Tarlov cysts, or perineural cysts, are nerve root lesions most frequently found in the sacral region, and are generally asymptomatic. They are usually detected incidentally and their association with sexual, bladder, or bowel dysfunction is frequently ruled out.
AimsWe describe the management of a series of patients with symptomatic Tarlov cysts using a protocol that combines multimodal rehabilitation (biofeedback+posterior tibial nerve stimulation+INDIBA® radiofrequency) and pharmacological treatment (Tiobec Dol® dietary supplement and/or acetazolamide) and evaluate the effect of this programme on pain and pelvic floor muscle strength.
Material and methodsRetrospective study of 5 patients with urinary or faecal incontinence and pelvic pain or dyspareunia, who presented Tarlov cysts.
The outcome variables were: (1) pain as measured with the visual analogue scale (0–10) and (2) maximal and mean pelvic floor muscle strength (in mm Hg) at baseline and after treatment completion.
ResultsTreatment improved mean pelvic floor muscle contraction from 9.2±6.01mmHg to 10.6±5.2mmHg (P=.3111) and maximal pelvic floor muscle contraction from 41.2±21.98mmHg to 45.8±17.51mmHg (P=.4430), and decreased pain from 8±1.26 points to 4.6±2.65 points (P=.0343).
ConclusionsTarlov cysts may cause pain and radiculopathy, although they are usually asymptomatic and, consequently, underdiagnosed. More rarely, they cause urinary, bowel, and sexual dysfunction. We describe the cases of 5 patients with Tarlov cysts presenting with incontinence and pain who were managed with multimodal rehabilitation plus pharmacological treatment. Cases of Tarlov cysts should be reported and studied; this will help to expand our understanding of their pathophysiology and to standardise effective multimodal treatment.
Los quistes perineurales de Tarlov son lesiones de las raíces nerviosas sobre todo sacras, generalmente asintomáticos. Su hallazgo es incidental y muchas veces la disfunción sexual, vesical e intestinal es desestimada.
ObjetivoPresentar el manejo en una serie de casos con quistes de Tarlov sintomáticos utilizando un protocolo de Rehabilitación multimodal (biofeedback + neuromodulación del tibial posterior + radiofrecuencia [INDIBA®]) más farmacoterapia (Tiobec-dol y/o Acetazolamida) y evaluar su efecto sobre la fuerza de la musculatura de suelo pélvico y sobre el dolor.
Material y MétodosEstudio retrospectivo a 5 pacientes con Incontinencia urinaria o faecal más dolor pélvico o dispareunia y quistes de Tarlov sacros.
Variables de resultado: a) dolor medido por escala EVA (0–10) y b) fuerza máxima y media de la musculatura de suelo pélvico (medida en mm Hg) al inicio y al término del tratamiento.
ResultadoLos pacientes mejoraron la fuerza media de 9.2±6.01 a 10.6±5.2mmHg (p=.3111) y máxima de 41.2±21.98 a 45.8±17.51mmHg (p=.4430); el dolor disminuyó de 8±1.26 a 4.6±2.65 puntos (p=.0343).
ConclusionesLos quistes de Tarlov causan dolor y síndrome radicular, aunque generalmente se consideren asintomáticos y por tanto infradiagnosticados. Con menos frecuencia producen disfunción vesical, intestinal y sexual. Hemos documentado cinco pacientes con quiste de Tarlov, incontinencia y dolor y pautado simultáneamente tratamiento rehabilitador multimodal y farmacológico. Se precisa reportar y estudiar los quistes de Tarlov para entender su fisiopatología y establecer manejo multimodal estandarizado y efectivo.
Tarlov cysts, or perineural cysts, are nerve root lesions most frequently found in the sacral region.1 They were first described by Tarlov in 1938, during an autopsy study of the filum terminale. Since then, at least 100 symptomatic cases have been reported, although very few describe faecal incontinence (<3%), pelvic pain, or urinary incontinence.2
Tarlov cysts are extremely rare, with greater incidence among women aged 30–50years; the most frequent symptoms are pain and radiculopathy. They are most frequently located in the sacral region. Less frequently, these cysts may cause bowel and bladder dysfunction, leading to urinary or faecal incontinence.1,2
The incidence of Tarlov cysts in the general population is 1%, increasing to 4.6% in magnetic resonance imaging (MRI) studies of patients with lower back pain; the lumbar and especially the sacral spine are the most frequently affected regions. Cysts are usually asymptomatic, requiring no treatment.3
Although the aetiology of Tarlov cysts is unknown, several trigger factors have been proposed, including repeated microtrauma,1 increased cerebrospinal fluid (CSF) pressure,1 and congenital malformation (Tarlov cysts are believed to be linked to such neural tube defects as myelomeningocele and spina bifida4).
