Teriflunomide is an oral immunomodulatory agent approved for the treatment of relapsing–remitting multiple sclerosis (RRMS). We examined teriflunomide outcomes in patients with RRMS under clinical practice conditions in Spain.
Material and methodsNon-interventional, retrospective study at 15 sites in the Autonomous Region of Madrid and nearby regions. Effectiveness (relapses, EDSS, gadolinium-enhancing T1 lesions and new/enlarged T2-weighted lesions), safety (adverse events), and reasons for discontinuation during the 24 months after teriflunomide initiation were reported.
ResultsA total of 776 patients were included (mean [SD] age was 43.3 (9.8) years; 69.3% were female). Two-thirds (67.7%) of patients had received a prior treatment, with beta-interferons or glatiramer acetate (BRACE) as the most frequent (93.5%) treatment. After 24 months, teriflunomide significantly reduced the annualized relapse rate (ARR) by 72% (mean [95% confidence interval] 0.12 [0.10, 0.14] vs 0.43 [0.40, 0.47] at baseline; P<.0001) and 81.8% of patients were relapse-free. The number of patients with gadolinium-enhancing T1 lesions and new/enlarged lesions on T2 was also reduced after 24 months of teriflunomide treatment (P<.001). Mean EDSS (SD) score was 1.9 (1.5) at teriflunomide initiation and 2.0 (1.6) at month 24. Half of patients (n=388) reported at least 1 adverse event (AE; gastrointestinal disorders: 26.2%; hair thinning: 25%; and elevation of ALT values: 12.9%). Most patients (91.5%) did not show fatigue increase during teriflunomide treatment. Among patients who discontinued treatment (n=262; 34.2%), the most common reasons were lack of effectiveness (58.0%), AEs (31.9%; n=82), and pregnancy desire (6.6%; n=17).
ConclusionsMost RRMS patients treated with teriflunomide in clinical practice had received prior treatments. Teriflunomide resulted in decreased clinical and radiological activity and disability stabilization. AE frequency and type were in line with prior reports. Most patients did not experience fatigue increases after teriflunomide initiation.
La teriflunomida es un agente inmunomodulador oral aprobado para el tratamiento de la esclerosis múltiple remitente-recurrente (EMRR).
Examinamos los resultados de teriflunomida en pacientes con EMRR en condiciones de práctica clínica en España.
Material y métodosEstudio observacional, retrospectivo en 15 centros de la Comunidad de Madrid y regiones próximas. Se recogió la efectividad (brotes, EDSS, lesiones T1 con realce de gadolinio y lesiones nuevas/aumentadas en T2), seguridad (acontecimientos adversos) y motivos de interrupción durante los 24 meses posteriores al inicio de teriflunomida.
ResultadosSe incluyeron 776 pacientes (la edad media [DE] fue de 43,3 (8, 9) años; el 69,3% eran mujeres). Dos tercios (67,7%) de los pacientes habían recibido un tratamiento previo, siendo los beta-interferones o el acetato de glatiramero el tratamiento más frecuente (93,5%). Después de 24 meses, la teriflunomida redujo significativamente la tasa anualizada de brotes (TAB) en un 72% (media [intervalo de confianza del 95%] 0,12 [0,10, 0,14] frente a 0,43 [0,40, 0,47] al inicio; p < 0,0001) y el 81,8% de los pacientes estaban libres de brotes. El número de pacientes con lesiones T1 con realce de gadolinio y lesiones nuevas/aumentadas en T2 también se redujo tras 24 meses de tratamiento con teriflunomida (p < 0,001). La puntuación media de la EDSS (DE) fue de 1,9 (1, 5) al inicio de la teriflunomida y de 2,0 (1, 6) en el mes 24. La mitad de los pacientes (n=388) notificaron al menos un acontecimiento adverso (trastornos gastrointestinales: 26,2%; adelgazamiento del cabello: 25%; y elevación de los valores de ALT: 12.9%). La mayoría de los pacientes (91,5%) no mostraron un aumento de la fatiga durante el tratamiento con teriflunomida. Entre los pacientes que interrumpieron el tratamiento (n=262; 34,2%), los motivos más frecuentes fueron la falta de eficacia (58,0%), los acontecimientos adversos (31,9%; n=82) y el deseo de embarazo (6,6%; n=17).
