The development of monoclonal antibodies against calcitonin gene-related peptide has represented a revolution in the treatment of migraine. Erenumab was the first of these drugs to be available in clinical practice in our setting.
MethodsWe performed a prospective study of patients from real clinical practice, measuring efficacy (headache days per month and migraine days per month), safety, and disease impact; data were collected at the onset of treatment with erenumab and at 3, 6, 9, and 12 months.
ResultsOur sample included 31 patients diagnosed with refractory migraine, presenting a mean of 18.5 headache days and 13.2 migraine days per month. A response rate of 50% was obtained in 58.6%, 65.2%, 69.2%, and 62.5% of patients at 3, 6, 9, and 12 months, respectively. According to Patient Global Impression of Change scale scores, 64.5% of patients rated their clinical improvement as good or excellent at 3 months, 65.2% at 6 months, 84.6% at 9 months, and 100% of cases at 12 months follow-up. A total of 45% of patients experienced mild adverse reactions, the most frequent being constipation. No severe adverse reactions were reported.
ConclusionsErenumab is an effective and safe option for the prevention of episodic and chronic migraine. Our results are similar to those obtained in real-world studies; however, results require confirmation in larger numbers of patients.
El tratamiento de la migraña ha experimentado una revolución tras el desarrollo de anticuerpos monoclonales (AcM) contra el péptido relacionado con el gen de la calcitonina (CGRP). Erenumab ha sido el primero de estos fármacos disponibles en la práctica clínica en nuestro medio.
MétodosEstudio prospectivo de práctica clínica real en el que se recogen las variables de eficacia (días con cefalea al mes: DCM y días con migraña al mes: DMM), seguridad e impacto al inicio del tratamiento con erenumab y a los 3,6,9 y 12 meses.
ResultadosSe obtuvieron resultados en 31 pacientes diagnosticados de migraña refractaria que presentaban de media 18,5 DCM y 13,2 DMM. Se obtuvo una tasa de respuesta del 50% en el 58,6%, 65,2%, 69,2% y 62,5% de los casos a los 3, 6, 9 y 12 meses, respectivamente. Los pacientes consideraban que habían presentado una mejoría clínica buena o excelente en la escala PGIC en el 64,5% de los casos a los 3 meses, 65,2% a los 6 meses, 84,6% a los 9 meses y 100% de los casos a los 12 meses de seguimiento. En total el 45% de los pacientes presentaron efectos adversos leves, siendo el más frecuente el estreñimiento. No se registró ningún efecto secundario grave.
ConclusionesErenumab es una opción eficaz y segura para la prevención de migraña episódica y crónica. Nuestros resultados son similares a los obtenidos en estudios de vida real pero estos resultados deberán confirmarse con mayor número de casos.
Migraine is one is the most common and most disabling diseases, and represents the leading cause of years lived with disability at ages of peak productivity.1,2
Fortunately, the therapeutic options for this condition have recently been expanded,3 particularly due to the clinical development of drugs targeting the action of calcitonin gene-related peptide (CGRP).4
CGRP is made up of 37 amino acids and is found in the central and peripheral nervous systems.4
CGRP receptors are diffusely expressed across all nervous system regions involved in the pathophysiology of migraine, including the cerebral cortex, subcortical regions, brainstem, meninges, and trigeminal pathways (peripherally, the dorsal root, trigeminocervical complex, and spinal cord). These findings have promoted the search for antagonists of CGRP and its receptors for the treatment of migraine.1
Specifically, four different monoclonal antibodies (mAb) inhibiting the activity of the peptide (galcanezumab, fremanezumab, and eptinezumab) or its receptor (erenumab) have been developed, and several clinical trials have demonstrated their effectiveness and safety.1,3
Erenumab, the only mAb targeting the CGRP receptor, was approved by the European Medicines Agency in 2018 and was the first of these drugs to be available for clinical practice in our setting, although under very different circumstances than those reported in pivotal trials. Real-world results are gradually being published;5–7 the aim of our study is to report our experience after one year of treatment with erenumab in a population of patients with refractory migraine.