In asymptomatic patients, the diagnostic technique of choice is MRI, which differentiates between solid and cystic lesions.5 The second most sensitive technique is computed tomography (CT) with or without contrast. Electromyography (EMG) may display reduced amplitude of sural sensory nerve action potentials. Reduced recruitment of the affected myotome and slowing of H-reflex latency have also been described.4
Although these patients are rarely referred to the rehabilitation department, rehabilitation therapy should focus on improving the most disabling symptoms (urinary or faecal incontinence, pain). Our research group recently designed a multimodal treatment protocol for pain management that combines biofeedback, radiofrequency, and posterior tibial nerve stimulation. We hypothesise that this treatment may be useful for the management of patients with incontinence and chronic pelvic pain (CPP), as is the case with patients with symptomatic Tarlov cysts.
This study describes the management of a small series of patients with symptomatic Tarlov cysts using a multimodal rehabilitation protocol (biofeedback+posterior tibial nerve stimulation+INDIBA® radiofrequency) and pharmacological treatment (Tiobec Dol® dietary supplement and/or acetazolamide) and evaluates the effect of this programme on pain (visual analogue scale [VAS]) and mean and maximal pelvic floor muscle contraction.
Material and methodsWe conducted a retrospective study of 5 patients with urinary and/or faecal incontinence plus CPP and/or dyspareunia who presented Tarlov cysts in the sacral region; these patients were referred to the rehabilitation department of Hospital Universitario Santa Cristina (Madrid, Spain) between January 2018 and January 2022. The study complies with the ethical principles of the Declaration of Helsinki. As this study is a critical analysis of clinical practice, approval by our hospital's research ethics committee was not necessary.
Inclusion criteria were as follows: (1) women older than 18years; (2) patients with signs of urinary and/or faecal incontinence, CPP, or dyspareunia of over 6months' progression; (3) patients with a radiological (MRI) diagnosis of Tarlov cysts in the sacral region (S1, S2, or S3); (4) patients referred to the rehabilitation department by the gynaecology, psychiatry, psychology, urology, or family medicine departments; and (5) written informed consent for treatment.
Exclusion criteria were as follows: (1) severe mental disorders limiting comprehension and/or cooperation with treatment; (2) severe neurological diseases (stroke, dementia, spinal cord lesion, etc) preventing voluntary contraction of pelvic floor muscles; (3) lack of VAS assessment, pelvic floor muscle manometry data at treatment onset/completion, and/or lack of radiological and/or neurophysiological assessment; (4) neoplastic lesions or active infection contraindicating application of radiofrequency in the pelvic region; and (5) any other formal contraindication for deep thermotherapy (pregnancy, deep vein thrombosis, hypoaesthesia, skin lesions, use of a pacemaker, or lumbosacral stimulator).
During the initial assessment, we gathered personal data, medical history, gynaecological and obstetric history (pregnancies, miscarriages, deliveries, or menopause), and history of surgery (gynaecological, urological, or abdominal surgery).
The multimodal rehabilitation treatment protocol consisted of manometric biofeedback-assisted pelvic floor muscle training supervised by a physiotherapist, followed by monopolar capacitive-resistive radiofrequency (INDIBA®) applied to the suprapubic and perineovaginal regions, plus at least 8 sessions of posterior tibial nerve stimulation.
Outcome variables. The outcome variables were: (1) pain as measured with the VAS (0–10) and (2) pelvic floor muscle strength (in mm Hg) at baseline and after treatment.
The VAS, designed by Scott and Huskisson in 1976, is a quick and easy-to-administer tool for measuring pain intensity. The patient is asked to rate his or her pain on a numerical scale from 0 to 10, represented on a 10-cm horizontal line, where 0 signifies “no pain” and 10 signifies “worst pain possible.” The VAS presents an intraclass correlation coefficient of 0.70 with respect to the verbal numerical rating scale.6
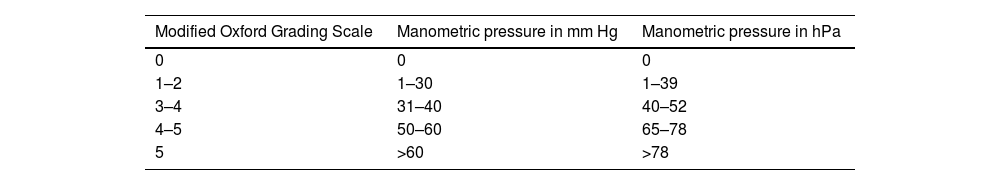
Manometric biofeedback was performed with the Myomed® 932 device and a transvaginal probe. This device produces auditory and visual (on a screen) cues that provide feedback of pelvic floor muscle contraction. This part of the assessment may be difficult to conduct in some cases, particularly in elderly or very young patients. The transvaginal probe was covered with a condom and coated with a lubricant gel to facilitate insertion into the vagina. Pelvic floor muscle contraction was evaluated by digital palpation, using a classification system ranging from 0 to 5, and correlated with the manometric values obtained with the biofeedback device (Table 1).7
Correlation between manual and manometric assessment of pelvic floor strength (Rioja-Toro,7 2012).