ConclusionesLa mayoría de los pacientes con EMRR tratados con teriflunomida en la práctica clínica habían recibido tratamientos previos. La teriflunomida produjo una disminución de la actividad clínica y radiológica y una estabilización de la discapacidad. La frecuencia y el tipo de acontecimientos adversos coincidieron con informes previos. La mayoría de los pacientes no experimentaron aumentos de la fatiga tras el inicio de teriflunomida.
Multiple sclerosis (MS) is the most common chronic neurological disorder in young adults, usually diagnosed when patients are between 20 and 40 years old. The disease is a potentially severe cause of disability throughout adult life, impairing work capacity1 and health-related quality of life (HRQoL),2 among other aspects of patients' lives. MS prevalence has increased in western Europe,3 including in Spain,4 in recent decades, and the disease affects at least 2.8 million people worldwide.5
A considerable number of disease modifying treatments (DMTs) that substantially improve the prognosis of MS are now available.6 Growing therapy options for patients have also posed challenges for clinicians in terms of disease management. The efficacy and safety profile of the DMT along with the patient's individual disease should be taken into consideration when designing personalized treatment strategies.7,8 Patient preferences are also a key factor for the DMT choice, since choosing a DMT according to patients priorities may increase treatment acceptance and adherence9 and decrease the healthcare resource use.10 For instance, route of administration plays an important role in patients' preference, and most MS patients have reported to prefer an oral DMT.11
Teriflunomide, a once-daily oral DMT, is approved for the treatment of relapsing forms of MS (RMS) or relapsing–remitting MS (RRMS), depending on the jurisdiction. The efficacy and safety of teriflunomide were established in several randomized clinical trials (RCT) and follow-up studies. The TEMSO and TOWER RCT showed that, compared to placebo, teriflunomide reduced the risk of relapses and the annualized relapse rate (ARR), and delayed disability worsening.12,13 Teriflunomide also was shown to be effective in reducing lesion volume and brain volume loss (BVL) observed on brain magnetic resonance imaging (MRI).12,14,15 Teriflunomide's efficacy was similar to high-dose subcutaneous interferon-beta-1a in the TENERE RCT, but patients' satisfaction with teriflunomide was higher.16 RCT and real-world evidence (RWE) has confirmed that teriflunomide has a manageable safety profile and is generally well-tolerated for up to 9 years of treatment.17–21 Most common (>10%) adverse events (AE)—nasopharyngitis, hair thinning, gastrointestinal disorders, and alanine aminotransferase (ALT) elevation—are usually transient and mild to moderate in severity.
Even though the safety and effectiveness profile of teriflunomide in the real-world patient had been confirmed by several RWE studies in Europe,20,22–24 only 2 of the published studies were conducted in Spain, and they were limited to the Valencian Community25 or the southern regions.26 Due to differences in MS management guidelines, treatment availability, and prescribing practices between regions, collecting data from the clinical practice of different regions will provide valuable insights into the outcomes of patients treated with teriflunomide. Here, we present data on teriflunomide safety and effectiveness in RRMS patients treated according to the clinical practice of the central regions of Spain (Community of Madrid and surrounding areas).
Materials and methodsStudy designThe TERICAM was a non-interventional retrospective study conducted at the Departments of Neurology of 15 hospitals in the Autonomous Region of Madrid and nearby Autonomous Regions (Castilla y León [Ávila and Segovia] and Castilla la Mancha [Toledo and Albacete]). Teriflunomide was prescribed according to routine clinical practice, following the local label. Patients were scheduled to make follow-up visits to their neurologist approximately 3 months after starting teriflunomide treatment and every 6 months thereafter, according to clinical practice. Data were retrieved from medical charts for patients who initiated treatment between January 2012 and April 2021.
EthicsThe study was approved by the ethics committee of the Hospital General Universitario Gregorio Marañón. It was conducted in accordance with the International Conference on Harmonization Guidelines for Good Clinical Practice, the Declaration of Helsinki, and the Spanish legislation for post-authorization studies.
