Mild–moderate cognitive impairment is a frequent pathology in the adult population. It is important to explore early expression biomarkers that contribute to early detection and benefit the prognostic outlook. Brain-derived neurotrophic factor (BDNF) is an essential regulator of synaptic plasticity, neuronal survival, and differentiation, and even a critical molecular target for drug development in neurological disorders. It is crucial to carry out studies in the early stages that better clarify the landscape of BDNF.
ObjectiveTo compare the serum levels of BDNF in healthy adult patients and those with mild to moderate cognitive impairment.
Materials and methodsA casecontrol study in patients over 55- years with cognitive impairment, obtained by the MMSE® and MoCA® screening scales. A total of 191 patients, 102 patients with cognitive impairment (cases) and 89 healthy ones (controls) participated in the study. Blood samples were collected to obtain serum, with the determination of BDNF concentrations. For differences in BDNF concentrations, a one-way analysis of variance was used. The significance level of p<.05.
ResultsThe mean serum concentration of BDNF in the case group was 277.68±75.09 vs. the control group, where the concentration was 354.97±66.06 pg/ml (p=.001).
ConclusionsThere is an association between decreased BDNF levels and mild and moderate cognitive impairment in our study population.
El deterioro cognitivo leve-moderado es una patología frecuente en la población adulta mayor. Es importante explorar biomarcadores de expresión temprana que contribuyan a la detección temprana y beneficien el pronóstico. El factor neurotrófico derivado del cerebro (BDNF) es un regulador esencial de la plasticidad sináptica, la supervivencia y la diferenciación neuronal e incluso un objetivo molecular crítico para el desarrollo de fármacos en trastornos neurológicos. Es fundamental realizar estudios en las primeras etapas que aclaren mejor el panorama del BDNF.
ObjetivoComparar los niveles séricos de BDNF en pacientes adultos sanos y aquellos con deterioro cognitivo leve a moderado.
Materiales y métodosEstudio de casos y controles en pacientes mayores de 55 años con deterioro cognitivo, obtenidos mediante las escalas de MMSE® y MoCA®. Participaron en el estudio un total de 191 pacientes, 102 pacientes con deterioro cognitivo (casos) y 89 sanos (controles). Se recogieron muestras de sangre para obtener suero, con determinación de concentraciones de BDNF. Para las diferencias en las concentraciones de BDNF, se utilizó un análisis de varianza unidireccional ANOVA. El nivel de significancia de p < 0,05.
ResultadosLa concentración sérica media de BDNF en el grupo de casos fue de 277,68 ± 75,09 vs. el grupo control, donde la concentración fue de 354,97 ± 66,06 pg. /mL (P = 0,001).
Conclusionesexiste asociación entre niveles disminuidos de BDNF y deterioro cognitivo leve y moderado en nuestra población de estudio.
Cognitive impairment is a high-risk condition for the onset of dementia, developed dependency, functional impairment, and a predictor of mortality, conditioning a burden for the primary caregiver. The brain-derived neurotrophic factor is a regulator of synaptic plasticity, neuronal survival, and differentiation, including a critical molecular target for drug development in neurological disorders. There are no studies on BDNF and mild to moderate cognitive impairment in the Mexican population. Therefore, it is essential to explore early expression biomarkers that contribute to early detection and benefit the prognostic outlook of these patients.
IntroductionAccording to World Population Prospects 2019 (United Nations, 2019), by 2050, 1 in 6 people in the world will be over the age of 65, up from 1 in 11 in 20191. This rapid demographic aging will increase the prevalence of disease and disabilities, with an expected particular emphasis on the impairment of cognitive functions2.
Cognitive deficit is a syndrome characterized by a decline in cognitive functions more significant than expected for age and individual educational level, which does not significantly interfere with activities of daily living3.
The term cognitive impairment was introduced in the late 1980s by Reisberg et al. (1988) to characterize people in this intermediate stage4. Petersen et al. (1999) further developed the concept by proposing criteria based on an observational study of aging5. In 2003, derived from the symposium, the international standards for moderate cognitive impairment were published6.