| Modified Oxford Grading Scale | Manometric pressure in mm Hg | Manometric pressure in hPa |
|---|---|---|
| 0 | 0 | 0 |
| 1–2 | 1–30 | 1–39 |
| 3–4 | 31–40 | 40–52 |
| 4–5 | 50–60 | 65–78 |
| 5 | >60 | >78 |
The manometric biofeedback protocol consisted of a 30-min session of pelvic floor muscle contractions, with 15min of tonic exercises (cycles of 3-s contraction and 6-s relaxation) and 15min of phasic exercises (5 fast contractions followed by 10-s relaxation), twice per week until completing 8 sessions. During the exercises, a transvaginal probe and a Myomed® 932 biofeedback device were used, and sessions were supervised by a physiotherapist. Visual cues provided feedback for motor learning.
At the baseline and final assessments, a 2-min manometric assessment (1min of tonic exercises followed by 1min of phasic exercises) was conducted to obtain mean and maximal muscle contraction values. These values served as an objective measure of pelvic floor muscle strength. Manometric biofeedback ensured correct execution of tonic and phasic exercises.8
The monopolar capacitive-resistive radiofrequency protocol used the INDIBA® Activ Pro Recovery HCR902 system (INDIBA S.A.; Barcelona, Spain). This device generates hyperthermia at a frequency of 448kHz, using 2 electrodes (active and passive). The active electrode is a rigid circular metallic electrode measuring 65mm in diameter, whereas the passive electrode is a large, flexible, rectangular metallic plate measuring 200×260mm. Two different active electrodes can be used to deliver either capacitive or resistive radiofrequency energy. The capacitive electrode has a polyamide coating that acts as a dielectric medium and insulates the metallic body from the skin surface, thus generating heat at the most superficial layers of the skin. The resistive electrode, in contrast, is uncoated, and transmits the radiofrequency energy directly through the body towards the passive electrode, thus generating heat in deeper tissues. The treatment protocol consisted of 15-min sessions, including 5min of capacitive radiofrequency and 10min of resistive radiofrequency, twice weekly. Sessions were conducted by a physiotherapist. The capacitive electrode was applied to the lower part of the abdomen (suprapubic region) and at the perineum, and was moved in circular motions over the target region. The resistive electrode was coated with conductive gel and placed inside the vagina, in contact with the vaginal wall. Finally, the passive electrode was placed over the lumbosacral region to transmit the heat to the pelvic floor. Treatment intensity was established at 6–7 on a subjective sensitivity analogue scale (SAS) ranging from 0 (no thermal sensation) to 10 (worst possible thermal sensation). A manufacturer-supplied conductive gel must be used as the conductive medium between the active electrode and the body surface to be treated during the intervention.8–10
Posterior tibial nerve stimulation was administered with the patient seated and the target leg elevated. We used 2 surface electrodes, one placed 5cm proximally to the medial malleolus on the trajectory of the posterior tibial nerve, and the other over the ipsilateral calcaneus. Stimulation was administered using the TENStem Eco Basic device (CE 0482; Germany). We applied a symmetrical biphasic rectangular current at an intensity of 0–9mA, frequency of 20Hz, and pulse width of 200μs, for 30min. Current intensity was regulated until patients either experienced a tingling sensation on the plantar surface or presented plantar flexion of the big toe or fanning of the toes.11,12
Data were analysed using the SPSS® statistical software, version 20.0. Quantitative variables were expressed as descriptive statistics (mean and standard deviation [SD]), and qualitative variables as frequencies and percentages. To evaluate the difference in quantitative variables before and after treatment, we used the Mann–Whitney U test. The threshold for statistical significance was set at P<.05 (confidence level of 95%).
ResultsWe studied a series of 5 patients with symptomatic Tarlov cysts. Mean age (SD) was 50.8 (8.0) years. Tarlov cysts were located at sacral nerve roots S1–S3 (57.1% located at the S2 nerve root, 28.6% at S3, and 14.3% at S1) (Table 2).