Material and methodsWe performed a prospective study of 31 patients diagnosed with high-frequency episodic migraine (5) and chronic migraine (26) according to the third edition of the International Classification of Headache Disorders (ICHD-3) and treated with erenumab between March 2020 and May 2021. We used a database to record the number of headache days per month, migraine days per month, Migraine Disability Assessment Scale (MIDAS) and Headache Impact Test-6 (HIT-6) scores, response rate, and score on the Patient Global Impression of Change (PGIC) scale, reported by patients in an interview and questionnaires administered at treatment onset and at 3, 6, 9, and 12 months.
All study patients were initially administered 70 mg of erenumab per month, which was increased to 140 mg after 3 months in those patients with a response rate below 50%.
The primary objective was to determine the rate of response to erenumab. The secondary objective was to assess the degree of disability, the impact of migraine, and PGIC score according to the information provided by patients.
The statistical analysis included a descriptive study of the main characteristics of patients at baseline; these data are expressed as frequencies and measures of central tendency and dispersion. Quantitative variables were tested for normal distribution using the Kolmogorov–Smirnov test. Qualitative variables are expressed as frequencies and percentages, whereas quantitative variables are expressed as mean and standard deviation (SD) or as median and interquartile range (Q1–Q3), as appropriate. We conducted a bivariate analysis with hypothesis testing, comparing frequencies when both variables were qualitative (chi-square, Fisher exact test), and comparing means and difference of means when one variable was quantitative (t test, and such non-parametric tests as the Mann–Whitney U test for non-normally distributed variables). Statistical significance for the association between two variables was set at P < .05. Data were analysed using the STATA 14 data analysis and statistical software.
Our study complied with the requirements of the Declaration of Helsinki, as well as the applicable Spanish legislation on the performance of observational studies. All participants received information about the study. Data were pooled for the analysis and dissemination of findings. Individual patient data were kept confidential at all times. The content of the questionnaires, as well as the documents and the database generated during the study will not be used by anyone not involved in the study and will therefore be considered strictly confidential.
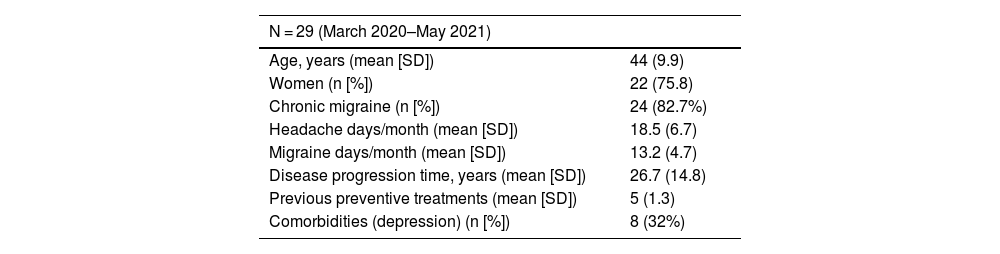
ResultsThe initial sample included 29 patients, and a further 2 patients were recruited at 3 months, in whom treatment with another mAb has failed. Baseline characteristics of the total sample are summarised in Table 1. The mean age in our sample was 44 (9.9) years, 75.8% (n = 22) were women, and the mean disease progression time was 26.7 (14.8) years.
Baseline characteristics of the sample.
| N = 29 (March 2020–May 2021) | |
|---|---|
| Age, years (mean [SD]) | 44 (9.9) |
| Women (n [%]) | 22 (75.8) |
| Chronic migraine (n [%]) | 24 (82.7%) |
| Headache days/month (mean [SD]) | 18.5 (6.7) |
| Migraine days/month (mean [SD]) | 13.2 (4.7) |
| Disease progression time, years (mean [SD]) | 26.7 (14.8) |
| Previous preventive treatments (mean [SD]) | 5 (1.3) |
| Comorbidities (depression) (n [%]) | 8 (32%) |
Patients presented a mean (SD) of 18.5 (6.7) headache days per month, meeting ICHD-3 criteria for migraine on 13.2 (4.7) days. Furthermore, patients presented treatment failure with a mean of five oral preventive drugs before starting treatment with erenumab. The main comorbidity detected was depression, which affected up to 32% (n = 8) of patients.