Cognitive impairment and dementia represent conditions that affect the quality of life of the elderly population and determine a greater use of health services. Cognitive impairment constitutes a high-risk condition for the appearance of dementia, with a probability of developing it of 10–15% per year, compared to healthy controls, where it is 1–2%7.
Dementia is one of the determinants for the development of dependency and functional deterioration in adults over 75 years of age, with the consequent burden for the primary caregiver, in addition to being a strong predictor of mortality, with a risk of 2–3 times greater when compared to other chronic diseases that reduce life expectancy8.
Brain-derived neurotrophic factor (BDNF) is one of the neurotrophic factors involved in the differentiation9, maturation10, and survival of neurons in the nervous system11. It shows a neuroprotective effect under adverse conditions, such as glutamatergic stimulation, cerebral ischemia, hypoglycemia, and neurotoxicity12. BDNF stimulates and controls neurogenesis13,14.
BDNF contains 11 exons. It encompasses approximately 70 kB and is located on chromosome 11p15, which consists of a mature isoform of 118 amino acids that specifically binds to the tropomyosin kinase B receptor, increasing the excitatory synapse and inhibiting GABAergic neuronal synapse16. BDNF protein and mRNA have been identified in most brain areas, including the olfactory bulb, cortex, hippocampus, basal forebrain, midbrain, hypothalamus, brainstem, and spinal cord. A critical role in regulating plastic changes in the adult brain, including regulation of NMDAR transport, phosphorylation, and expression levels. BDNF has been postulated to be an essential part of the cellular mechanism for memory formation and maintenance by promoting synaptic consolidation17.
BDNF plays a vital role in cognitive alterations related to hippocampal atrophy and structures involved in memory. Low serum levels have been associated with reduced Folstein Mini-Mental State Examination (MMSE) scores in patients with dementia. Komulainen et al. (2008) found that BDNF levels were associated with cognitive function in older women, suggesting that plasma BDNF may be a clinically relevant biomarker18. Shimada et al. (2014) reported lower levels of BDNF associated with lower scores and moderate cognitive impairment19.
This study aims to compare BNDF serum levels in a Mexican adult population with mild to moderate cognitive impairment and controls.
MethodsCase–control study in patients diagnosed with mild to moderate cognitive impairment and patients without cognitive impairment as a control group in a highly specialized hospital in northwestern Mexico.
Study populationOur study evaluated 191 people who were users of the Instituto Mexicano del Seguro Social (IMSS) in the period from February 2020 to February 2021. To be included, each participant was 55 years old or older at the time of the study and did not have participated in other studies. Patients with current severe medical conditions (e.g., recent myocardial infarction, decompensated heart failure, metabolic imbalance, hydroelectrolytic disturbances), neurological or psychiatric history (e.g., cerebrovascular accident, Alzheimer's disease, schizophrenia, mental disorder, bipolar, depression) were excluded. Patients who do not sign an informed consent letter or with missing data were excluded.
Cognitive impairment criteria and cognitive function testsWe defined cognitive impairment based on previous studies (Petersen et al., 2014), using the following criteria: (1) self-reported or family-reported memory complaint; (2) objective memory impairment (indicated by an age-adjusted score of at least 1.5 SD below the lower reference limit of tests used for neuropsychological assessments); (3) no evidence of functional dependency; (4) exclusion of people with criteria for dementia.
- 1.
Screening for cognitive impairment includes a standardized face-to-face interview to collect sociodemographic, anthropometric, medical history, and functional status data, along with tests of cognitive function using the Mini-Mental State Examination (MMSE®) and Montreal Cognitive Assessment (MoCA®), scales are validated in the Mexican population;20,21 both scales simultaneously, considering the patient's comfort and compliance to continue with the evaluations.
The study complied with the principles of the Declaration of Helsinki. All participants gave their informed consent to participate in this study; the study protocol was authorized with the registration number (R-2020-1002-010) by the ethics committee in health research of the High Specialty Medical Unit (IMSS- UMAE No. 71), Torreon, Coahuila, Mexico.