PatientsPatients were eligible for inclusion in the study if they met all the selection criteria: age≥18 years, diagnosis of RRMS, have received teriflunomide treatment according to clinical practice conditions, and have signed written patient informed consent for using their medical data for the purpose of this research. No explicit exclusion criteria were specified to avoid selection bias.
Outcomes measures collectedThe primary effectiveness outcome was the ARR (defined as the number of confirmed relapses per patient-year) during the 2 years after teriflunomide initiation compared with the 2 prior years. Time until first relapse and number of relapses that occurred up to each follow-up visit (from: 0–3 months, 3–6 months, 6–12 months, and 12–24 months) were also collected. A relapse was defined as any new neurologic symptom not associated with fever or infection lasting for at least 24 h and accompanied by new neurologic signs.
Other effectiveness outcomes were changes in disability as measured by the Kurtzke Expanded Disability Status Scale (EDSS) from teriflunomide initiation to months 3, 6, 12, 18, and 24, and changes in the number of gadolinium-enhancing (Gd+) T1 and new/enlarged T2-weighted lesions on MRI from teriflunomide initiation to months 12 and 24.
The frequency of most common AEs (hepatic enzyme elevation, lymphopenia, alopecia, infections, gastrointestinal disorders, peripheral neuropathy, and increased fatigue) over teriflunomide treatment and reasons for discontinuation were collected. Data at teriflunomide initiation (demographics, medical history of MS, previous DMT, and reasons for switching from previous DMT) and teriflunomide treatment adherence were also included.
Statistical analysisA descriptive analysis of the study variables was performed. Description of quantitative variables was performed using mean (standard deviation [SD] or 95% confidence interval [95% CI]) and/or median (interquartile range [IQR]) values. For the description of qualitative variables, absolute and relative frequencies were used. For relative frequencies, 2 percentages were calculated: the total percentage (i.e., the percentage of the sum of valid responses plus missing values) and the valid percentage (i.e., the percentage of the valid responses). Valid percentages are reported here, unless specified otherwise.
At different times of follow-up, quantitative variables were compared using the Wilcoxon test (non-parametric) or t-test (parametric), according to the distribution of the sample; qualitative variables were compared using Chi-square test, Fisher test, McNemar's test or the matrix for the marginal homogeneity based on the sample distribution.
Post-hoc analysis included the description of patient characteristics at teriflunomide initiation (demographics, medical history of MS, previous treatment, and ARR) in patients who had fatigue increase as an AE during teriflunomide treatment and in patients who did not have this AE. Mann–Whitney test (non-parametric) or t-test (parametric) were used for quantitative variables and Fisher test or Chi-square test were used for qualitative variables.
Values of P<.05 were considered statistically significant. No imputations for missing data were performed. All statistical analyses were performed using Statistical Package for Social Sciences (SPSS) version 22.
ResultsPatient characteristics and prior treatmentA total of 776 patients met the selection criteria and were included in the analysis. Detailed information on patient characteristics at initiation of teriflunomide treatment is provided in Table 1. Briefly, 69.3% of patients were females and the mean (SD) age was 43.3 (9.8) years. The mean time from first MS symptom was 10.2 (8.4) years and the mean number of MS relapses in the 2 prior years was 0.9 (1.1).
Demographic and clinical characteristics at teriflunomide initiation.
| Characteristic | Value | Na |
|---|---|---|
| Age (years), mean (SD) | 43.3 (9.8) | 770 |
| Gender (female), n (%) | 538 (69.3) | 776 |
| Time from first MS symptom (years), mean (SD) | 10.2 (8.4) | 751 |
| Patients with relapses in the 2 prior years, n (%) | 407 (52.9) | 769 |
| Number of relapses in the 2 prior years, mean (SD) | 0.9 (1.1) | 769 |
| EDSS score, mean (SD) | 1.8 (1.4) | 759 |
| T1 Gd+ lesions (yes), n (%) | 176 (26.3) | 668 |
| Number of T2-lesions (≤3 months), n (%) | 660 (98.1) | 673 |
| <9 | 149 (26.6) | 561 |
| 9–15 | 124 (22.1) | 561 |
| >15 | 288 (51.3) | 561 |
Almost one-third of patients (32.3%) were treatment naïve. In patients previously treated (67.7%), the most frequently used agents were BRACE (beta-interferons or glatiramer acetate) (93.5%) and the most common reason for discontinuation was the occurrence of AEs (73.1%). All previous treatments are shown in Table 2.