Demographic, clinical, and treatment-related data from our series of 5 patients with Tarlov cysts.
| Age | Location of Tarlov cyst | Neurophysiological study (EMG/ENG, RSC) | Symptoms/comorbidities | Rehabilitation treatment |
|---|---|---|---|---|
| 65years | S2 | Pudendal neuropathy (S2, S3, S4) | Faecal incontinenceHistory of genital herpes zosterObstetric history: G2P2A0 | BFB+EMS (12 sessions)Tiobec Dol® (1month) |
| 49years | S2S3 | Normal EMG/ENG findingsSSR 60% | SUI+UUIDyspareunia (10/10)Irritable bowel syndromeLumbar disc herniationObstetric history: G2P2A0 | BFB+RF+PTNS (8 sessions)Acetazolamide (3months) |
| 42years | S3 | SSR 60% | SUIChronic pelvic pain (8/10)Dyspareunia (8/10)Obstetric history: G2P2A0 | BFB+RF+PTNS (8 sessions)Tiobec Dol® (1month) |
| 53years | S2 | SSR 100% | Chronic pelvic pain (6/10)Dyspareunia (6/10)HeadacheHysterectomy (2months previously)Obstetric history: G3P3A0 | BFB+RF+PTNS (8 sessions)Acetazolamide (1month) |
| 49years | S1S2 | SSR 60% | Chronic pelvic pain (8/10)Dyspareunia (8/10)HeadacheObstetric history: G0P0A0 | BFB+RF+PTNS (8 sessions)Acetazolamide (1month) |
| Average values | ||||
| 50.8 (8.0) years (mean [SD]) | S1: 14.3%S2: 57.1%S3: 28.6% | EMG/ENG: 40%SSR: 60% | Pelvic pain: 80%Urinary incontinence: 40%Faecal incontinence: 20%Pregnancy: 80% | 8 (1.6) sessions (mean±SD) |
BFB: biofeedback; EMG: electromyography; EMS: electrical muscle stimulation; ENG: electroneurography; GPA: gravida/para/abortus; PTNS: posterior tibial nerve stimulation; RF: radiofrequency; SD: standard deviation; SSR: sympathetic skin response (normal value: ≤60%); SUI: stress urinary incontinence; UUI: urgency urinary incontinence.
The most frequent symptoms were pain (80%) and incontinence (60%). Urinary incontinence was observed in 40% of patients, and faecal incontinence in 20%. Four patients (80%) had obstetric history (pregnancy) (Table 2).
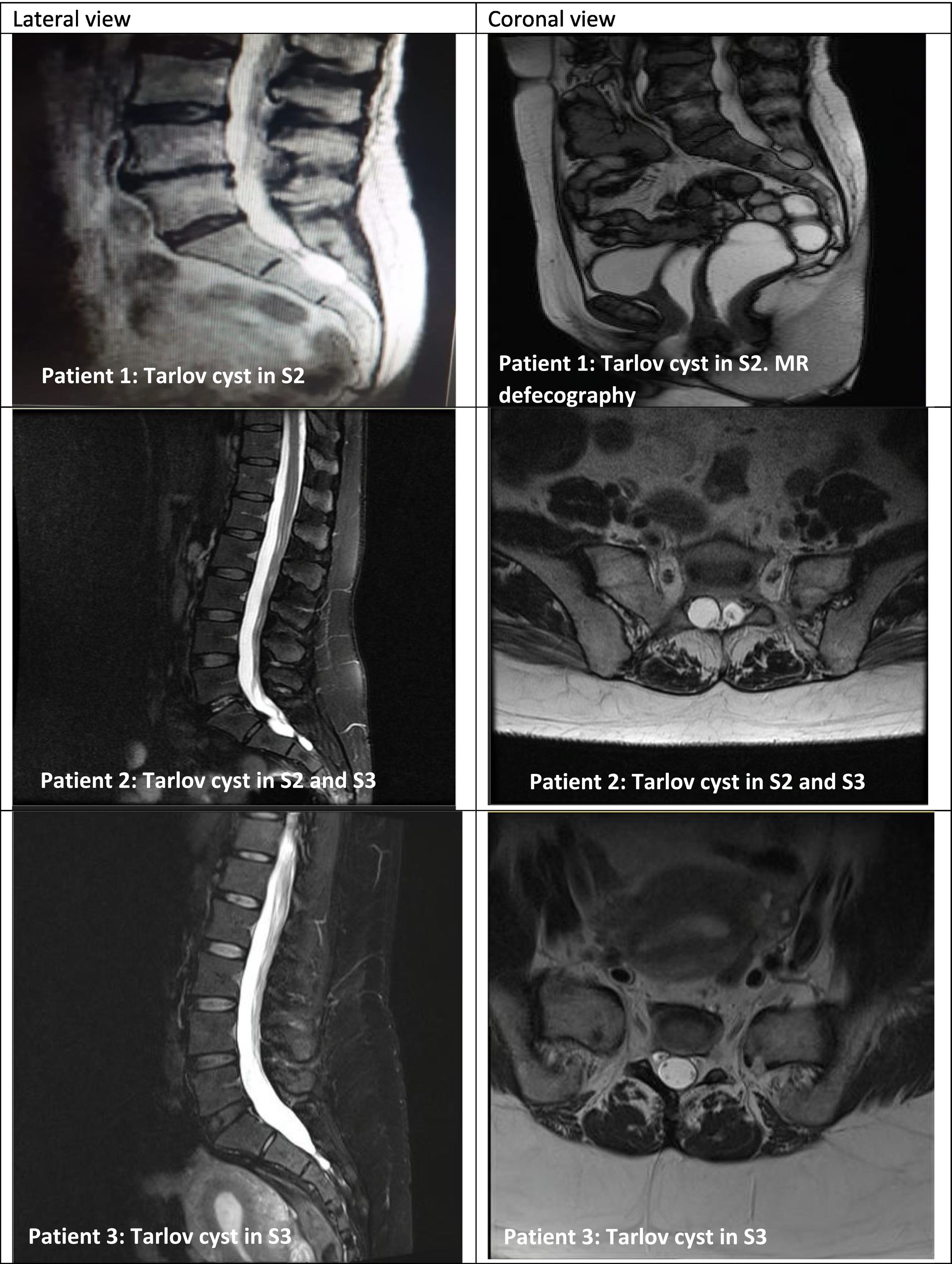
All patients had undergone radiological (MRI in 100% of cases) and neurophysiological assessment (EMG/electroneurography in 40% and sympathetic skin response in 60%) (Table 2, Fig. 1).
