The objective of the present study was to evaluate the effectiveness and safety of teriflunomide in relapsing–remitting multiple sclerosis (RRMS) patients treated in a real-world setting.
MethodsThis retrospective study was conducted at neurology departments of 15 hospitals in 2 Spanish Autonomous Regions. The primary endpoint was annualized relapse rate (ARR) during teriflunomide treatment. Secondary endpoints included changes in Expanded Disability Status Scale (EDSS), radiological activity, and adverse events (AEs).
Results485 patients (72.2% women, mean of 36.5 years) were included; 74.8% had previously received other disease-modifying treatment. EDSS score at inclusion was 2.0. Mean time receiving teriflunomide was 2.5 years. The ARR during teriflunomide treatment was 0.16, a 20% lower than at baseline (0.20), although the difference did not reach statistical significance (P = 0.098). The mean number of relapses significantly decreased after teriflunomide initiation, with 0.17 relapses at month 12, 0.11 at month 24, and 0.13 at month 36, compared to 0.50 in the year before teriflunomide initiation (P < 0.001). EDSS scores were maintained over the study period. The percentage of patients without gadolinium-enhanced T1-weighted lesions was significantly higher after teriflunomide (P = 0.01), and the percentage of patients without new/enlarged lesions on T2 remained stable. The proportion of patients with AEs was 41.9% (1.4% serious), being hair thinning (19.4%) and gastrointestinal disorders (18.4%) the most frequent.
DiscussionOver teriflunomide treatment, the ARR was low, radiologic evidence of disease activity decreased, and disability stabilized. These findings, together with the acceptable safety profile observed, support the use of teriflunomide in RRMS patients.
El objetivo de este estudio es evaluar la efectividad y seguridad de teriflunomida en pacientes con esclerosis múltiple remitente-recurrente (EMRR) en un contexto del mundo real.
MétodosRealizamos un estudio retrospectivo de pacientes atendidos en los servicios de neurología de 15 hospitales localizados en dos comunidades autónomas de España. La variable principal fue la tasa anualizada de brotes (TAB) durante el tratamiento con teriflunomida. Como variables secundarias analizamos los cambios en la puntuación de la escala Expanded Disability Status Scale (EDSS), la actividad radiológica y los efectos adversos.
ResultadosNuestra muestra incluyó 485 pacientes (72,2% mujeres; edad media de 36,5 años); 74,8% de los pacientes habían recibido otro tratamiento modificador de la enfermedad con anterioridad. La puntuación media en la EDSS al inicio fue de 2,0. Los pacientes recibieron teriflunomida durante una media de 2,5 años. Durante el tratamiento, la TAB se redujo en un 20% respecto al inicio (0,16 frente a 0,20), aunque la diferencia no fue estadísticamente significativa (P = 0,098). El número medio de brotes se redujo significativamente tras iniciar el tratamiento con teriflunomida, pasando de 0,50 brotes en el año anterior al inicio del estudio a 0,17 brotes a los 12 meses de tratamiento, 0,11 brotes a los 24 meses y 0,13 brotes a los 36 meses (p < 0,001). Las puntuaciones en la EDSS se mantuvieron estables a lo largo del estudio. El porcentaje de pacientes que no mostraron lesiones captadoras de gadolinio en secuencias potenciadas en T1 fue significativamente mayor tras el tratamiento con teriflunomida (p = 0,01), mientras que el porcentaje de pacientes que no presentaron lesiones nuevas o un aumento en el tamaño de lesiones previas en secuencias potenciadas en T2 permaneció estable. Se reportaron efectos adversos en 41,9% de los pacientes (graves en 1,4%); los más frecuentes fueron la pérdida de cabello (19,4%) y los problemas gastrointestinales (18,4%).
ConclusiónDurante el periodo de tratamiento con teriflunomida, la TAB y la actividad radiológica de la enfermedad disminuyeron, mientras que el grado de discapacidad permaneció estable. Estos hallazgos, junto con el aceptable perfil de seguridad de teriflunomida, apoyan el uso del fármaco en pacientes con EMRR.
Multiple sclerosis (MS) is a lifelong, inflammatory, demyelinating, and neurodegenerative disease of the central nervous system. It typically affects young adults at working age,1 substantially reducing their work productivity and impairing their quality of life.2 Relapsing–remitting multiple sclerosis (RRMS) is the most common form of MS, and it is characterized by the occurrence of unpredictable but reversible episodes of neurological deficits (relapses) and the risk of disability progression. During the past 3 decades, the prevalence of MS has increased in many regions, including western Europe.3 In Spain, this increasing trend has also been observed, with a current MS prevalence as high as 80 to 180 cases per 100, 000 inhabitants.4
The number of disease-modifying treatments (DMTs) aimed at reducing disease activity and disability progression has considerably risen from the 1990s, with more than 12 DMTs available.5 The growing therapy armamentarium has simultaneously broadened treatment options for patients and poses disease management challenges for clinicians. When customizing treatment strategies, the efficacy and safety profile of the agent together with patient's individual disease must be considered.6
Teriflunomide 14 mg is a once-daily oral immunomodulator approved by the European Medicines Agency in August 2013 for the treatment of relapsing forms of MS or RRMS, depending on the local label. Randomized controlled trials consistently showed the efficacy of teriflunomide in reducing the annualized relapse rate (ARR),7,8 magnetic resonance imaging (MRI) markers of disease activity,7,9 brain volume loss,10 and delaying disability worsening.7,8 Its efficacy was also demonstrated in patients with a first clinical episode suggestive of MS.11 The consistent safety and tolerability profile of teriflunomide has been observed for up to 9 years of treatment, without unexpected adverse events (AEs).12,13
Real-world data (RWD) on the effectiveness and safety of teriflunomide in everyday clinical use, with a more heterogeneous patient population, is still limited, and published RWD mainly comes from studies conducted in central and northern Europe.14–17 Since MS management differs by region in terms of treatment guidelines, availability of DMTs, and prescribing practices, collecting RWD from routine clinical practice in different regions will provide further insights on the clinical outcomes of teriflunomide. The aim of the present study was to assess the effectiveness and safety profile of teriflunomide in RRMS under clinical practice conditions in two Spanish regions.
