep, and neuronal plasticitySome authors suggest that alterations in waking mechanisms or those promoting NREM sleep may explain some types of epilepsy. Childhood absence epilepsy is an epilepsy affecting the system that promotes NREM sleep (the corticothalamic system), where spike-wave discharges would use the same circuit as sleep spindles. Similarly, nocturnal frontal lobe epilepsy is an epileptic variant of NREM parasomnias, involving the cholinergic forebrain arousal system. An example of this is autosomal dominant nocturnal frontal lobe epilepsy, caused by a mutation in nicotinic acetylcholine receptor gene subunits.9
Sleep as a diagnostic toolSleep may induce seizures and interictal abnormalities. Sleep deprivation increases diagnostic performance and is known to be an independent factor in the activation of IEDs.1
Sleep disorders and sleep alterations in patients with epilepsyNearly one-third of patients with epilepsy present sleep disorders or sleep alterations. Excessive daytime sleepiness is a common complaint, regardless of the number of antiepileptic drugs (AED) used, seizure frequency, epileptic syndrome, or presence of nocturnal seizures1; its prevalence ranges from 11% to 28%.10 Such respiratory disorders as obstructive sleep apnoea syndrome (OSAS), with a prevalence of 10%–30%,10 also contribute to excessive daytime sleepiness. Up to one-third of patients undergoing presurgical assessment meet diagnostic criteria for OSAS.1 Several retrospective studies have found improvements in the management of refractory seizures in patients treated with continuous positive airway pressure, with results comparable to those of adding an AED. Older age, male sex, obesity, oropharyngeal stenosis, greater neck circumference, focal epilepsy, secondary generalisation, and longer progression time seem to increase the risk of OSAS.1,2,10 Furthermore, a positive correlation has been described between probable OSAS and risk of sudden death, as measured with the revised SUDEP risk inventory score.11 Insomnia, present in 30%–40% of patients,10 may be influenced by presence of concomitant mood disorders, particularly anxiety and depression,1 and even increased risk of suicide.12 Frequent awakenings may be explained by multiple causes, including epilepsy itself, medication, or use of toxic substances, and may act as trigger factors or even be a manifestation of epileptic seizures.1