Series of 5 patients with Tarlov cysts confirmed by MRI (lateral and coronal views). MRI of the sacrum revealed perineural cysts at the level of the S1, S2, or S3 nerve roots, which were hypointense on T1-weighted sequences and hyperintense on T2-weighted sequences (consensus among radiologists).
Rehabilitation treatment consisted of a mean of 10.4 sessions of multimodal rehabilitation (biofeedback+posterior tibial nerve stimulation+radiofrequency combined with pharmacological treatment [Tiobec Dol® in 40% and/or acetazolamide in 60%]).
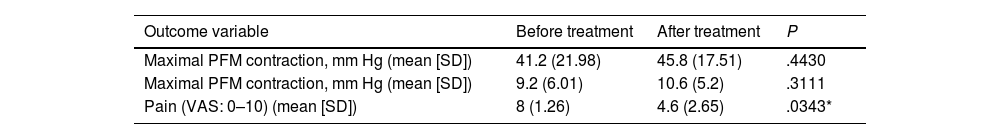
Treatment improved mean pelvic floor muscle contraction from 9.2±6.01mmHg to 10.6±5.2mmHg (P=.3111) and maximal pelvic floor muscle contraction from 41.2±21.98mmHg to 45.8±17.51mmHg (P=.4430), and decreased pain from 8±1.26 points to 4.6±2.65 points on the VAS (P=.0343) (Table 3).
Effects of multimodal rehabilitation treatment (biofeedback+posterior tibial nerve stimulation+INDIBA® radiofrequency) combined with pharmacological treatment (Tiobec Dol® and/or acetazolamide) on outcome variables.
| Outcome variable | Before treatment | After treatment | P |
|---|---|---|---|
| Maximal PFM contraction, mm Hg (mean [SD]) | 41.2 (21.98) | 45.8 (17.51) | .4430 |
| Maximal PFM contraction, mm Hg (mean [SD]) | 9.2 (6.01) | 10.6 (5.2) | .3111 |
| Pain (VAS: 0–10) (mean [SD]) | 8 (1.26) | 4.6 (2.65) | .0343* |
P-value for the Mann–Whitney U test. PFM: pelvic floor muscle; SD: standard deviation; VAS: visual analogue scale. *P<.05.
To our knowledge, this is the first Spanish series of patients with symptomatic Tarlov cysts managed with multimodal rehabilitation (biofeedback+posterior tibial nerve stimulation+radiofrequency) and pharmacological treatment (Tiobec Dol®/acetazolamide).
Tarlov cysts are nerve root lesions involving the dorsal root ganglion. Although these cysts had already been described in 1902 during autopsy studies, it was Isadore Tarlov who in 1938 gave a more extensive description during an autopsy study of the filum terminale, assuming that these lesions were clinically irrelevant. In 1948, however, Tarlov reconsidered his position and suggested an association between these lesions and radicular symptoms, reporting for the first time in the literature the clinical case of a patient whose symptoms improved following complete resection of the cyst.7
Since then, there has been controversy around whether Tarlov cysts cause pain or radicular symptoms. In 1956, Strully argued that Tarlov cysts are the least suspected, most frequently ignored, and rarely treated of all causes of lower back pain. This explains why Tarlov cysts are frequently overlooked; more than 80years since Tarlov's first description, little has changed in this respect.13
Most researchers consider Tarlov cysts to be asymptomatic.1–5 Symptomatic cases are extremely rare, with only around 100 cases having been reported to date.1,2 Tarlov cysts are more frequent in male patients around the second decade of life, although few cases have been reported2; on the contrary, they have been reported more frequently in women, particularly between the ages of 30 and 50years.2–4 According to Murphy and Ryan, Tarlov cysts are more frequent among white women, and their frequency increases with age, ranging from 77% to 88% and even 89.9%, as reported by Landon, Marino, and Murphy, respectively.14 In our series, mean age was 50.8 (8.01) years, in line with previous studies, and all patients were women.