Materials and methodsStudy designThis was an observational retrospective study conducted at the neurology departments of 15 hospitals in 2 Spanish Autonomous Regions (Andalucía and Extremadura). Teriflunomide was prescribed according to routine clinical practice and to the approved Spanish label.
The study was approved by the ethics committee of Andalucía and conducted in accordance with the International Conference on Harmonization Guidelines for Good Clinical Practice, the Declaration of Helsinki, and the Spanish legislation for post-authorization studies.
PatientsAll RRMS patients meeting selection criteria were recruited between January 15, 2019 and April 1, 2020. The inclusion criteria were: had received teriflunomide treatment according to clinical practice conditions, and had provided written informed consent regarding the use of their medical data for the purpose of this research. There were no exclusion criteria. All patients had been diagnosed with RRMS according to the McDonald criteria.6,7
Endpoints and assessment schedulePatient information was retrospectively retrieved from their medical charts. Data from baseline (i.e. teriflunomide treatment initiation) up to the last annual follow-up visit at 12, 24, and 36 months (±3 months) were collected, when available. Data at each month included data from the previous 12 months (i.e. month 12 included data from month 0 to month 12, month 24 included data from month 12 to month 24, and month 24 included data from month 24 to month 36; ±3 months).
Data collected from before teriflunomide initiation included: demographics, medical history of MS, and previous DMT. ARR, number of relapses, expanded disability status scale (EDSS) scores, and gadolinium-enhancing (Gd+) T1 lesions and new/enlarged lesions on T2-weighted MRI scans were collected from before teriflunomide initiation and over teriflunomide treatment. AEs related to teriflunomide treatment and reasons for discontinuation were also collected over treatment. A relapse was defined as a neurologic deficit typical of MS that lasts at least 24 h in the absence of fever and infection.
The primary endpoint was ARR during teriflunomide treatment. Secondary endpoints were EDSS changes, radiological activity changes, and frequency and characterization of AEs over teriflunomide treatment.
The ARR at baseline was calculated considering the total number of relapses during the previous 24 months before teriflunomide initiation divided by the total period (2 years) before teriflunomide initiation at risk of relapse. The ARR after teriflunomide initiation was defined as the total number of relapses during teriflunomide treatment divided by the total period (years with follow-up) after teriflunomide initiation at risk of relapse. Mean of relapses was calculated as the total number of relapses divided by the number of patients.
Statistical analysisDescription of quantitative variables was performed using mean (standard deviation [SD] or 95% confidence interval [95% CI]) and/or median (minimum [min] and maximum [max]) values). For the description of qualitative variables, absolute and relative frequencies were used. For relative frequencies, 2 percentages were calculated: the total percentage, which was the percentage of the sum of valid responses plus missing values, and the valid percentage, which was the percentage of the total valid responses. Valid percentages are reported here.
Changes in the ARR, number of relapses, and EDSS were calculated using the non-parametric Wilcoxon test. Changes in the percentage of patients with T1 Gd + lesions and new/enlarged lesions on T2) were assessed using the non-parametric Cochran's Q test. These comparisons (effectiveness analyses) were performed for the sample of patients with available data at all follow-up visits. Changes in the number of relapses were also analyzed for those patients with available data only at month 12, month 24, or month 36. Descriptive analysis for EDSS scores and MRI assessments for patients with available data only at month 12, month 24, or month 36 are also presented.
Values of P < 0.05 were considered statistically significant. No imputations for missing data were performed. All statistical analyses were performed using Statistical Package for Social Sciences (SPSS) version 22.
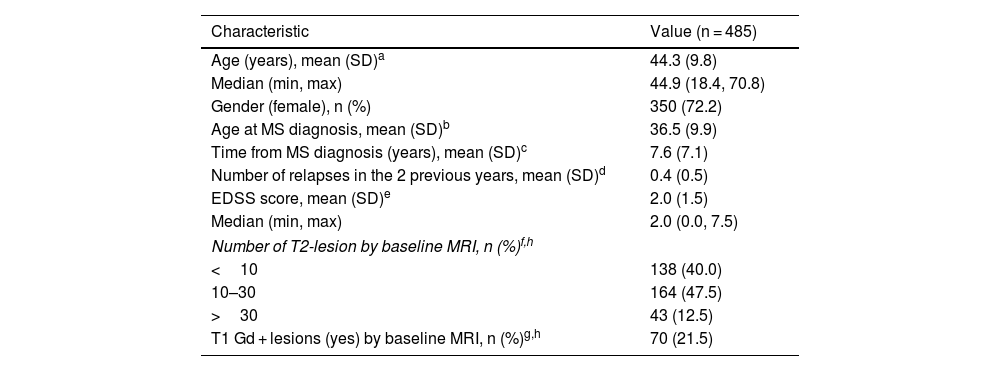
ResultsDemographic and clinical characteristicsA total of 485 RRMS patients treated with teriflunomide were included and analyzed in the study. Table 1 shows the patients' demographic and clinical characteristics. Briefly, most of the patients were women (72.2%), with a mean (SD) age of 36.5 (9.9) years at MS diagnosis. At teriflunomide initiation the mean (SD) time from MS diagnosis was 7.6 (7.1) years. The ARR over the 24-month before teriflunomide initiation was 0.20 (95% CI: 0.18, 0.24). The mean (SD) EDSS at baseline was 2.0 (1.5).