Effects of epilepsy on sleep quality and architecturePatients with epilepsy present poorer sleep quality and greater prevalence of sleep disorders, affecting their quality of life3; this phenomenon is more marked among patients with refractory epilepsy.13 Sleep disorders, in turn, influence psychiatric comorbidities; these patients present an increased risk of suicide (OR: 10).12 Paediatric patients also experience sleep alterations, which have an impact on the cognitive, behavioural, and emotional domains, with similar data to those observed in adults. Although several predictors of sleep disorders have been proposed (epilepsy severity, number and type of seizures, number of AEDs, secondary effects), they are not universally accepted; other studies have found no correlation with clinical or sociodemographic variables.14–16 Regarding sleep microstructure, some studies have reported increases in sleep onset latency and awake time during sleep, instability of sleep stages, increase in stages N1 and N2 of NREM sleep, lower sleep spindle density, increased REM sleep latency, and reduced REM sleep time. Alterations in sleep quality and architecture have been described in animal models of multiple epileptic syndromes17 and in patients with epilepsy.1 Similar observations have been made in patients with juvenile myoclonic epilepsy18 and refractory mesial temporal lobe epilepsy,19 and children with epileptic encephalopathy.20
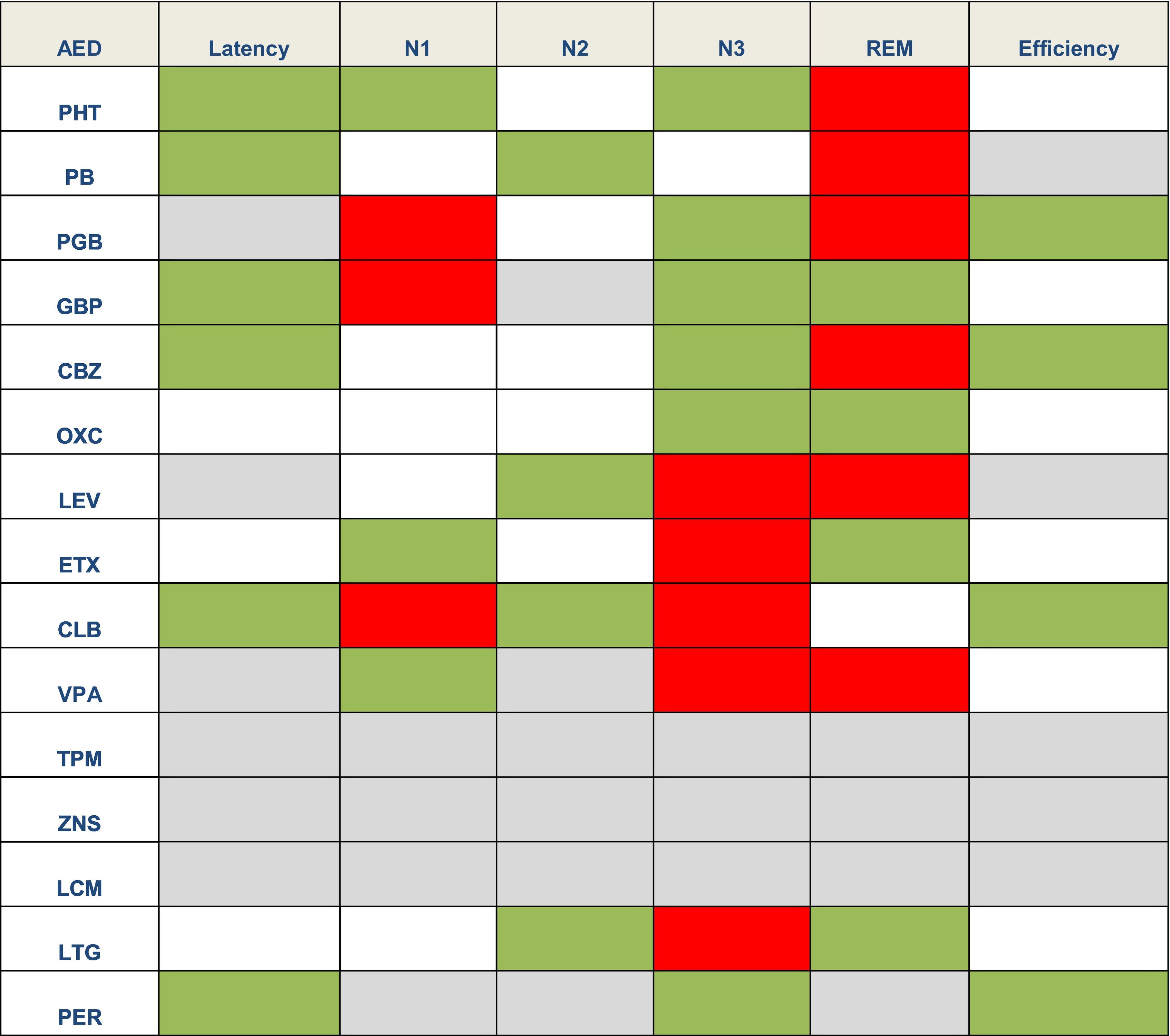
Effect of antiepileptic treatment on sleepAEDs may have different effects on sleep architecture.2 In general terms, first-generation AEDs may cause greater sleep disruption, whereas new-generation AEDs seem to have a more favourable profile or even no effect on sleep. However, few studies have analysed this issue,4 and it is difficult to determine whether the changes observed constitute adverse effects of AEDs or rather a consequence of epilepsy. Table 2 lists the main adverse effects of AEDs on sleep.
Effects of antiepileptic drugs on sleep latency, sleep stages, and sleep efficiency.1,2,4,21
Green: favourable effects (reduced sleep latency or longer duration of a given sleep stage). Grey: neutral. White: no data. Red: deleterious effects (shorter duration of a given sleep stage).
