The SARS-CoV-2 pandemic has been affecting the world since January 2020. Although its pathogenesis is primarily directed to the respiratory tract, other organs may be affected, including the nervous system. It has also been shown that the social context (confinement, lack of treatment) has affected neurological patients during this period. The aim of the study it was to assess the subjective worsening of neurological/psychiatric diseases in the context of the SARS-Cov-2 pandemic.
MethodsThree groups of neurological/psychiatric patients were included: Patients who had symptomatic COVID-19 (n = 89), patients who had asymptomatic COVID-19 (n = 40), and a control group (n = 47), consisting of neurological/psychiatric patients without a history of SARS-Cov-2 infection.
Results30.7% of the included individuals considered that their basal pathology had worsened during the study period. This feeling was significantly more frequent (P = 0.01) in patients with symptomatic COVID-19 (39.3%) than in patients of the other 2 groups (21.8%). Worsening was not related to the severity of COVID-19. The neurological conditions that significantly worsened after COVID-19, comparing symptomatic COVID-19 with the other 2 groups, were demyelinating and degenerative diseases.
ConclusionsThese results confirmed the impact of the SARS-Cov-2 pandemic on patients with neurological/psychiatric diseases. Confinement, lack of medical care, and the threat of diagnosis are surely contributing factors. Although the finding of a higher frequency of worsening in symptomatic COVID-19 patients may be related to greater anxiety/depression in this group of patients, we cannot exclude the role of direct affectation of the nervous system by the virus or damage due to neuroinflammation.
La pandemia por SARS-CoV-2 afecta al mundo desde enero de 2020. Aunque su patogenia se dirige principalmente a las vías respiratorias, otros órganos pueden verse afectados, incluido el sistema nervioso. También se ha demostrado que el contexto social (confinamiento, falta de tratamiento) ha afectado a los pacientes neurológicos durante este periodo. El objetivo del estudio fue evaluar el empeoramiento subjetivo de enfermedades neurológicas/psiquiátricas en el contexto de la pandemia por SARS-Cov-2.
MétodosSe incluyeron tres grupos de pacientes neurológicos/psiquiátricos: pacientes que tenían COVID-19 sintomático (n = 89), pacientes que tenían COVID-19 asintomático (n = 40) y un grupo control (n = 47), formado por pacientes neurológicos/psiquiátricos sin antecedentes de infección por SARS-Cov-2.
ResultadosEl 30,7% de los individuos incluidos consideró que su patología basal había empeorado durante el período de estudio. Este sentimiento fue significativamente más frecuente (p = 0,01) en pacientes con COVID-19 sintomático (39,3%) que en pacientes de los otros 2 grupos (21,8%). El empeoramiento no estuvo relacionado con la gravedad de COVID-19. Las condiciones neurológicas que empeoraron significativamente después de la COVID-19, comparando la COVID-19 sintomática con los otros 2 grupos, fueron las enfermedades desmielinizantes y degenerativas.
Conclusionesestos resultados confirmaron el impacto de la pandemia del SARS-Cov-2 en pacientes con enfermedades neurológicas/psiquiátricas. El encierro, la falta de atención médica y la amenaza del diagnóstico son seguramente factores contribuyentes. Aunque el hallazgo de una mayor frecuencia de empeoramiento en pacientes sintomáticos de COVID-19 puede estar relacionado con una mayor ansiedad/depresión en este grupo de pacientes, no podemos excluir el papel de la afectación directa del sistema nervioso por el virus o el daño por neuroinflamación.
The novel 2019 coronavirus disease (COVID-19) caused by severe acute respiratory syndrome coronavirus (SARS-CoV-2) has attracted attention not only because of its respiratory symptoms, but also because it affects other organs.1 The most common symptoms are fever, cough, fatigue, and headache.2 Severe cases of the disease can induce respiratory distress, renal, hepatic, and cardiac failure, and a multisystemic inflammatory syndrome.3,4 Asymptomatic cases are also common.5
Several studies have shown that COVID-19 was associated with neurological manifestations, sometimes in a large proportion of patients.6 Different mechanisms have been implicated in the pathophysiology of neurological disorders. While not exclusive, 2 of them are mainly evoked: Damage linked to the direct presence of the virus in the nervous system, and neuroinflammation as consequence of the systemic inflammatory reaction. Regarding the former, the neuroinvasive capacity of SARS-CoV-2 has been demonstrated,7 and viral material (RNA, antigens, particles) has been observed in different compartments/cells of the nervous system.8 Binding of SARS-CoV2 to angiotensin-converting enzyme (ACE) 2 located on epithelial cells is known to be the primary mechanism of virus entry into the host.9 ACE-2 is also expressed in various cells of the nervous system, potentially allowing for direct affectation of brain.10 Regarding the inflammatory hypothesis, the presence of the virus induces a systemic pro-inflammatory response that, in some cases, can lead to an uncontrolled “cytokine storm” that can severely affect different organs.11 In the case of the brain, this systemic inflammatory response alters the blood–brain barrier, which can enable a significant neuroinflammatory response.12,13 As it is well known that the neuroinflammatory response is part of the pathology of most nervous system diseases,14–18 this phenomenon could be associated with worsening of previous neurological or psychiatric diseases. On the other hand, the COVID-19 pandemic has been shown to worsen the situation of neurological/psychiatric patients, whether infected or not.19–22 The main factors involved are related to the effect of confinement, social distancing, lack of adequate treatment, and increased anxiety/depression.