Demographic and clinical characteristics.
| Characteristic | Value (n = 485) |
|---|---|
| Age (years), mean (SD)a | 44.3 (9.8) |
| Median (min, max) | 44.9 (18.4, 70.8) |
| Gender (female), n (%) | 350 (72.2) |
| Age at MS diagnosis, mean (SD)b | 36.5 (9.9) |
| Time from MS diagnosis (years), mean (SD)c | 7.6 (7.1) |
| Number of relapses in the 2 previous years, mean (SD)d | 0.4 (0.5) |
| EDSS score, mean (SD)e | 2.0 (1.5) |
| Median (min, max) | 2.0 (0.0, 7.5) |
| Number of T2-lesion by baseline MRI, n (%)f,h | |
| <10 | 138 (40.0) |
| 10–30 | 164 (47.5) |
| >30 | 43 (12.5) |
| T1 Gd + lesions (yes) by baseline MRI, n (%)g,h | 70 (21.5) |
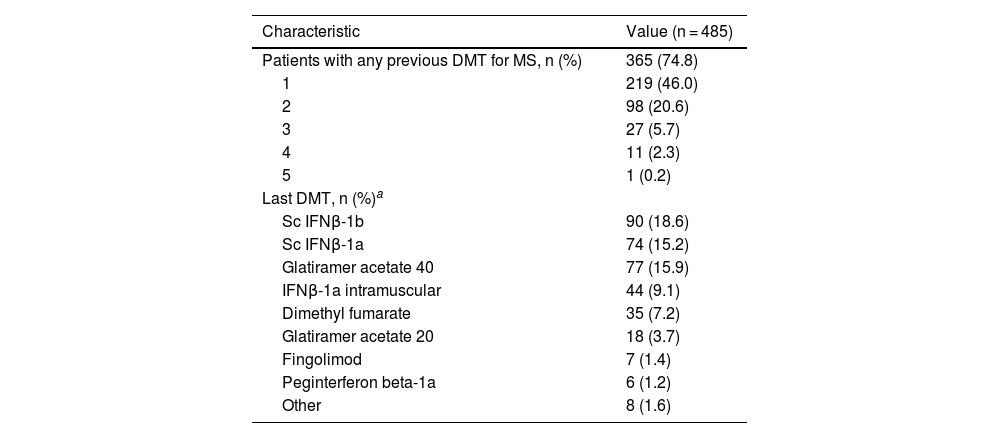
DMT use before teriflunomide was registered in 74.8% of patients. The most frequently used DMTs were subcutaneous (sc) interferon (IFNβ)-1b (18.6%), sc IFNβ-1a (15.2%) and glatiramer acetate 40 mg/ml (15.9%) (see Table 2). The most common reasons for switching to teriflunomide were AEs (75.2%; n = 270), lack of effectiveness (16.2%; n = 58), and patient choice (8.6%; n = 31).
Previous DMT.
| Characteristic | Value (n = 485) |
|---|---|
| Patients with any previous DMT for MS, n (%) | 365 (74.8) |
| 1 | 219 (46.0) |
| 2 | 98 (20.6) |
| 3 | 27 (5.7) |
| 4 | 11 (2.3) |
| 5 | 1 (0.2) |
| Last DMT, n (%)a | |
| Sc IFNβ-1b | 90 (18.6) |
| Sc IFNβ-1a | 74 (15.2) |
| Glatiramer acetate 40 | 77 (15.9) |
| IFNβ-1a intramuscular | 44 (9.1) |
| Dimethyl fumarate | 35 (7.2) |
| Glatiramer acetate 20 | 18 (3.7) |
| Fingolimod | 7 (1.4) |
| Peginterferon beta-1a | 6 (1.2) |
| Other | 8 (1.6) |
The mean (SD) observation period (i.e. period of time receiving teriflunomide) was 2.5 (1.4) years. During this period, 107 (22.8%) patients had discontinued teriflunomide treatment. The most common reasons for discontinuation were lack of effectiveness (50.5%; n = 54), AEs (33.6%; n = 36), pregnancy desire (3.7%; n = 4), patient choice (3.7%; n = 4), or other (8.3%, n = 9). AEs that led to the discontinuation of teriflunomide included gastrointestinal events (n = 11), hair loss (n = 9), liver function test alterations (n = 6), hematologic events (n = 5), hypertension (n = 5), lumbar pain (n = 2), infections (n = 1), tachycardia (n = 1), jejuno-ileitis (n = 1), polyneuropathy (n = 1), and anxiety (n = 1). Temporary interruption of treatment was reported in 16 (3.4%) patients, mainly due to AEs (25.1%; n = 4), pregnancy (18.9%; n = 3), lack of effectiveness (6.3%; n = 1), or other (31.3%; n = 5). Reasons for temporary interruption were not available for three patients. Lack of effectiveness was considered at the discretion of the physician.