Symptomatic Tarlov cysts cause local or radicular pain. Pain may affect the buttock or radiate to the leg, as occurs in sciatica, and be associated with such other symptoms as paraesthesia in the penis or vagina. Rarer symptoms include bladder and bowel dysfunction, which may lead to urinary or faecal incontinence.15 In our series, 80% of patients presented pain and 60% presented incontinence (urinary incontinence in 40% and faecal incontinence in 20%).
Tarlov cysts, or perineural cysts, are extradural cysts formed between the endoneurium and perineurium of the posterior root of the spinal nerve distally to the dorsal root ganglion or at the junction with the latter structure. They are most frequently located at the second or third sacral nerve root (S2 and S3), although they have also been identified in the thoracic or lumbar spine.16 In our series, the most frequent locations were the S2, S3, and S1 nerve roots, in that order.
Asymptomatic Tarlov cysts are identified incidentally, during studies conducted to rule out lumbar or sacral spinal cord disease. Sensory alterations constitute the predominant manifestation of symptomatic cysts, due to their proximity to the dorsal root ganglion. When a Tarlov cyst is sufficiently large as to compress the ventral root, it can cause motor deficits. If the cyst continues to grow, it may affect several nerve roots.17 The associated symptoms include lower back pain, perineal pain, sciatica, neurogenic claudication, limb paraesthesia or weakness, bladder or bowel dysfunction,17 and even painful intercourse2 and impotence.3 In our series, the main symptoms were sexual dysfunction with pain and/or dyspareunia (80%), and bladder (40%) or bowel dysfunction (20%).
The main aetiological factor contributing to the formation of Tarlov cysts is CSF hydrostatic and pulsatile forces, which are transmitted unidirectionally from the subarachnoid space to the Tarlov cyst, leading to continuous dilation of the cyst.14,17,18 According to some authors, Tarlov cyst formation involves a valvular mechanism,4,16 while others suggest that this increased pressure may be the cause of such symptoms as headache.14 This may explain why symptoms are initially intermittent, worsening with standing, walking, and such Valsalva manoeuvres as coughing, with improvement during sleep.15
Many researchers continue to believe that Tarlov cysts are asymptomatic19; they are frequently overlooked in radiological studies and rarely identified as the cause of lumbar pain, paraesthesia, bladder or bowel dysfunction, dyspareunia, or even impotence.13 In fact, according to Murphy et al.,14 many patients with Tarlov cysts are told that these lesions are not the cause of pain and that their condition has no cure, leaving them untreated. In the light of this, we decided to report a series of patients with Tarlov cysts presenting pain and bladder/bowel dysfunction, in whom we believe a causal association between the lesions and their symptoms is sufficiently clear.
The prevalence of Tarlov symptoms in adults is estimated at 1%–5%, with higher rates among women; Tarlov cysts in children are extremely rare. Dayyani19 was the first to report a Tarlov cyst in an 8-month-old girl. Between 70% and 86% of patients with Tarlov cysts are women. Women present greater permeability and interstitial fluid leakage in the dorsal root ganglion than men. Therefore, they are more vulnerable to mechanical pressure or toxic agents. These harmful stimuli may cause chronic neuropathic pain. Increased permeability may promote the formation of Tarlov cysts due to CSF leakage between the endoneurium and the perineurium.13 In our series, all patients were adult women.
Three main hypotheses have been proposed on the pathogenesis of Tarlov cysts: (1) acquired, with increased hydrostatic pressure being due to trauma or inflammation; (2) congenital, due to proliferation of the arachnoid membrane (the recent report of an 8-month-old girl with a Tarlov cyst would support this hypothesis19); (3) associated with congenital anomalies, such as spina bifida or some connective tissue disorders.15,16
No specific criteria are currently available for differentiating between symptomatic and asymptomatic Tarlov cysts.16 Diagnosis of Tarlov cysts is established based on imaging findings.4 Radiography usually detects no alterations, although it may show regions of bone erosion secondary to pressure of the cyst against the vertebral body15; this finding may be confirmed by bone scintigraphy.20 Bone erosion caused by large cysts may result in insufficiency fractures or stress fractures, due to insufficient elastic resistance in osteoporotic bones.20 MRI is the gold-standard for detecting these lesions.1–5,13–19 Among the advantages of MRI, it provides high-resolution multiplanar images and is non-invasive; however, studies are costly.16 Furthermore, cysts display low signal intensity on T1-weighted sequences and high signal intensity on T2-weighted sequences, similarly to CSF (Fig. 1).15 CT is another possibility, although it is less sensitive.15,16 CT may display isodense cystic masses, as well as bone erosion, as observed with radiography.15,16 CT myelography revealing a contrast-filled hernial sac an hour after contrast medium administration is highly suggestive of perineurial cyst.15,16 This technique is used to demonstrate the connection between the cyst and the subarachnoid space.17 In our series, Tarlov cysts were diagnosed and confirmed by MRI in all cases.