Assessment of cognitive impairmentThe Folstein Mini-Mental State (MMSE®) and Montreal Cognitive Assessment (MoCA®) scales were applied to determine the cognitive function and screening for cognitive impairment. Standard cut-off points for MMSE® normal (MMSE 24–30), mild (20–23), moderate (MMSE 10–19), and severe (MMSE <9) were used based on previous studies (Benoit et al., 2020). For MoCA®, the following cut-off points were used for normal (MoCA >26), mild (MoCA 18–25), moderate (MoCA 10–17), and severe (MoCA <10) classification according to provider recommendations.
Based on the scores obtained, the participants were classified into 2 large groups: those with cognitive impairment (cases) and participants without cognitive impairment (controls). The flow of participants can be seen in Table 1.
Demographic characteristics between study groups.
| Measure | Case | Control |
|---|---|---|
| n=102 | n=89 | |
| Age, years, mean (SD) | 67.50 (8.43) | 63.79 (7.38) |
| Education, years, mean (SD) | 8.98 (3.96) | 13.15 (4.17) |
| Male sex, n (%) | 52% | 64% |
| MMSE® score (0–30) mean (SD) | 23.46 (3.53) | 28.20 (1.45) |
| MoCA® score (0–30) mean (SD) | 21.92 (3.06) | 27.10 (1.20) |
| Hypertension, % | 69% | 42% |
| Diabetes, % | 41% | 36% |
| Heart disease, % | 66% | 17% |
| BMI mean (SD) | 27.88 (3.56) | 28.26 (3.56) |
| Normal weight (BMI <25) % | 17% | 20% |
| Overweight (BMI 25–30) % | 58% | 53% |
| Obesity (BMI >30) % | 25% | 27% |
| BDNF expression, mean (SD) | 277.68 (75.09) | 354.97 (66.06) |
Participants were instructed to attend the sample collection after fasting for 8 h. The blood sample was obtained by venipuncture, with a pool in a BD Vacutainer® SST™ tube of 6 mL whole blood. The model coagulation was at room temperature for 30 min and subsequent centrifugation at 2500 rpm/min for 20 min; the serum was stored at a temperature of −80 °C for further analysis.
Quantitative determination of serum levels of BDNFQuantitative determination of BDNF levels in serum was analyzed using a commercial kit (BDNF Human ELISA kit, Aviscera Biosciences, Santa Clara, CA, USA) following the manufacturer's instructions. Serum samples were diluted in polypropylene test tubes 40 times (10 μL sample+390 μL dilution buffer). Samples were added to the 96-well flat bottom plate and then shaken for 2 h at room temperature. Subsequently, after washing 4 times, a detection antibody was added to each well, and the plates were incubated for 2 h under constant agitation at room temperature. After another washing step, horseradish peroxidase-conjugated streptavidin was added, and the plates were incubated for 60 min at room temperature, protected from light. Unbound streptavidin was discarded, and TMB substrate solution was added. The reaction was stopped 15 min later. Optical density was measured in a microplate reader (Stat Fax® 4700 Awareness Technology, USA) at a 450±3 nm wavelength proportional to the BDNF concentration. The intra- and inter-assay variations of BDNF were less than 9%. BDNF concentrations were determined according to the BDNF standard curve. All assays were duplicated, and results were expressed in pg/ml.
Statistical analysisThe Chi-square homogeneity test was used to compare cognitive impairment with the categorical variables of the control group and cases. Student's t-test was applied to compare cognitive impairment with BDNF levels with normal distribution or Mann–Whitney's U test for non-normal distribution. Differences in serum BDNF concentrations were performed by one-way analysis of variance (ANOVA) in both sexes. All statistical comparisons were performed at a significance level of p<.05, and all statistical and data management calculations were performed using the scikitlearn®, scipy®, and pandas® Library Software Package in the scikitlearn®, scipy®, and pandas® programming language Python®.