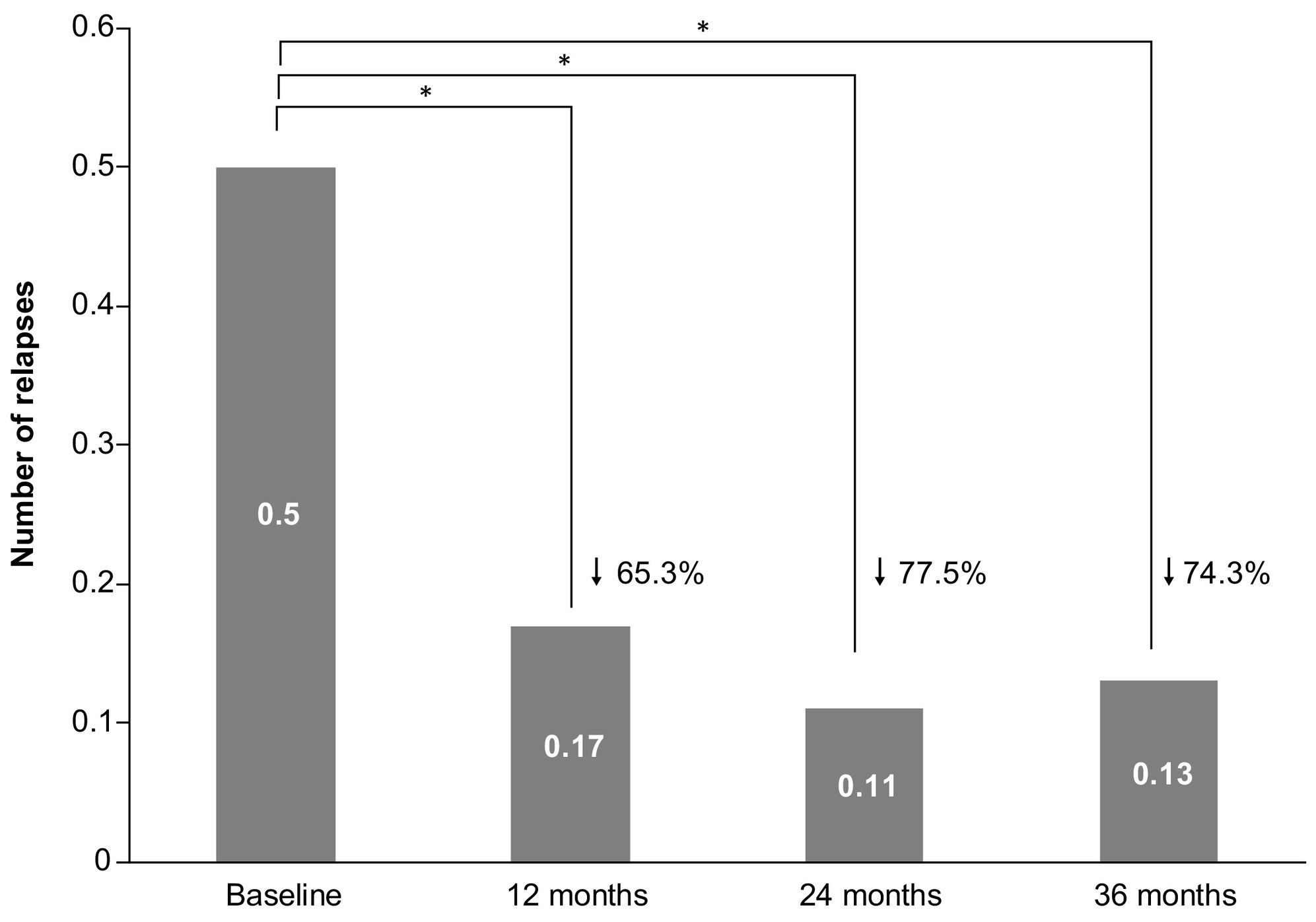
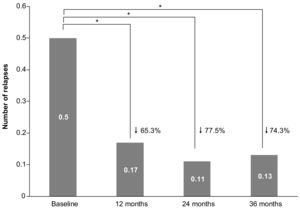
EffectivenessRelapsesThe ARR during the complete treatment period with teriflunomide was 0.16 (95% CI: 0.14–0.20), which was a 20% lower than the ARR at baseline (0.20 [0.18, 0.24]), but the difference did not reach statistical significance (P = 0.098). When only patients with available data at all follow-up visits were considered (n = 176), the mean (SD) number of relapses significantly decreased after teriflunomide initiation (P < 0.001), with 0.17 (0.42) relapses after 12 months, 0.11 (0.35) after 24 months, and 0.13 (0.40) after 36 months, compared to 0.50 (0.64) relapses in the year before teriflunomide initiation collected at baseline (see Fig. 1).
When patients with available data only at month 12, month 24, or month 36 were analyzed, similar results were obtained. The mean (SD) number of relapses was significantly lower during teriflunomide treatment, compared to baseline, at month 12 (0.21 [0.46] vs 0.42 [0.55]; n = 395), month 24 (0.14 [0.41] vs 0.46 [0.59]; n = 279), and month 36 (0.14 [0.42] vs 0.51 [0.65]; n = 178) (P < 0.001). Most patients did not have any relapse during teriflunomide treatment, with 81.7% of relapse-fee patients at month 12, 88.2% at month 24, and 88.5% at month 36.