Previous treatment.
| Characteristic | Value (n=776) |
|---|---|
| Previous treatment (yes), n (%) | 525 (67.7) |
| Number of previous treatments, mean (SD) | 1.0 (0.9) |
| 1, n (%) | 327 (42.1) |
| 2, n (%) | 148 (19.1) |
| 3, n (%) | 35 (4.5) |
| 4, n (%) | 15 (1.9) |
| Last treatment, n (%) | |
| BRACE | 427 (81.3) |
| Dimethyl fumarate | 75 (14.3) |
| Azathioprine | 12 (2.3) |
| Laquinimod | 4 (0.8) |
| Fingolimod | 4 (0.8) |
| Mycophenolate | 1 (0.2) |
| Natalizumab | 1 (0.2) |
| Rituximab | 1 (0.2) |
| Reasons for discontinuationa | |
| AEs | 378 (73.1) |
| Lack of effectiveness | 126 (24.4) |
| Convenience | 21 (4.1) |
| End of clinical trial | 4 (0.8) |
| Lack of adherence | 4 (0.8) |
| Physician's choice | 2 (0.4) |
The mean (SD) observation period (i.e., period of time from teriflunomide initiation to study data collection) was 1.9 (0.8) years. During this period, 262 (34.2%) patients had discontinued teriflunomide treatment. The most common reasons for discontinuation were lack of effectiveness (58.0%; n=149), AEs (31.9%; n=82), and pregnancy desire (6.6%; n=17). Lack of effectiveness was observed as: clinical activity (86 [64.7%] patients), radiological activity (13 [9.8%]), clinical and radiological activity (3 [2.3%]), progression to secondary progressive MS (16 [10.7%]), and not specified (31 [23.3%]) The characteristics of patients who discontinued teriflunomide treatment due to lack of effectiveness are presented in Table 3.
Characteristics and previous treatment of patients who discontinued teriflunomide due to lack of effectiveness (n=149).
| Characteristic | Value | N |
|---|---|---|
| Age (years), mean (SD) | 40.0 (9.8) | 147 |
| Gender (female), n (%) | 104 (68.9) | 149 |
| Time from first MS symptom (years), mean (SD) | 9.5 (7.2) | 142 |
| Patients with relapses in the 2 prior years, n (%) | 97 (65.1) | 149 |
| Number of relapses in the 2 prior years, mean (SD) | 1.3 (1.4) | 149 |
| Previous treatment (yes), n (%) | 114 (76.5) | 149 |
| Number of previous treatments, mean (SD) | 1.6 (0.8) | 114 |
| 1, n (%) | 66 (44.3) | |
| 2, n (%) | 34 (22.8) | |
| 3, n (%) | 10 (6.7) | |
| 4, n (%) | 4 (2.7) | |
| Last treatment, n (%) | 114 | |
| BRACE | 109 (95.6) | |
| Dimethyl fumarate | 20 (17.5) | |
| Azathioprine | 3 (2.6) | |
| Natalizumab | 3 (2.6) | |
| Fingolimod | 2 (1.8) | |
| Laquinimod | 1 (0.9) |
Relapses. The mean (95% CI) ARR was significantly lower during the first 24 months of teriflunomide treatment (0.12 [0.10, 0.14]) compared to the 24 months prior to teriflunomide treatment (0.43 [0.40, 0.47]), showing a 72% reduction (P<.0001) (Fig. 1A). A total of 623 (81.8%) patients were relapse-free during teriflunomide treatment (88.6% [95% CI: 88.3–88.8%] at month 12 and 82.8% [95% CI: 82.7–82.8% at month 24) (Fig. 1B). In those patients who had at least a relapse (139; 18.3%) during teriflunomide treatment, mean time to first relapse was 0.9 (0.7) years.
EDSS. Mean (SD) EDSS score at teriflunomide initiation was 1.9 (1.5) and 2.0 (1.6) after 24 months of treatment (P=.001; n=477). When all patients with available EDSS were considered, EDSS scores remained stable up to month 24 (Fig. 2).