ResultsOne hundred ninety-one patients were included in the study; the main characteristics of the participants are in Table 1. The mean age of the participants was 65.77±8.15. The average schooling was 10.92±4.55 years. Eighty-one women (42%) and 110 men (58%) were included in the study. The average score of the participants in general in the MMSE® instrument was 25.67±3.63, and using the MoCA® tool, 24.34±3.51.
The mean age for the case group was 67.5±8.43, and for the control group, it was 63.7±7.38. Regarding gender, in the case group, there are 53 men (52%) and 49 women (48%); in the control group, there are 57 men (64%) and 32 women (36%). The number of years of study in the case group was 8.98+3.96; for the control group, it was 13.15+4.17.
Regarding the score obtained by the MoCA® instrument in the group of cases, 94 patients were classified as having a mild cognitive impairment (92.1%); 8 patients were classified as having a moderate cognitive impairment (7.8%), and no patients were classified as having severe cognitive impairment. Using the MMSE® instrument, 94 patients with mild cognitive impairment (92.1%), 6 with moderate cognitive impairment (5.8%), and 2 patients with severe cognitive impairment (1.9%) were classified from the case group. The mean serum concentration of BDNF in the case group was 277.68±75.09 vs. the control group, where the concentration was 354.97±66.06 pg/ml (p=.001).
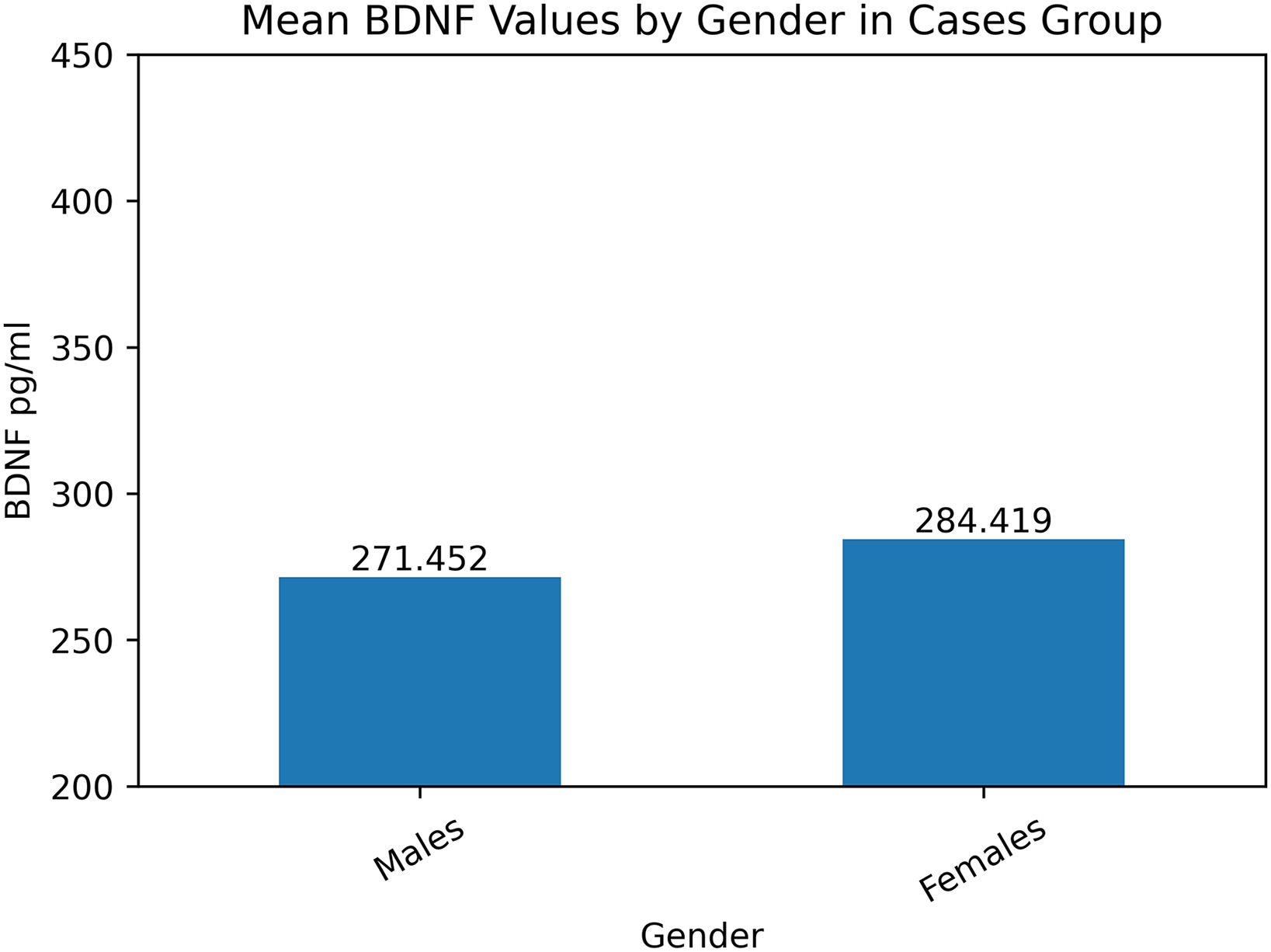