AED: antiepileptic drug; CBZ: carbamazepine; CLB: clobazam; ETX: ethosuximide; GBP: gabapentin; LCM: lacosamide; LEV: levetiracetam; LTG: lamotrigine; OXC: oxcarbazepine; PB: phenobarbital; PER: perampanel; PGB: pregabalin; PHT: phenytoin; TPM: topiramate: VPA: valproic acid; ZNS: zonisamide.
Research has been conducted into the effects of surgery for temporal lobe epilepsy. Patients undergoing successful epilepsy surgery and presenting a good clinical response show improvements in sleep quality and architecture (increased total sleep time, increased REM sleep time, fewer awakenings, shorter sleep onset latency, improved apnoea–hypopnoea index), which in turn improves their quality of life.1,2,4,22 Results vary in the case of patients receiving vagus nerve stimulation. Low-intensity stimulation (<1.5 mA) improves sleep profiles, decreasing sleep onset latency, increasing the duration of NREM sleep (N1–N3), and reducing excessive daytime sleepiness. However, use of these devices worsens respiratory function, especially at higher intensities (>1.5 mA), predisposing to or worsening OSAS, or inducing respiratory sinus arrhythmia. It is therefore essential to screen for respiratory disorders prior to implantation of the vagus nerve stimulator.1,2,4,22 Deep brain stimulation targeting the anterior thalamic nucleus seems to increase the number of microarousals in a voltage-dependent manner, although the evidence on this subject is limited.4,22 Insufficient data are available on the effects of responsive cortical neurostimulation.4 The ketogenic diet and its variants seem to increase REM sleep, although contradictory results have been reported and the underlying mechanisms are unclear.2,4
NeuropeptidesA wide range of neuropeptides are known to be involved in the sleep–wake cycle. In fact, some drugs regulating this cycle have been proposed as potential antiepileptic agents. Adenosine is reported to decrease IEDs, and it has been suggested that prostaglandin D2 may play a role in seizure suppression. Melatonin may modulate neuronal electrical activity by acting on glutamatergic and GABAergic neurotransmission, although the available evidence is controversial. Trials of beprodon, a melatonin receptor agonist, have been conducted to determine itsefficacy for the treatment of refractory focal epilepsy. Furthermore, although serotonin and histamine promote wakefulness, they may also have an antiepileptic effect. Pitolisant, a histamine H3-receptor inverse agonist used to treat drug-resistant daytime sleepiness in patients with other neurological diseases, may act as an antiepileptic agent by suppressing photoparoxysmal responses in EEG.4 Orexin, which also promotes wakefulness, seems to promote seizures. In recent years, attention has been drawn to the orexinergic system due to its numerous physiological functions, especially in the sleep–wake cycle.23 Neurotransmission changes in this system may be involved in sleep disorders and epilepsy. In 1998, two research groups (de Lecea et al. and Sakurai et al.) independently published their findings on a neuropeptide that was expressed in a group of neurons located in the lateral hypothalamus. The neuropeptide was called hypocretin due to its location, origin, and a sequence similar to that of the gastrointestinal hormone secretin. Given that the lateral hypothalamus regulates metabolism and food intake, this neuropeptide was initially thought to regulate appetite and was called orexin, from the Greek word orexis, meaning “appetite.” Both research groups identified the precursor peptide prepro-hypocretin, also known as prepro-orexin. The prepro-orexin gene is located on chromosome 17q21.2 and codes for a peptide of 131 amino acids that results in 2 small peptides, hypocretin 1 and 2 (orexin A and B), comprising 33 and 28 amino acids, respectively. Orexin A binds to receptor 1 (OXR1), and both orexin A and orexin B bind to receptor 2 (OXR2). Orexin has been shown to regulate food intake, the sleep–wake cycle, metabolism, response to stress, addiction, and analgesia. OXR1 is involved in arousal and maintaining wakefulness, whereas OXR2 promotes wakefulness.24,25 Orexinergic neurons secrete orexin in three different ways (oscillatory, phasic, and tonic firing patterns) via excitatory mediators (glutamate or galanin). Oscillatory activity is regulated by the circadian rhythm and associated with multiple functions unrelated to sleep. Phasic activity, in the form of bursts, promotes hypersynchronisation or transition between states. Tonic activity maintains consciousness without interruptions, preventing sudden lapses into sleep. Orexinergic tone is highest during wakefulness, moderate during NREM sleep, and absent during REM sleep. The mechanisms of the “orexi-cortical” axis are represented in Fig. 4.