A neurophysiological study may display reduced sural sensory nerve action potential amplitude. Other findings include reduced recruitment of the affected myotome and slowing of H-reflex latency.4,16 However, cases have been reported of symptomatic Tarlov cysts associated with normal neurophysiological study results.4 In our series, the neurophysiological study revealed pudendal neuropathy in one patient (20%) and abnormal sympathetic skin response in another (20%). Abnormal sympathetic skin response (persistence >60%) is linked to probable central sensitisation syndrome, and would explain pain.11 The remaining patients (60%) presented normal neurophysiological study results; this is consistent with previous reports in the literature.
There is controversy on whether Tarlov cysts should be treated.1 Most Tarlov cysts are found incidentally on radiological studies.17 However, the hypothesis that these lesions should be treated has gained support in recent years. It was Tarlov, in 1948, who first reported improvement in a patient with a symptomatic perineural cyst after complete surgical resection.13 In 1956, Strully asserted that most Tarlov cysts are not associated with clinical symptoms, and are therefore overlooked and left untreated.13 However, as denounced by Murphy and Ryan,14 the situation has not changed over 70years later: Tarlov cysts are still believed to be asymptomatic, and their association with perineal or perilabial pain continues to be rejected, leaving these patients untreated. In our series, all patients had undergone multiple radiological and EMG studies, despite which all previous physicians overlooked the causal association between the cyst and pain/dyspareunia and bladder/bowel dysfunction.
In a recent review, Hulens et al13 listed 10 reasons for which Tarlov cysts are frequently overlooked: (1) Tarlov cysts are frequently assumed to be asymptomatic; however, 25% of Tarlov cysts are symptomatic. (2) It is assumed to be difficult to determine whether a Tarlov cyst is responsible for the patient's pain; neurophysiological studies are extremely useful to this end. (3) MRI or neurophysiological studies focus on the lumbar and S1 nerve roots, omitting the remaining sacral nerve roots (S2, S3, S4). (4) Tarlov cysts detected by MRI are not reported by radiologists, who consider them clinically irrelevant. (5) Lower back pain and sciatica are frequently assumed to be caused by lumbosacral degenerative alterations, rejecting a possible causal association between sacral perineural cysts and sacral pain. (6) Small Tarlov cysts may also be symptomatic; the associated pain and paraesthesia are due to changes in hydrostatic pressure (the cause of Tarlov cysts) rather than to the size of the cyst itself. (7) Increased CSF hydrostatic pressure is the underlying pathogenic mechanism of Tarlov cysts; this is demonstrated by the fact that symptoms improve when CSF hydrostatic pressure decreases. (8) Physicians do not frequently question patients about symptoms associated with injury to the sacral nerve roots (S2, S3, S4), such as genital pain, bladder/bowel dysfunction, or impotence. (9) Pain of unknown cause is attributed to depression rather than to an organic cause (symptomatic Tarlov cysts). (10) Physicians wrongly assume that Tarlov cysts are asymptomatic, attributing atypical physical symptoms (bladder, bowel, or sexual dysfunction) to psychological, rather than to organic, factors, particularly in women.13,14 In the light of the above, Tarlov cysts are probably underdiagnosed, as demonstrated by our small case series.
To date, there is no consensus regarding treatment for Tarlov cysts.13,14,17–19 However, physicians do agree that asymptomatic Tarlov cysts require no treatment, but must be followed up.1,16 Furthermore, Voyadzis et al2 found that patients with Tarlov cysts measuring over 15mm present more evident neurological symptoms and better response to surgical treatment. Therefore, these authors recommend resecting symptomatic cysts measuring over 15mm, although this recommendation continues to be controversial.1–4,19
Pina-Montoya et al4 classify treatment for Tarlov cysts as either conservative or surgical. Conservative treatment includes pain management and rehabilitation therapy.4 More invasive approaches involve CT-guided percutaneous drainage4 and corticosteroid3 or fibrin infiltration.4,14 Surgical treatment involves laminectomy combined with complete resection, particularly in patients with cysts measuring over 15mm, presenting symptoms of incontinence, and in whom conservative treatment has failed.4
In a 2019 article, Dayyani notes that treatment for Tarlov cysts continues to be controversial.19 Treatment options include anti-inflammatory medication, corticosteroids,3 acetazolamide to reduce CSF pressure,13 and even electroacupuncture.21 Other more invasive treatments include percutaneous cyst drainage15; external CSF drainage; percutaneous injection of fibrin glue14; cyst-subarachnoid, lumboperitoneal, and cyst-peritoneal shunts; and cyst wall resection, imbrication, and cauterisation.19
The treatment of choice for faecal incontinence is manometric biofeedback, which aims to recover anal sensitivity and strengthen the external anal sphincter and pelvic floor muscles.22 Surface EMG biofeedback has also proved to be effective in the management of faecal incontinence.23 It was for this reason that we used biofeedback to treat bladder and bowel dysfunction in our series (urinary/faecal incontinence).