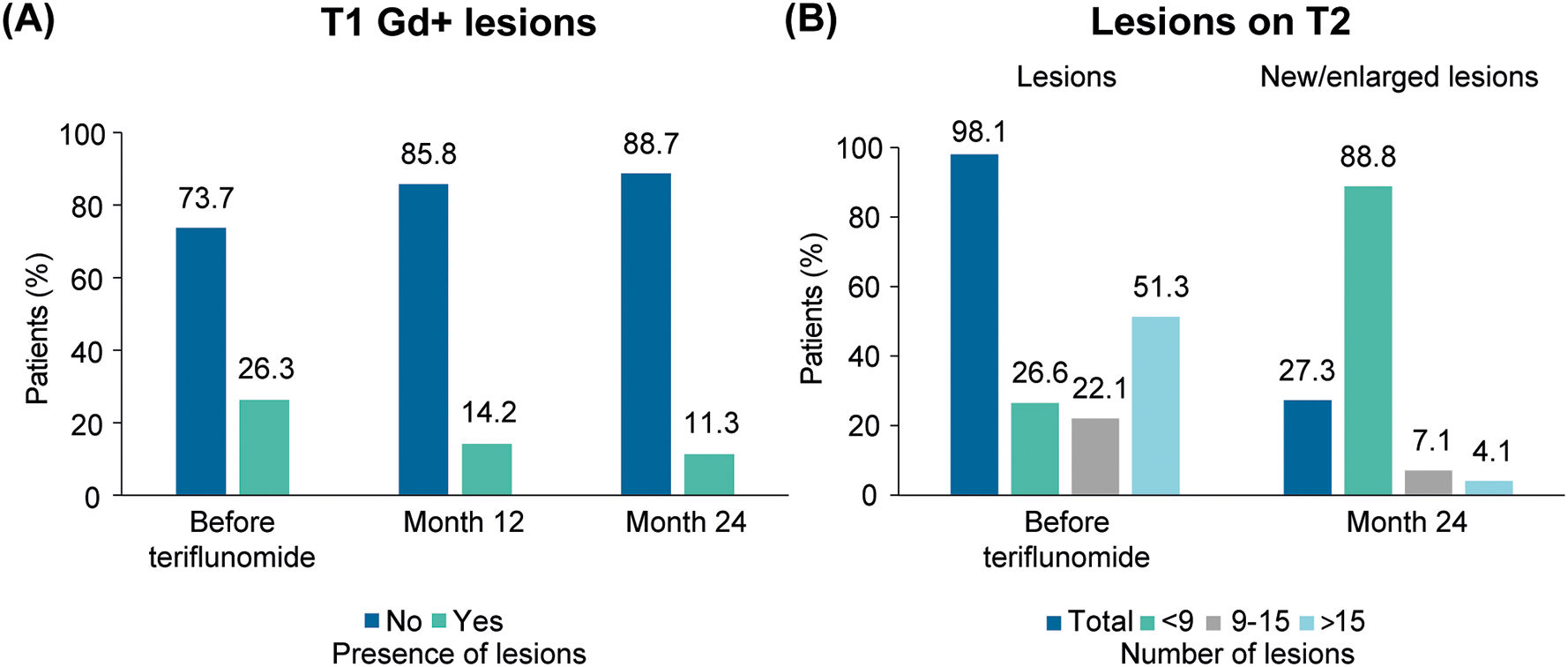
Lesions by MRI. The number of patients with Gd+ T1 lesions decreased after 24 months of teriflunomide treatment (P<.001; n=363). Among those patients with Gd+ T1 lesions before (≤3 months) teriflunomide initiation (n=92), most patients (88%; n=81) did not present Gd+ T1 lesions 24 months after teriflunomide treatment. Among those with Gd+ T1 lesions, the mean (SD) number of lesions was 1.9 (1.5) before teriflunomide treatment (≤3 meses; n=172), 1.9 (1.4) at month 12 (n=74), and 1.8 (1.2) at month 24 (n=44). Fig. 3A shows the percentage of patients with and without Gd+ T1 lesions available up to month 24.