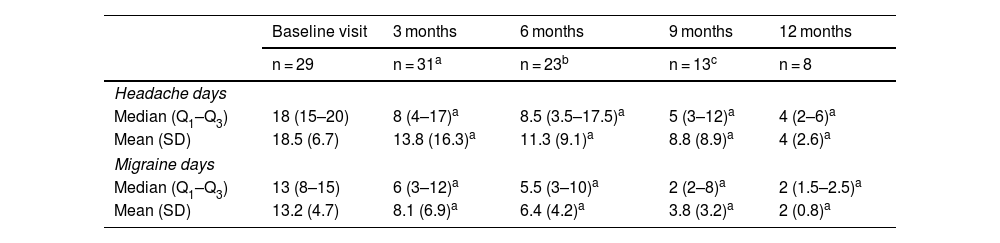
At 3 months, the mean (SD) number of headache days and migraine days per month was 13.8 (16.3) and 8.1 (6.9), respectively; erenumab dose was increased to 140 mg in 18 patients due to suboptimal response with doses of 70 mg.
At 6 months, results were obtained for 23 patients, who reported a mean (SD) of 11.3 headache days and 6.4 migraine days per month. Of the 23 patients analysed at 6 months, 5 switched to another mAb.
We collected data on 13 patients at a third consultation held 9 months after onset of erenumab treatment, showing a mean (SD) of 8.8 (8.9) headache days and 3.8 (3.2) migraine days per month. At this visit, four patients diagnosed with high-frequency episodic migraine discontinued treatment due to ineffectiveness.
At 12 months, 8 patients were still receiving treatment with erenumab; the mean (SD) number of headache days and migraine days reported per month was 4 (2.6) and 2 (0.8), respectively. Table 2 summarises the results of the reviews.
Headache days and migraine days per month at baseline and at 3, 6, 9, and 12 months after starting preventive treatment with erenumab.
| Baseline visit | 3 months | 6 months | 9 months | 12 months | |
|---|---|---|---|---|---|
| n = 29 | n = 31a | n = 23b | n = 13c | n = 8 | |
| Headache days | |||||
| Median (Q1–Q3) | 18 (15–20) | 8 (4–17)a | 8.5 (3.5–17.5)a | 5 (3–12)a | 4 (2–6)a |
| Mean (SD) | 18.5 (6.7) | 13.8 (16.3)a | 11.3 (9.1)a | 8.8 (8.9)a | 4 (2.6)a |
| Migraine days | |||||
| Median (Q1–Q3) | 13 (8–15) | 6 (3–12)a | 5.5 (3–10)a | 2 (2–8)a | 2 (1.5–2.5)a |
| Mean (SD) | 13.2 (4.7) | 8.1 (6.9)a | 6.4 (4.2)a | 3.8 (3.2)a | 2 (0.8)a |
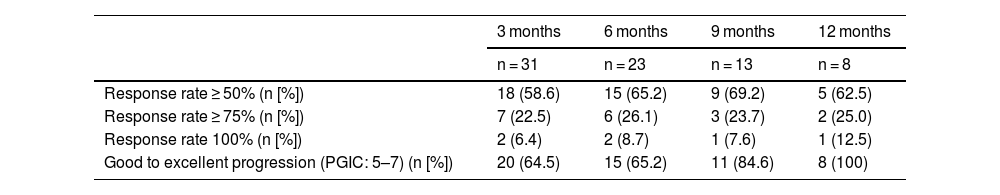
PGIC scores and the rate of response to erenumab treatment were measured using the data provided by patients during follow-up. Table 3 summarises these data.
Response rates and PGIC scores at 3, 6, 9, and 12 months after onset of erenumab treatment.
| 3 months | 6 months | 9 months | 12 months | |
|---|---|---|---|---|
| n = 31 | n = 23 | n = 13 | n = 8 | |
| Response rate ≥ 50% (n [%]) | 18 (58.6) | 15 (65.2) | 9 (69.2) | 5 (62.5) |
| Response rate ≥ 75% (n [%]) | 7 (22.5) | 6 (26.1) | 3 (23.7) | 2 (25.0) |
| Response rate 100% (n [%]) | 2 (6.4) | 2 (8.7) | 1 (7.6) | 1 (12.5) |
| Good to excellent progression (PGIC: 5–7) (n [%]) | 20 (64.5) | 15 (65.2) | 11 (84.6) | 8 (100) |
PGIC: Patient Global Impression of Change.