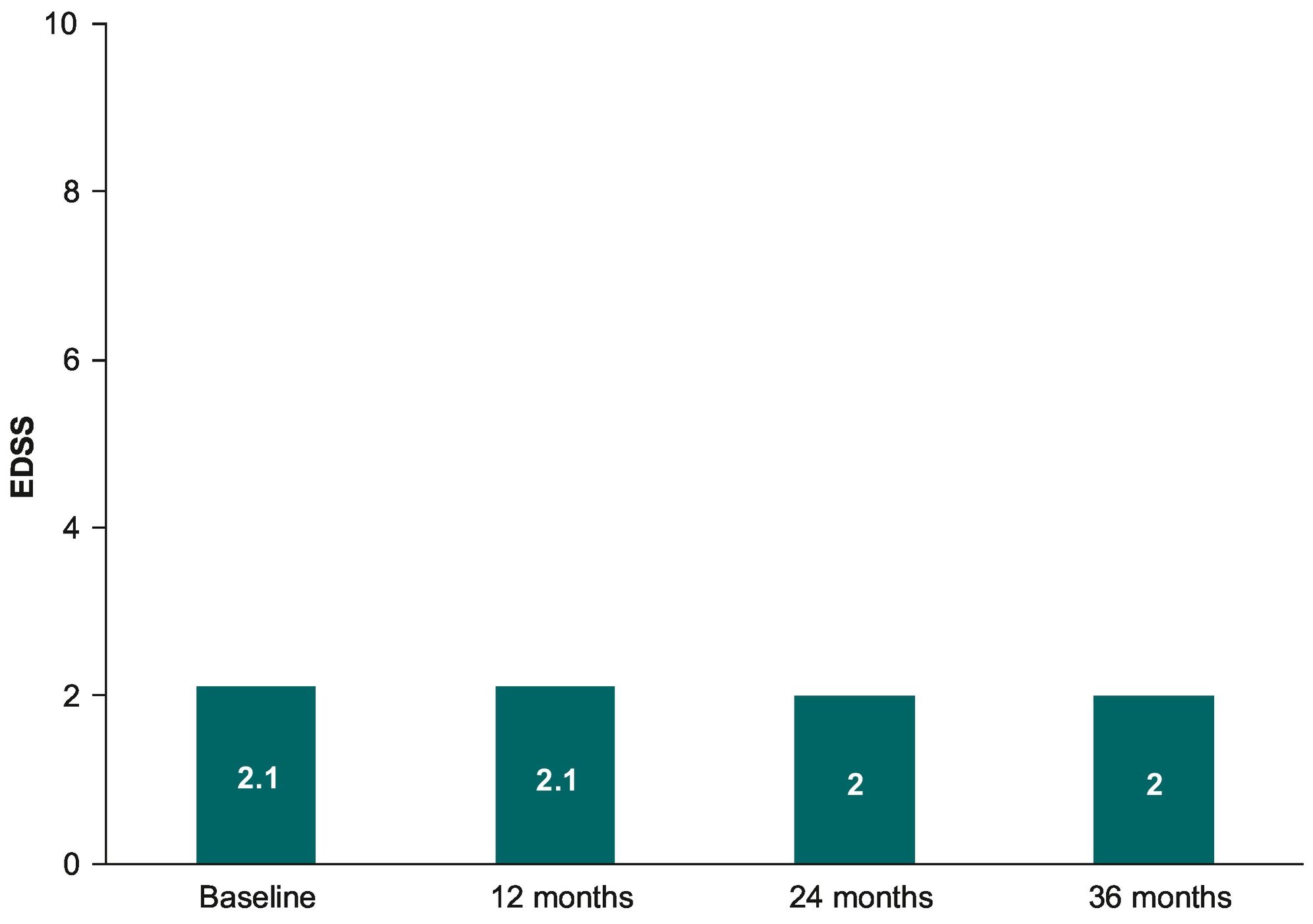
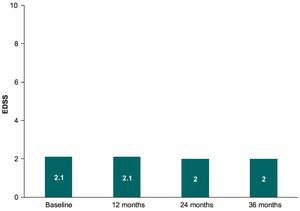
EDSSWhen patients with available data up to 36 months were analyzed (n = 177), no changes between the EDSS scores were observed over teriflunomide treatment (P = 0.587), with a similar mean (SD) EDSS at month 12 (2.1 [1.5]), month 24 (2.0 [1.5]), and month 36 (2.0 [1.6]) (see Fig. 2). Mean (SD) EDSS scores over teriflunomide treatment in those patients with follow-up only up to month 12 (n = 402), month 24 (n = 279), or month 36 (n = 182) were 2.0 (1.6), 2.1 (1.7), and 2.1 (1.7), respectively.