Most patients with lesions on T2 at teriflunomide initiation (73.5% (n=261)) did not show new/enlarged lesions after 24 months of treatment. Fig. 3B presents the percentage of patients with lesions on T2 before teriflunomide treatment and of patients with new/enlarged lesions on T2 after 24 months of teriflunomide treatment (<9, 9–15, or >15 lesions).
SafetyA total of 388 (50%) patients reported at least 1 AE during treatment. The most frequently reported AEs (>10%) were gastrointestinal disorders (26.2%), hair thinning (25%), and elevation of ALT values (12.9%). The AEs reported are displayed in Table 4. Among those patients who presented infections (8.7%), 32.7% (n=16) were considered as not related/probably not related/or to have an uncertain relationship to teriflunomide.
Among those patients who discontinued treatment with teriflunomide due to AEs (n=82; 21.3%), the most common AEs leading to discontinuation were gastrointestinal disorders (n=34), hair thinning (n=19), and ALT increase (n=18) (see Table 5). In 18 patients, the specific AEs that lead to discontinuation were not available.
Adverse events leading to teriflunomide discontinuation (n=82).
| AEs | Number (%) |
|---|---|
| Gastrointestinal disorders | 34 (41.5) |
| Hair thinning | 19 (23.2) |
| ALT increase | 18 (22.0) |
| <3 | 7 (8.5) |
| 3–5 | 4 (4.9) |
| >5 | 7 (8.5) |
| Lymphopenia | 9 (11.0) |
| Grade 1 | 3 (3.7) |
| Grade 2 | 1 (1.2) |
| Grade 3 | 4 (4.9) |
| Infections | 5 (6.1) |
| Fatigue increase | 5 (6.1) |
| Peripheral neuropathy | 4 (6.3) |
Grade 1 (>800 lymphocytes), grade 2 (500–800), grade 3 (200–500).
Each patient could have had more than 1 AE leading to discontinuation, and therefore, the number of AEs is higher than the number of patients. Moreover, 18 patients with an AE, did not have the specific AE reported.
Most patients (91.5%) did not show fatigue increase during teriflunomide treatment. Post-hoc analysis conducted to compare the profile (demographics, medical history of MS, previous DMT, and ARR) at teriflunomide initiation of patients who had an increase of fatigue during treatment with patients who did not have this AE revealed no statistically significant differences between the subgroups (Table 6).
Baseline profile of patients with fatigue increase and with no fatigue increase during teriflunomide.
| Characteristic | Fatigue increase (n=50) | No fatigue increase (n=536) | P value |
|---|---|---|---|
| Age (years), mean (SD) | 44.2 (10.0) | 43.3 (9.7)a | .524 |
| Gender (female), n (%) | 40 (80.0) | 365 (68.1) | .108 |
| Time from MS first symptom (years), mean (SD) | 10.5 (9.3) | 10.4 (8.3)b | .681 |
| Previous treatment (yes), n (%) | 35 (70.0) | 375 (70.0) | >.999 |
| Number of previous treatments, mean (SD) | 1.0 (0.9) | 1.1 (1.0) | .678 |
| Patients with relapses in the 2 prior years, n (%) | 30 (60.0) | 266 (50.1) | .187 |
| Number of relapses in the 2 prior years, mean (SD) | 0.9 (1.1) | 0.9 (1.1)c | .533 |
| ARR in the 2 prior years | 0.450 | 0.434c | .863 |
N=fatigue increase (yes), 50; fatigue increase (no), 536; a532; b526; c531.
The TERICAM study retrospectively collected data from 776 adults diagnosed with RRMS receiving teriflunomide according to the clinical practice of 15 hospitals in Spain. The study provides insights into the real-world outcomes of teriflunomide over 24-months, confirming the effectiveness and safety reported in RCTs12,13,17 and other real-world studies.20,25–30 The main value of real-world studies, such as the present study, is that the effect of treatment can be observed in a more heterogeneous sample of patients (usually older and with a longer history of prior treatments), providing further evidence to data from RCTs and other observational studies conducted in different clinical settings.