As shown in Fig. 1, mean BDNF levels were higher in females. Likewise, we can observe in Fig. 2 that according to the level of schooling, the level of BDNF increases.
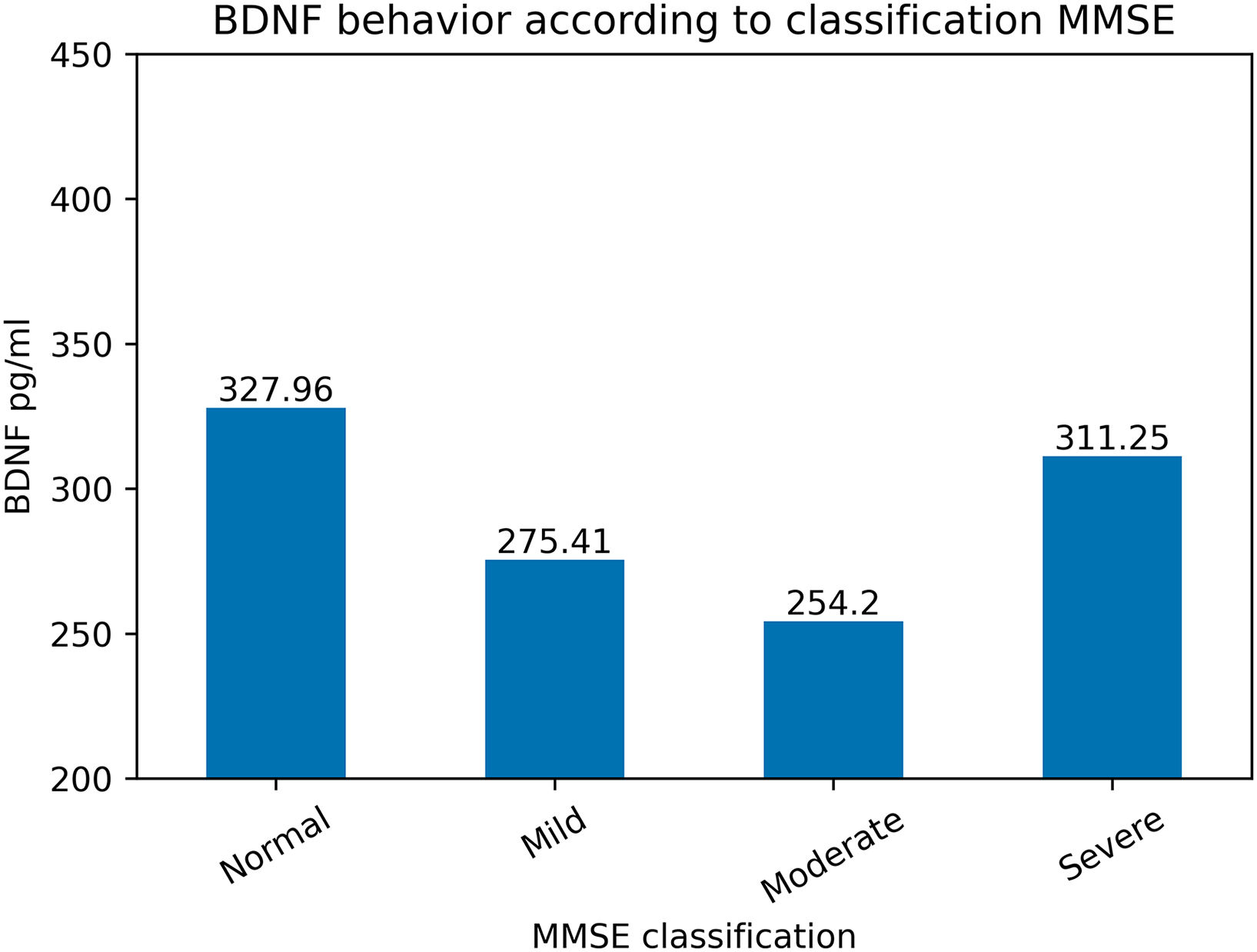
BDNF concentrations varied according to the classification of cognitive impairment based on the MMSE® and MoCA® tests (Figs. 3 and 4).
The correlation was made between the variables with the serum level of BDNF, shown in Table 2. where it was found that there is a correlation between BDNF and cardiovascular diseases, years of schooling, MMSE score, and MOCA score. Moreover, the general distribution of the BDNF marker shows a normal distribution curve (Fig. 5), which is why the ANOVA F value analysis is performed (Fig. 6).
Correlation of numerical variables concerning BDNF pg/ml.
| Correlation of numerical variables concerning BDNF (pg/ml) | |
|---|---|
| Medical and demographic variables | BDNF (pg/ml) |
| Cardiovascular diseases | −0.275441 |
| Age | −0.116012 |
| Arterial hypertension | −0.072353 |
| Type II diabetes | −0.029303 |
| Sex | 0.005882 |
| BMI | 0.049353 |
| Schooling (Years) | 0.21489 |
| MMSE® score | 0.342508 |
| MoCA® score | 0.426976 |
| BDNF (pg/ml) | 1 |
The statistical analysis presented in Table 3 and the accompanying ANOVA F value in Fig. 6 is a comprehensive view of the significance of the variables present in our study. The p-values in Table 3 reveal that ‘Education’, ‘MOCA score’, ‘MMSE score’, and ‘BDNF pg/ml’ with values below 0.05, indicating significant statistical relevance. This is supported by the ANOVA F-value analysis in Fig. 6, where the corresponding F-values for these variables are markedly high, with the ‘MOCA score’ peaking at 41.62, highlighting its substantial influence. Conversely, ‘BMI’ demonstrates a p-value of 0.492, paired with a relatively low F-value, indicating that it does not have a significant association. The alignment of low p-values with high F-values confirms the robustness of the variables' impact, underscoring their importance in the research findings. These statistical measures collectively highlight the variables most critical to the study's conclusions and those that may be considered less influential.
DiscussionOur study aimed to compare serum BDNF levels in Mexican patients with mild–moderate cognitive impairment and a control group from a high-specialty hospital.
The number of people worldwide with cognitive impairment in 2000 was approximately 20 million; this figure will reach 81.1 million in 2040. Several studies have shown that neurotrophic factors such as BDNF are involved in the pathophysiology of neurodegenerative diseases. Higher serum BDNF levels are associated with better MoCA® / MMSE® test performance.
In the present study, we found that patients with moderate, mild cognitive impairment determined by the MoCA® / MMSE® score have a lower serum level of BDNF than subjects with normal scores in these instruments. The mean serum concentration of BDNF in the case group was 277.68±75.09 vs. the control group with 354.97±66.06 pg/ml (p=.001).