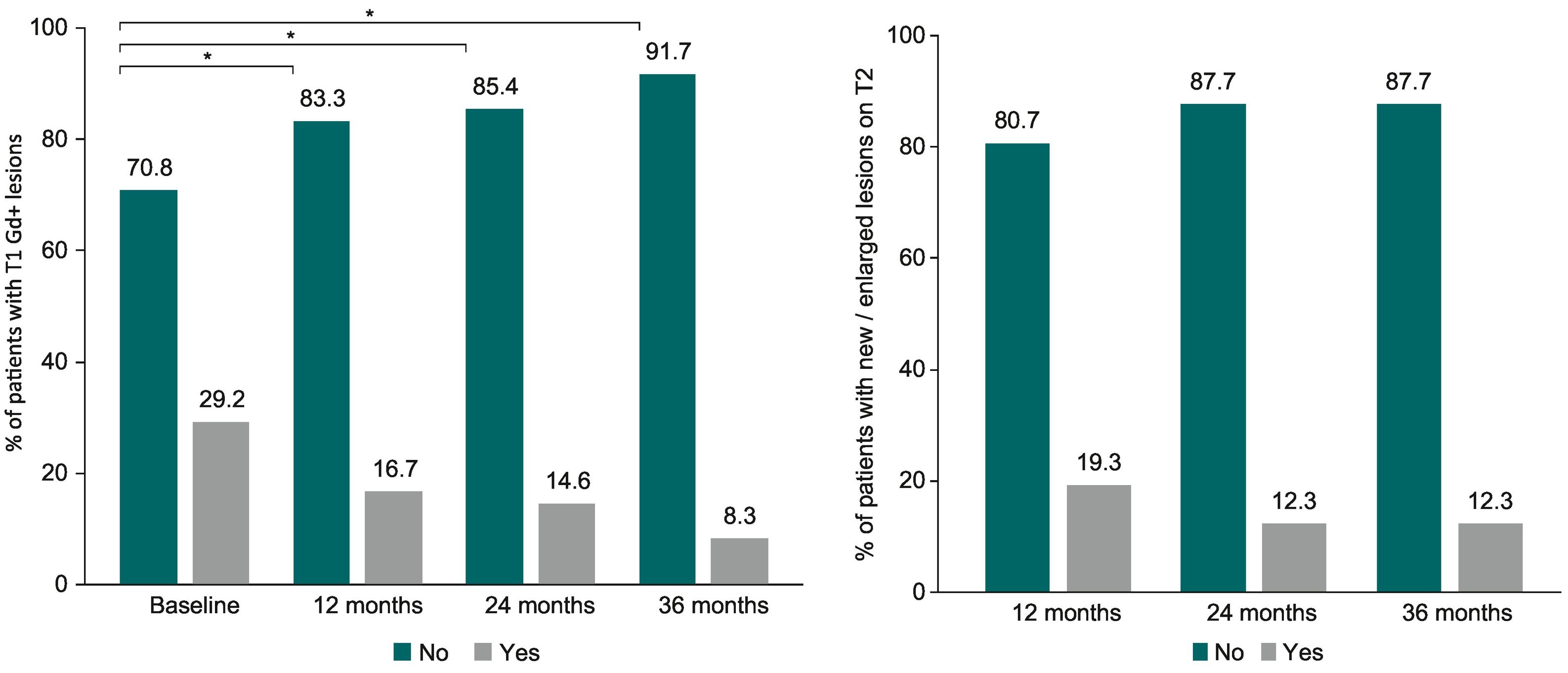
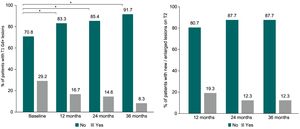
MRIAs Fig. 3A shows, the percentage of patients without T1 Gd + lesions were significantly higher after teriflunomide initiation, with 83.3% at month 12, 85.4% at month 24, and 91.7% at month 36 compared to 70.8% at baseline (p = 0.01) (patients with available data up to 36 months, n = 48). Most patients included in the study did not present any T1 Gd + lesion after 12 months (88.7%; n = 259), 24 months (83.9%; n = 130), or 36 months (92.4%; n = 61) over teriflunomide treatment.
As presented in Fig. 3B, the percentage of patients without new/enlarged lesions on T2 over follow up did not change (P = 0.39), being 80.7% at month 12, and 87.7% at month 24 and month 36 (patients with available data up to 36 months; n = 57). New/enlarged lesions on T2 were not observed in 251 (77.7%) patients at month 12 (n = 323), 132 (83.5%) patients at month 24 (n = 158), and 58 (89.2%) patients at month 36 (n = 65).
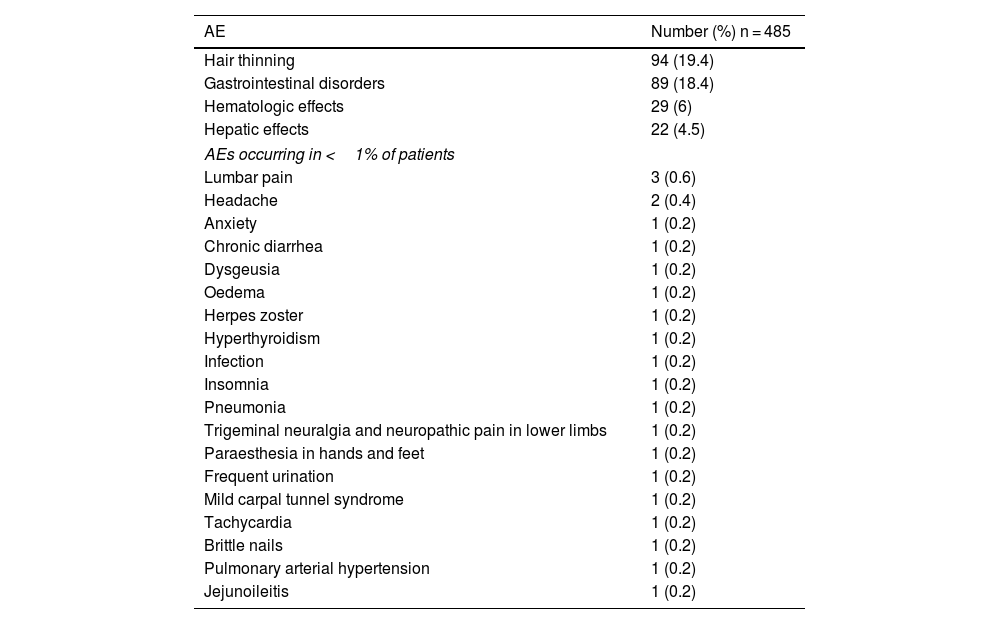
SafetyTwo hundred seventy-one AEs were reported by 203 patients (41.9%) and were mostly mild to moderate in nature. Most patients (n = 144; 70.9%) presented one AE, 50 patients (24.6%) two AEs, and 9 patients (4.4%) three AEs. The most frequently reported AEs were hair thinning (19.4%), gastrointestinal disorders (18.4%), hematologic effects (6%), hepatic effects (4.5%), and other (4.7%) (Table 3). Seven (1.4%) AEs were considered to be severe: diarrhea (n = 3), alopecia (n = 1), alanine transaminase (ALT) values increase x6 (n = 1), and pulmonary arterial hypertension (n = 1). Data for one severe AE was missing.