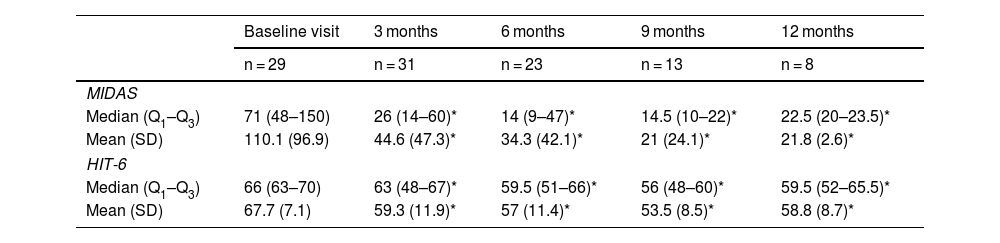
MIDAS and HIT-6 scores were used to measure the impact of migraine and the degree of disability associated with the disease.
Before starting treatment with erenumab, mean MIDAS and HIT-6 scores were 110.1 (96.9) and 67.7 (7.1), respectively. At 3 months, mean scores were 44.6 (47.3) on the MIDAS scale and 59.3 (11.9) on the HIT-6 scale. At 6, 9, and 12 months, mean scores were 34.3 (42.1) and 57 (11.4); 21 (24.1) and 53.5 (8.5); and 21.8 (2.6) and 58.8 (8.7), respectively. Table 4 summarises the scores obtained on these scales.
MIDAS and HIT-6 scores at baseline and at 3, 6, 9, and 12 months.
| Baseline visit | 3 months | 6 months | 9 months | 12 months | |
|---|---|---|---|---|---|
| n = 29 | n = 31 | n = 23 | n = 13 | n = 8 | |
| MIDAS | |||||
| Median (Q1–Q3) | 71 (48–150) | 26 (14–60)* | 14 (9–47)* | 14.5 (10–22)* | 22.5 (20–23.5)* |
| Mean (SD) | 110.1 (96.9) | 44.6 (47.3)* | 34.3 (42.1)* | 21 (24.1)* | 21.8 (2.6)* |
| HIT-6 | |||||
| Median (Q1–Q3) | 66 (63–70) | 63 (48–67)* | 59.5 (51–66)* | 56 (48–60)* | 59.5 (52–65.5)* |
| Mean (SD) | 67.7 (7.1) | 59.3 (11.9)* | 57 (11.4)* | 53.5 (8.5)* | 58.8 (8.7)* |
HIT-6: Headache Impact Test-6; MIDAS: Migraine Disability Assessment Scale.
Regarding the safety of erenumab, 45% of patients presented mild adverse reactions, but no severe reaction was reported. The most frequent adverse reaction was constipation, which manifested in 10 patients (7.5%). Other adverse reactions, in order of frequency, included asthenia (3 patients), local reaction at the injection site (2), dizziness (1), headache (1), and nasopharyngitis (1).
DiscussionThe results of our study show erenumab to be an effective and safe treatment in real clinical practice, in a heterogeneous population of patients with refractory migraine.
Treatment of migraine with anti-CGRP mAbs presents multiple advantages over conventional oral preventive treatments, including the following: they are more specific therapeutic options; their half-life is longer than that of other preventive treatments, enabling monthly or even quarterly administration; they are metabolised in the reticuloendothelial system, with low potential for hepatic or renal toxicity; and as they are large molecules, they do not cross the blood–brain barrier at high concentrations, thereby reducing the likelihood of adverse reactions in the central nervous system. The only significant disadvantage is the administration route, as they must be administered by subcutaneous injection or intravenous infusion. However, this aspect seems to be beneficial from the perspective of treatment adherence.1
Our experience is consistent with the results reported in recent articles on real-life experience. For instance, a prospective multicentre cohort study including nine Italian hospitals showed that erenumab 70 mg is effective, safe, and well-tolerated in patients with high-frequency episodic migraine or chronic migraine and ≥3 previous treatment failures, as it reduces the number of headache/migraine days, the monthly intake of analgesics, pain severity, and disability.6
Similarly, the results of a retrospective study in a headache centre in the United States suggested that the drug was effective in reducing the number of headache days and migraine days per month in 69.47% of patients, although 70% of patients presented some adverse reaction, with constipation being the most frequent, as in our sample.7
Another recent publication on the effectiveness of erenumab in real clinical practice was a prospective study at an Italian centre, including 30 patients diagnosed with episodic or chronic migraine who started treatment with erenumab after failure of at least two oral preventive treatments. The rate of reduction in the number of headache days and migraine days per month was analysed at 3, 6, and 12 months after treatment onset, and the authors observed a significant reduction in both measures. This study also reported mild adverse reactions, with constipation and local reactions at the injection site being the most frequent. Only one patient presented a severe adverse reaction, and had to discontinue erenubmab treatment.8
According to the recommendations on clinical trials studying migraine, we considered the treatment to have been effective when the frequency of headache days or migraine days was reduced by more than 50%.9 Such a reduction was obtained in 58.6%, 65.2%, 69.2%, and 62.5% of patients at 3, 6, 9, and 12 months, respectively; up to 6.4% of the patients showed a 100% reduction at 3 months after treatment onset.