Orexinergic neurons preserve wakefulness by sending projections to wake-promoting areas, acting on the tuberulomamillary nucleus, laterodorsal tegmental nucleus, pedunculopontine nucleus, forebrain, dorsal raphe nucleus, locus coeruleus, and ventral tegmental area, which express both OXR1 and OXR2. These regions generate negative feedback by producing GABA, noradrenaline, dopamine, and serotonin.17,25 Lack of orexinergic activity promotes REM sleep onset and diffuse cortical desynchronisation, which reduces the opportunity for spatial and temporal summation of aberrant neuronal activity into IEDs and epileptic seizures. A growing body of evidence suggests that the orexinergic system may play a major role in epilepsy.25 Experimental studies have reported a seizure-inducing effect of orexin and other orexin receptor agonists,26,27 and studies with different mouse models have shown that orexin receptor antagonists act as powerful seizure inhibitors.28–30 Other studies have reported decreased levels of orexin A in the cerebrospinal fluid (CSF) in patients with recurrent generalised seizures.31 Research has also been conducted into the effects of status epilepticus on the orexin/hypocretin system. CSF orexin A levels were analysed in patients with convulsive status epilepticus and compared against the levels found in patients with controlled epilepsy and healthy controls; levels were significantly lower in the former group. Likewise, orexin A levels were inversely correlated with modified Rankin Scale scores at one month; therefore, CSF orexin A levels were proposed as a biomarker of neuronal damage after status epilepticus, which suggests that the orexinergic system is involved in the pathogenesis of this condition.23 Furthermore, blood orexin A levels have been proposed as a biomarker for differentiating between epileptic seizures and psychogenic non-epileptic seizures, as they are significantly higher in patients with history of epileptic seizures, with no differences between patients with psychogenic seizures and controls.32 Selective OXR1 antagonists also seem to reduce anxiety.33 Suvorexant was the first selective dual orexin receptor antagonist to be approved by the United States Food and Drug Administration for the treatment of insomnia in humans, in 2013.34,35 Its efficacy36,37 and safety38 have been evaluated in patients with such respiratory comorbidities as OSAS. Subsequent studies have analysed the usefulness of the drug for controlling pentylenetetrazol- and electroshock-induced seizures; the drug exhibited an antiepileptic effect.39
Conclusions- •
Sleep and epilepsy are closely interconnected at the pathophysiological level.
- •
Patients with epilepsy frequently present alterations in sleep quality and architecture, as well as a higher prevalence of sleep disorders.
- •
Antiepileptic treatments also seem to affect sleep.
- •
Epilepsy behaves differently in each sleep stage: NREM sleep promotes the propagation of IEDs and epileptic seizures, whereas REM sleep hinders it.
- •
The neuropeptides involved in the sleep–wake cycle may play a role in epileptic activity.
- •
In view of the seizure-inducing effect of orexin, blocking orexin receptors may constitute a new therapeutic target.
This study complies with the principles of the Declaration of Helsinki on medical research involving humans. No personal data from patients have been included.
FundingNone.
Declaration of Competing InterestNone.
To all neurologists at the Epilepsy Monitoring Unit of London Health Sciences Centre (London, Ontario, Canada). Special thanks to Dr Ana Suller Martí, Dr Manuel Herrera Aramburi, Dr Miguel Arévalo Astrada, and Dr Jorge Burneo de las Casas.