All reported adverse events.
| AE | Number (%) n = 485 |
|---|---|
| Hair thinning | 94 (19.4) |
| Gastrointestinal disorders | 89 (18.4) |
| Hematologic effects | 29 (6) |
| Hepatic effects | 22 (4.5) |
| AEs occurring in <1% of patients | |
| Lumbar pain | 3 (0.6) |
| Headache | 2 (0.4) |
| Anxiety | 1 (0.2) |
| Chronic diarrhea | 1 (0.2) |
| Dysgeusia | 1 (0.2) |
| Oedema | 1 (0.2) |
| Herpes zoster | 1 (0.2) |
| Hyperthyroidism | 1 (0.2) |
| Infection | 1 (0.2) |
| Insomnia | 1 (0.2) |
| Pneumonia | 1 (0.2) |
| Trigeminal neuralgia and neuropathic pain in lower limbs | 1 (0.2) |
| Paraesthesia in hands and feet | 1 (0.2) |
| Frequent urination | 1 (0.2) |
| Mild carpal tunnel syndrome | 1 (0.2) |
| Tachycardia | 1 (0.2) |
| Brittle nails | 1 (0.2) |
| Pulmonary arterial hypertension | 1 (0.2) |
| Jejunoileitis | 1 (0.2) |
Five patients reported pregnancies during teriflunomide therapy. Three of them discontinued teriflunomide treatment and two continued during pregnancy (one pregnancy was unwanted and ended up in abortion and the other one discontinued the treatment during pregnancy and neither the course nor the outcome were affected). Among those who discontinued, two reinitiated treatment after delivery. No birth defects or miscarriage were reported by patients who conceived after teriflunomide discontinuation.
DiscussionThe EFFECT study showed that teriflunomide administered to RRMS patients under clinical practice conditions enabled clinical effectiveness to be achieved for up to 36 months. The ARR during teriflunomide treatment was 0.16, the incidence of relapses was lower after teriflunomide treatment, and more than 81% of patients were relapse-free at all follow-up visits. Disability stabilization was also observed, as EDSS scores remained constant over treatment. Additionally, the percentage of patients with MRI markers of disease activity decreased over teriflunomide treatment, supporting its clinical effectiveness.
These results confirm the efficacy of teriflunomide 14 mg reported in the pivotal clinical trials TEMSO7,9 and TOWER.8 In fact, the ARR after teriflunomide initiation in our study was numerically lower (0.16) than the ARR in the TEMSO (0.37) and TOWER (0.32) studies. It is worth noting that patients in our study were older (44.3, max: 70.8 years) than in TEMSO (37.8) and TOWER (38.2), where only patients up to 55 years were included. The absence of changes in the EDSS score observed here is also consistent with the slowdown in disability accumulation previously reported.7,8,18
Effectiveness of teriflunomide was not only clinically confirmed, but also radiologically. Active inflammatory lesions decreased after teriflunomide treatment, with approximately 92% of patients free from T1 Gd + lesions and 90% free from new/enlarged T2 lesions at 36 months. These findings provide further evidence for the beneficial effect of teriflunomide in attenuating MRI activity observed in clinical trials, which also reported that other MRI outcomes, such as total lesion volume or volume of T1-hypointense lesions, were lower in the teriflunomide groups compared to placebo.7,9 Similar to findings from the TEMSO study,9 the effect of MRI activity was consistent with the magnitude of relapses in our study, as both measures decreased at each follow-up visit compared to baseline. Altogether, our findings suggest that the effectiveness of teriflunomide in everyday clinical practice is similar to that observed in clinical trials.