Regarding patient-reported data on the impact of migraine and the disability associated with the disease, we should underscore that the reduction in MIDAS and HIT-6 scores was considerable and early; furthermore, it persisted at 12 months after onset of erenumab treatment. Other studies have found mAbs to significantly reduce the disability associated with migraine, as we observed in our sample. PGIC scores were rated as good to excellent in more than 64% of patients after treatment onset and in up to 100% of the patients at 12 months.
The most frequent adverse reactions to erenumab reported in previous studies were constipation and local reactions at the injection site.5 We observed no severe adverse reactions in our sample, and the main reason for treatment discontinuation was ineffectiveness.
One outstanding issue in the preventive treatment of migraine using mAbs is when treatment should be discontinued. In general, preventive drugs for migraine are used for at least 6 months. The available data from clinical trials suggest that in patients who are responsive to mAbs, this response occurs after 3 months of treatment, although in some cases a relevant clinical response is achieved during the first month of treatment. Some studies suggest that if migraine frequency or severity do not decrease within 1–3 months, treatment should be discontinued.10
Considering the natural fluctuations of migraine over the course of the disease, and based on the principle of minimising medication whenever possible, discontinuation of treatment with mAbs should be considered in patients who do not achieve a sustained optimal response.5 There is no consensus on the most appropriate time to discontinue erenumab treatment or any other antibody, due to the lack of information on outcomes after treatment discontinuation.
Several studies report structural and functional changes in the brains of patients with migraine, especially those with chronic migraine. These changes affect regions participating in the pathophysiology of the disease, and may lead to differences in response both to specific treatment and to its discontinuation.11 Therefore, it is essential to obtain further data on this subject and to identify biomarkers that may help to predict the response to treatment and discontinuation, in order to establish a specific and individualised treatment. Some response predictors reported to date include pain unilaterality, dopaminergic symptoms, and the number of headache days per month as positive factors; and psychiatric comorbidity and the number of previous treatment failures as negative factors.10
We should highlight certain issues that have emerged with regard to mAbs in the context of the COVID-19 pandemic. The healthcare situation we have experienced since March 2020 has led experts to consider whether CGRP antagonists may alter the body's immune response to the virus. Based on the available data, the prevalence of COVID-19 appears to be similar between patients receiving treatment with mAbs and those who are not receiving this treatment; furthermore, anti-CGRP treatment seems not to be associated with poorer clinical progression of the infection.12
Furthermore, treatment with mAbs does not seem to interfere either with immunogenicity, safety, or effectiveness of any vaccine against COVID-19. Therefore, considering the risks imposed by the infection and the proven effectiveness of mAbs in the preventive treatment of migraine, delaying these interventions is not recommended.13,14
ConclusionsErenumab is an effective and safe treatment option for patients with episodic and chronic migraine. We obtained similar results to those reported by other studies on clinical practice; however, further information is needed on the long-term results of mAb treatment for refractory migraine. These results require confirmation in a larger number of patients.
Ethical considerationsThe authors declare that they have followed their center's protocols on the publication of patient data and have obtained the corresponding permissions.
Conflicts of interestThe authors have no conflicts of interest to declare.