In a recent review by our research group, multimodal treatment (biofeedback+radiofrequency) was shown to improve pain in patients with dyspareunia and CPP.11 This approach may be useful for pain management in patients with Tarlov cysts. This hypothesis has been confirmed by the improvements observed in our series.
Biofeedback is the main treatment for the management of CPP and dyspareunia. This technique aims to train patients to activate and relax their muscles; in other words, it seeks to decrease resting muscle tone, which is frequently increased in these patients (negative biofeedback), and promote active muscle tone, which tends to be decreased (positive biofeedback). This technique provides visual feedback on muscle tension, enhancing voluntary muscle control and enabling personalised treatment under the supervision of a physiotherapist. Fernández-Cuadros et al11 showed that a multimodal rehabilitation protocol (biofeedback+radiofrequency) improves pain and pelvic floor muscle tone in patients with CPP and dyspareunia. This motivated our decision to use biofeedback for the treatment of patients with symptomatic Tarlov cysts.
Fernández-Cuadros et al11 reported that radiofrequency is widely used in clinical practice due to its thermal effects, which reduce pain and inflammation and improve tissue extensibility. Local thermotherapy increases pain tolerance; this analgesic effect is due to the gate control theory postulated by Melzack and Wall. According to this theory, pain perception is modulated in the dorsal column by competition between signals from large-diameter non-nociceptive Aβ fibres that transmit superficial, mechanical, or electrical stimulation information, and signals from small nociceptive Aδ and C fibres that transmit painful information. The Aδ fibres activated by thermal stimulation from the capacitive-resistive radiofrequency currents reduce pain transmission and, consequently, pain intolerance decreases. Furthermore, Melzack and Wall reported that the biological effects of capacitive-resistive radiofrequency include increased cell repair, increased lipolytic activity, and decreased inflammation, pain, and oedema. In fact, a combination of biofeedback and radiofrequency improved pain in 37 patients with CPP and dyspareunia.10 This led us to consider radiofrequency as an alternative in the multimodal treatment of pain in patients with symptomatic Tarlov cysts.
In our series, we administered Tiobec Dol® to one patient (20%) with neuropathic pain, as it has been suggested that the drug acts on nerve compression symptoms (pudendal neuropathy) and promotes myelin regeneration. In another patient, Tiobec Dol® was prescribed for pain management due to its analgesic properties.24,25
In the present series, we prescribed acetazolamide to 3 patients (60%), due to its ability to decrease CSF pressure, as pain in these cases is believed to be caused by dorsal nerve root compression.13 The drug was withdrawn in one of these due to intolerance (hypotension). One patient underwent an MRI study after treatment, which revealed lack of radiological improvement (the cyst did not decrease in size) despite clinical improvement. In the light of this, we did not perform post-treatment MRI studies in the remaining patients, but rather evaluated outcome variables only (pain [VAS] and maximal and mean pelvic floor muscle contraction, as described by Hulens).13,26,27
One limitation of our study is the small size of the sample, which is due to the low frequency of Tarlov cysts and to the fact that their symptoms are frequently overlooked or underestimated by the treating physicians, to the extent that they do not consider their pathogenic role and assume that symptoms are functional or even psychogenic.13 However, the small size of our series does not discredit our results, with treatment achieving significant improvements in pain (P<.05).
Patients with Tarlov cysts should be assessed thoroughly to determine whether the cysts are asymptomatic; symptomatic cases should be referred to the rehabilitation department for appropriate management of pain and bladder and bowel dysfunction, since these patients may benefit from multimodal rehabilitation treatment.16,28
ConclusionTarlov cysts are a known cause of pain and radiculopathy; however, they are frequently overlooked due to the erroneous assumption that they are asymptomatic. Tarlov cysts may also cause bladder, bowel, and sexual dysfunction. We documented the cases of 5 patients with Tarlov cysts who presented bladder/bowel dysfunction and pelvic pain, and who received multimodal rehabilitation therapy combined with pharmacological treatment. Tarlov cysts should be reported and studied; this will help to expand our understanding of their pathophysiology and, most importantly, to standardise effective multimodal treatment.
We would like to thank Saturnino Díaz Trujillo, librarian of Hospital Universitario Santa Cristina in Madrid, for his assistance with the literature search.