The effectiveness of teriflunomide in our study was also similar to that reported in other RWD studies in Europe.14,16,17,19 TAURUS-MS was a prospective observational study conducted in Germany that included a larger cohort of patients (n = 1128) but with similar demographic and clinical characteristics, including age (44.3 years in our study versus 44.9 years in TAURUS-MS), previous DMT use (74.8% in our study versus 75.2% in TAURUS-MS), and median EDSS at baseline (2.0 in both).14 In both studies, the ARR was lower after teriflunomide treatment, although this reduction achieved statistical significance in the TAURUS-MS study, and only a trend towards statistical significance in our study. The lack of statistical significance in our study could be due to our smaller sample size and lower ARR prior to teriflunomide (0.20 in our study versus 0.87 in the TAURUS-MS study). The low ARR in our study is consistent with the ARR observed in other three real-world studies: 0.16 at month 12 in the TACO study—conducted in Switzerland—16 0.17 in the teri-LIFE study—conduced in Norway, Sweden, and Denmark—17 and 0.17 at year 2 in the teri-CARE study—conducted in Spain.20 Importantly, 75% of patients switched to teriflunomide due to AEs, which could also contribute to explain the low ARR observed in our study, both at baseline and during teriflunomide treatment. Stable EDSS scores up to 24 months of teriflunomide treatment were observed in these real-world studies.14,16,17,20 Our study confirmed stable EDSS at month 24 and provided further evidence of the absence of disability progression up to 36 months.
The percentage of patients who had discontinued teriflunomide treatment in our study (22.8%) was similar to the one reported in the TAURUS-MS study (21.5%).14 This percentage is within the 16% to 27% discontinuation rate range associated with first-line DMTs.21 Among reasons for discontinuing teriflunomide, AEs were reported for 34% of our patients, which was inferior to the 53%15 and 40.1%14 reported in previous real-world studies, but superior to the 11% described in the extension phase of the TEMSO trial.12 A recently published retrospective study has shown that 29% discontinued teriflunomide (37% due to AEs and 15% to poor tolerability).19
Overall, teriflunomide had a good tolerability and favorable safety profile. Most of the reported AEs were consistent with the safety profile documented in the summary of product characteristics.22 The most frequent AEs (occurring in ≥10%) were hair thinning, gastrointestinal disorders, which were also among the most frequently reported AEs in the TEMSO.7 The AEs not previously reported (dysgeusia, hyperthyroidism, insomnia, and jejunoileitis) had an incidence of 0.2 and none of them were serious. Though the overall percentage of patients with AEs in our study was within the range of previous studies,14,15 the incidence of serious AEs (1.4%) was considerably lower compared to the 12.1% and 13% reported in the real-world,14,23 the 15.9% in the TEMSO,7 and the 22% in the TEMSO extension,12 which provides further evidence of the good tolerability of teriflunomide. Case-reports of MS patients treated with teriflunomide with SARS-CoV-2 infection have suggested that these patients can continue receiving treatment with teriflunomide even if they have relevant comorbidities or clinical conditions that require hospitalization.24–26
Despite the Spanish Society of Neurology recommends teriflunomide, among other DMTs, as a first-line treatment for RRMS, our results revealed that only one-quarter of these patients received teriflunomide as their first DMT. Available data from the teri-CARE study were consistent with our findings, although the percentage of treatment naïve patients was slightly higher (36%) in that study.20 In our study, among those previously treated, the majority received interferons (18.6% sc IFNβ-1b, 15.2% sc IFNβ-1a, intramuscular 9.1% IFNβ-1a). A rater-blinded trial showed that the efficacy of teriflunomide was comparable to sc IFNβ-1a, but patient satisfaction was higher in those treated with teriflunomide.27 The DMT choice should be a shared-decision process, where not only efficacy, safety, drug accessibility, and disease activity, but also patient preferences are considered and balanced. RRMS patients prefer daily oral administration (when treatment frequency and frequency of side effects were held constant) over other routes of administration.28 Whether RRMS patient preferences are considered during treatment decision-making and satisfied over teriflunomide treatment should be investigated by future studies in the Spanish routine clinical practice.
Several limitations are associated with the present study. Due to its observational nature and the retrospective collection of data, all the study variables were not collected in every patient included in the study, and the number of patients with available data decreased at each follow-up visit, which might limit the interpretation of results. The relatively low ARR in our study might reflect a selection bias, as patients with high disease activity might be underrepresented. Data on potential factors that could affect relapses were not collected and the ARR according to previous treatment was not included, despite both aspects could have affect the ARR. The absence of a control group also prevents definitive conclusions on effectiveness and safety. Additionally, the study was conducted in several hospitals of two Spanish regions, which limits the generalizability of the results to other regions in the country. Future studies are warranted to further compare the effectiveness and safety of teriflunomide to other oral DMTs to treat patients with RRMS and to further analyze teriflunomide effectiveness and safety according to prior treatments.
ConclusionsOverall, results from the EFFECT study showed the sustained effectiveness and safety of teriflunomide up to a 36-month period in a real-world clinical practice setting. These findings add to the body of evidence supporting a favorable benefit–risk profile for teriflunomide, and its long-term use as a DMT option for RRMS patients.
Ethical considerationsThe authors declare that the procedures followed conformed to the ethical standards of the responsible human experimentation committee and in accordance with the World Medical Association and the Declaration of Helsinki and have obtained the informed consent of the patients and/or subjects referred to in the article.
Conflicts of interestMª Carmen Durán Herrera has received consulting fees and research support from Roche Novartis; Montserrat Gómez has received consulting fees from Genzyme, Biogen, Merck and Novartis; Francisco Padilla Parrado has received consulting fees and research support from Novartis, Sanofi and Biogen. The rest of the authors declare no conflict of interests.
Medical writing support was provided by Laura Prieto del Val from Dynamic Science (Evidenze Clinical Research). The authors would also like to thank to Antonio Jose Uclés Sánchez and José Luis Casado Chocán for their help in patient recruitment, and to Cristina Conde Gavilán and Fernando Acebrón Sánchez Herrera for their help in the database development.