Laske et al. (2006) found that early-stage patients with MMSE scores ≥21 (mean 25.5) had significantly higher serum BDNF levels compared with late-stage patients with MMSE scores <21 (mean 25.5). and age-matched healthy controls. The mean age of their case group was 71.7±7.0, and the control group was 70.3±5.4. The mean MMSE® score for the case group was 19.2±7.0, and the control group was 29.5±0.2. It was found that in the control group, the mean concentration of BDNF was 20.7±5.2 ng/ml; in the initial stages of the disease, it was 25.1±4.1 ng/ml vs. the group in the final stages, where the mean concentration was 17.3±4.4 ng/ml (p<.0001). There was a correlation between the serum level of BDNF and the score obtained in the MMSE® instrument (r=0.486; p<.0001).
Yu et al. (2008) looked for an association between serum BDNF levels and patients with mild cognitive impairment. They recruited 99 patients with a previous diagnosis of mild cognitive impairment and 99 healthy patients. Serum levels of BDNF were determined. They reported in their study that serum BDNF concentrations in patients with mild cognitive impairment ([interquartile range]=4.37 [2.35–6.40] ng/ml) were significantly lower than in healthy controls (4.98 [3.50–7.33] ng/ml) (z=−2.449, p=.014). with a positive correlation between BDNF and delayed memory scores on the auditory verbal learning test, reflecting episodic memory (r=0.264, p=.008). This study suggests that reduced levels of BDNF may play a role in the pathophysiology of mild cognitive impairment.
Murillo-Ortiz et al. (2016) in their study included 40 patients with type 2 diabetes mellitus (mean age 51.88±12.81) and 40 healthy patients (mean age 42.30±12.87). MMSE® was performed to classify them into 2 groups: with or without cognitive impairment. The mean score using the MMSE® instrument was 20.26±2.15 for the case group and 25.44±1.50 for the control group (p=.0001). BDNF determination was performed in both groups, finding a statistically significant difference (31.55±10.24 vs. 43.78±9.05, p=.005).
The mentioned authors' results support our research findings, showing that lower scores in instruments such as MMSE® and MoCA® were associated with lower serum concentrations of BDNF. These studies use MMSE® to measure cognitive status and classify patients with or without cognitive impairment, which is consistent with our research methodology. They report meaning MMSE® scores of 20.26±2.15 for the case group and 25.44±1.50 for the control group. In our study, the mean score for the case group was 21.92±3.06; for the control group, it was 28.2±1.45. The serum concentrations of BDNF were lower in the case group vs. the control group, showing a difference with statistical significance. These results agree with what was previously reported. There is a certain degree of variability between their groups' scores and serum BDNF concentrations concerning ours, but they generally present the same trend.
As secondary results, it can be observed that there is a correlation between BDNF and cardiovascular diseases, schooling in years, MMSE® score, and MOCA® score. Moreover, the p-value and ANOVA F value analysis confirm these variables' importance and impact on the BDNF levels.
There is disagreement between the different publications on BDNF levels concerning gender. Some publications suggest that estrogens have a positive regulatory effect on BDNF expression and signaling. However, these observations were generated from fragmented studies, which were not primarily designed to examine the tissue distribution of BDNF in different sexes22. In our study, estrogen levels were not quantified, so we cannot infer this relationship.
Hashimoto (2016) conducted a study to determine if there is a difference between serum levels of BDNF based on ethnic origin; according to the study by Yoshida (2012), the Japanese population shows a mean level of BDNF of 23.11±5.90 ng/ml; Sodersten (2014) reported 30.3±7.2 ng/ml for the Swedish people and Levin (2015) for the Jewish population 14.0±8.9 ng/ml. Therefore, there is evidence that the concentration varies according to ethnic origin.