Here, and in line with other real-world studies,25–28 patients were older and with longer disease duration than in RCTs.12,13,17 Even in this population, the ARR after teriflunomide treatment observed here was lower than in RCT,12,13,17 and it was comparable with the very low ARR observed in the routine clinical practice.25,26,28,31 ARR decreases during teriflunomide treatment seem to be independent of age. In fact, analysis of the TAURUS-MS I study showed significant improvements in clinical activity both in younger and in older patients.32
In our cohort, the proportion of patients who received another treatment prior to teriflunomide (usually a BRACE) was considerably higher than the percentage of treatment-naïve patients. This was the case even if teriflunomide was recommended by the Spanish guideline as initial treatment, similarly to the recommendation regarding initiating BRACE or dimethyl fumarate treatment.33 The fact that teriflunomide was beneficial, even if it was not always used as recommended by the Spanish guideline, provide further evidence of its effectiveness in RRMS patients regardless of treatment line. The effectiveness of teriflunomide in patients who have discontinued other DMTs has been shown by post-hoc analysis from the TEMSO and TOWER RCTs34 and by a recent analysis of the real-world TAURUS-MS I study.32 We did not have data on the satisfaction of previously treated patients, but the TERI-PRO real-world study showed that patients switching from other DMTs to teriflunomide were more satisfied after teriflunomide initiation (assessed after 4 and 48 weeks), regardless of the reason for switching.35 The recently published Teri-LIFE study also showed that satisfaction with teriflunomide resulted in high treatment adherence and decreased healthcare utilization.10 Future studies assessing patient satisfaction after switching to teriflunomide are warranted.
The effect of teriflunomide on radiological activity was consistent with the magnitude of improvement in relapses. Measures of clinical (ARR, relapse-free patients) and radiological activity (number of patients with Gd+ T1 lesions or new/enlarged T2 lesions) decreased throughout the 24-month follow-up compared to baseline. A significant reduction of these MRI lesions after 2 years was also observed in patients from the TEMSO trial across age groups.36 Few studies from the clinical practice have provided MRI data during teriflunomide treatment,25–27 and hence the availability of radiological data in a high number of patients here constitutes a strength of the present study.
At teriflunomide initiation, the mean EDSS score was below 2 (2=minimal disability in 1 functional system), suggesting that even if most patients were previously treated, their degree of disability was still mild. In terms of disability worsening, the EDSS did not show clinically significant changes during teriflunomide treatment. Even if the Wilcoxon test revealed a statistically significant result when comparing the EDSS at baseline (1.9) with the EDSS at month 24 (2.0), an increase of 0.1 points in the EDSS after 2 years is not considered clinically meaningful and therefore our data showed that disability was sustained during the 24-months period. Similar EDSS scores at teriflunomide initiation and during the following 2 years of treatment were observed in real-word studies from Germany27 and Spain.25
Concerning safety outcomes, half of the patients experienced AEs after teriflunomide initiation. The proportion of patients with AEs is identical to the real clinical practice in Denmark,20 but slightly higher than in Germany,27 and other regions in Spain.26 As expected, the number of AEs was considerably lower than in the core trials.26 No new or unexpected AEs were observed with teriflunomide treatment, supporting a safety profile consistent with outcomes from controlled37 and real20,26,27,37 clinical contexts. Gastrointestinal disorders were the most common AEs in general and the most common AE leading to discontinuation. Gastrointestinal disorders as the most frequent AE causing discontinuation is in agreement with studies from clinical practice in Denmark20 and Italy,28 but was rarely a cause of discontinuation in the pivotal trials.18
When all potential reasons for teriflunomide discontinuation were considered, lack of effectiveness, and not AEs, was the most frequent reason. The description of the characteristics of those patients discontinuing teriflunomide due to lack of effectiveness showed that these patients had a more active disease than the overall study population (66.9% patients with relapses in the 2 prior years in the subgroup of patients who discontinued due to lack of effectiveness vs 52.9% patients in the overall study population). Considering other real-world studies of teriflunomide in Spain, lack of effectiveness was the main cause of withdrawal in the study by Landete et al., where 82% of those who discontinued did so for this reason,25 but was only reported by 16% in the study by Duran et al.26 This apparent inconsistency might be explained, at least in part, by the follow-up time, which was longer in the former study25 than in the latter.26 Buceli et al. distinguished between discontinuation of teriflunomide occurring before and after 3 months of treatment and observed that early discontinuation was mainly due to AEs and tolerability, whereas later discontinuation was mainly due to disease activity.25 However, since in both studies the observation time was superior to 3 months, another possible explanation for this discrepancy could be the methodological limitations inherent to retrospective studies. That is, reasons for teriflunomide discontinuation might not have been consistently collected and in a structured format in the clinical practice. In line with this argument, almost one-quarter of our patients did not have the reason for teriflunomide discontinuation specified in their health records.