Shimada et al. (2014) reported that serum BDNF levels are lower in patients with mild cognitive impairment (OR, 95%, CI 1.41, 1.00–1.99). Patients older than 65 years were included in this study, and those with established neurological diseases were excluded. They found that BDNF levels varied by gender for men (20.8±5.6 ng/ml) and women (21.2±5.2 ng/ml; t=2.162, df=4394, p=.031). In both groups, serum BDNF levels were associated with age (β=0.123, t=5.750, p<.001). Serum levels of BDNF were associated with a decrease in memory and calculation tests (β=0.027, t=1.958, p<.05). Finding that age presented an OR for mild cognitive impairment of 1.00 (95% CI 0.99–1.02; p=.977), gender of 0.85 (95% CI 0.71–1.00; p=.051), education 0.82 (95% CI 0.79–0.85; p=<.001) and type 2 diabetes mellitus of 1.04 (95% CI 0.82–1.31; p=.23).
Villela-Nunes et al. (2018) included 80 deceased participants with a diagnosis of dementia and depression in their study, compared with a control group of 80 deceased patients without these diagnoses. Patients with traumatic death, neoplasms, severe systemic diseases, and unknown causes of death were excluded. The mean age for the case group was 73.9±11.3, for the control group 74.9±11.1 (p=.58), in the case group, there were 15 patients with cardiovascular disease (18.75%), and in the case group 14 patients with this condition (17.5%) (p=.84). BDNF determination was performed in the prefrontal cortex, reporting lower levels in the case group vs. the control group (ß=-0.106, 95%CI=−0.204; −0.009, p=.034). They did not note differences in other variables, such as diabetes mellitus, cardiovascular diseases, and depression.
Concordance is found between the characteristics of our population and the previously reported studies, except for cardiovascular disease, since 56% of our participants declared themselves with this diagnosis. According to national statistics in Mexico, it is estimated that 70.3% of the adult population has at least one cardiovascular disease. The prevalence of obesity in the country in the adult population is 33.6%, according to ENSANUT data; in our study, a majority of 26% was reported.
A difference can be observed between the mean concentration of BDNF in our population and that of other studies. The enormous heterogeneity of published serum BDNF levels is mainly due to methodological issues, which need to be more noticed in quite a few publications (e.g., sample storage conditions, effects of repeated freeze–thaw cycles, and the different ELISA kits used).
The strengths of the present study include the large sample size, as we matched the size of previous studies—comprehensive measurement of cognitive function by implementing both widely validated tests in the Mexican population. The analysis of other variables that could affect the result, such as diabetes mellitus and cardiovascular diseases, is closely correlated with mild cognitive impairment—having rigorous exclusion criteria that could influence the outcome. Blood samples were taken at times reported in other studies to avoid the influence of circadian cycles on the result.
The limitations of the present study include that it is a cross-sectional study, which did not have a database, and limited blinding since the same person who applied the tests to measure cognitive abilities was involved in processing samples and results. The heterogeneity of our population with a medical affiliation is evident, which limited the power to match the groups, showing in the clinical characteristics a tendency of the group of cases to suffer more cardiovascular diseases.
Although our study was population-based, further prospective research is needed to validate the use of serum BDNF levels to discriminate the risk of cognitive decline.
ConclusionIn patients from the study population of cases and controls, utilizing the MoCA® and MMSE® screening test and serum marker analysis BDNF, there is a significant difference between control groups and patients with cognitive impairment in any of its clinical stages.
The mean serum concentration of BDNF in the case group was 277.68±75.09 vs. the control group of 354.97±66.06 pg/ml (p=.001).
The level of BDNF was found in both groups, with a lower concentration in the male gender compared to the female gender, as well as a higher level of biomarker related to schooling (Master's degree) in both groups.
The following are the supplementary data related to this article.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.neurop.2024.100158.
Financial SupportThe researchers have yet to receive financial support for this article's research, authorship, and publication.
Approval by the Ethics CommitteeRegistration number R-2020-1002-010.
Author contributionsStudy design and conception by ER, CA, HA, FJ, and MP; Experiments were performed by ER, CA, HA, FJ, and MP; and Data analysis by MP, ER, and CA. All authors contributed to the writing and review.
https://orcid.org/0000-0001-7677-5361.