Importantly, most patients did not have fatigue increases while treated with teriflunomide, as shown by the absence of fatigue reported as an AE. Stabilization of fatigue was also observed in the TAURUS-MS study, as assessed by the Fatigue Severity Scale (FSS).27 The presence of severe relapses has been associated with increases in fatigue and HRQoL worsening.38 In our study, most patients were relapse-free, which might have contributed to fatigue control. A prospective study that evaluated fatigue using the Modified Fatigue Impact Scale (MFIS) in France showed that fatigue scores remained stable during teriflunomide treatment over 2 years.39
Methodological limitations need to be considered, however, for a proper interpretation of the study findings. First, due to the retrospective nature of the study, study variables were not available in every patient; moreover, the AEs reported here were collected using a list of pre-defined AEs, and some AEs outside of that list might have occurred and not have been reported here. Second, disability outcomes were only assessed using the EDSS, which, even if it is the most widely used tool, is not free from limitations. Future real-world studies should aim to provide results from other disability function tests such as the Timed 25-Foot Walk (T25FW) or the 9-Hole Peg Test (9HPT), together with assessments of cognitive status and patient-reported outcomes (PROs). The use of PROs to assess symptoms such as fatigue would have provided a better understanding of these symptoms. Moreover, not all patients had an EDSS assessment at baseline, and confirmed disability progression (CDP) was not assessed. Third, a comparator group treated with a different DMT was not included. Fourth, patient's drop-out may have led to incomplete AE reporting and a potential underestimation of the true incidence of AEs in our study population. Another limitation of our study is the lack of data on the number of patients lost to follow-up. Not having the exact number of patients lost to follow-up at each stage of the study may have influenced our results. The absence of this information makes it challenging to assess the potential impact of attrition bias on our findings, as well as to estimate the true proportion of patients who remained relapse-free during the study period. Lastly, we only described the characteristics of patients who discontinued teriflunomide due to lack of effectiveness but did not analyze the characteristics of those who did not discontinue teriflunomide for these reasons, nor did we compare both groups. This analysis is of interest and could be the objective of future studies. Despite these limitations, the present study still contributes to the overall understanding of teriflunomide effectiveness and safety in a real-world setting. Real-world studies describing outcomes of patients who benefit the most from teriflunomide treatment will enable healthcare providers to offer personalized treatment and better inform their patients about their options.
ConclusionsMost RRMS patients treated with teriflunomide had previously received other treatments for their MS. Our findings suggest that teriflunomide was associated with decreased clinical and radiological activity and a stabilization of disability. However, it is important to acknowledge that the lack of a comparator group in our study design limits our ability to draw definitive conclusions regarding the effectiveness of teriflunomide compared to other treatments. The safety profile was in line with prior observations. Most patients did not report fatigue increases after teriflunomide initiation. Overall, the benefit–risk profile of teriflunomide remains favorable. Future research involving comparator groups would be valuable in further establishing the effectiveness and safety of teriflunomide in the management of RRMS.
Credit authorship statement- •
Conceptualization: Mª Luisa Martínez Ginés, Alberto Lozano Ros
- •
Data curation: Mª Luisa Martínez Ginés, Alberto Lozano Ros
- •
Investigation: All authors
- •
Supervision: Mª Luisa Martínez Ginés, Alberto Lozano Ros
- •
Writing - review & editing: All authors
This study received funding from Sanofi. The funder was not involved in the study design, collection, analysis, interpretation of data, the writing of this article or the decision to submit it for publication.
Patient consent (informed consent)All patients signed written patient informed consent for using their medical data for the purpose of this research.
Medical writing support was provided by Laura Prieto del Val from Evidenze Health Spain, funded by Sanofi.