In this context, our study aims to evaluate the effect of COVID-19 on the subjective impression of progression of neurological/psychiatric diseases in 3 groups of patients: symptomatic COVID-19 patients, asymptomatic COVID-19 patients, and controls, in order to obtain more information regarding the factors involved in disease progression.
MethodsThis study was conducted between August 2020 and October 2021 at the Instituto Nacional de Neurología y Neurocirugía Manuel Velasco Suárez (INNNMVS) in Mexico City, a national referral tertiary care center for patients older than 15 years of age without social security. During the pandemic, the outpatient clinic was closed from March 23, 2020 to May 31, 2020, as Mexico City had a high level of viral transmission. After this period, face-to-face consultations resumed slowly to avoid congestion of patients in the waiting area. A telemedicine service was implemented, but not all patients were able to attend consultations via this service. In the end, 55.8% of the planned consultations in 2020 could be carried out.
The study was approved by the INNN ethics committee, and all patients gave informed consent before inclusion.
PatientsThree groups of patients were considered: patients with COVID-19 who met the World Health Organization (WHO) case definition (probable and definite cases of COVID-19) (https://apps.who.int/iris/bitstream/handle/10665/337834/WHO-2019-nCoV-Surveillance_Case_Definition-2020.2-eng.pdf), patients with asymptomatic COVID-19, and control subjects (patients at the institute with no history of symptoms consistent with COVID-19, no contact with people affected by COVID-19, and no specific antibodies in serum).
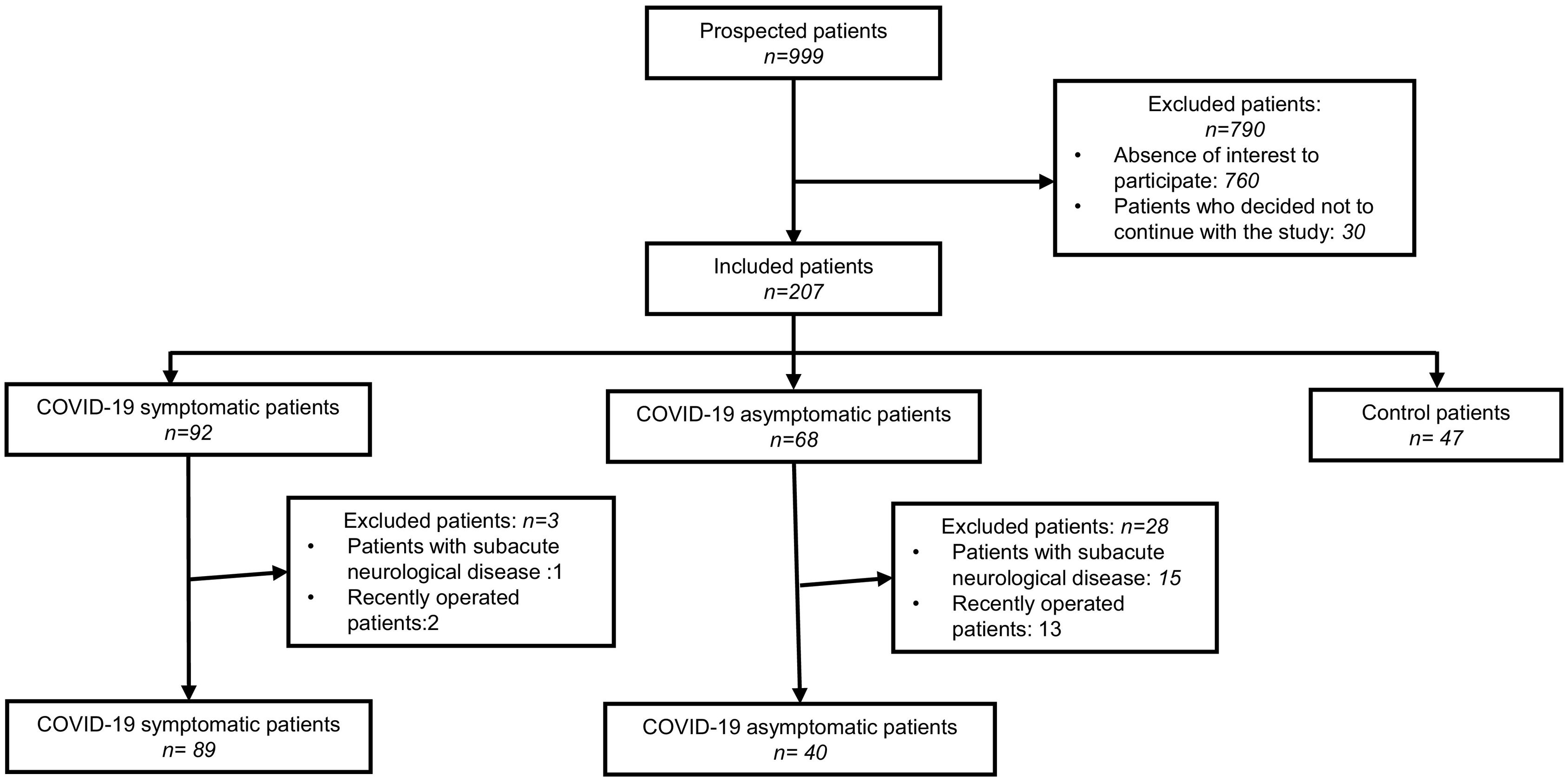
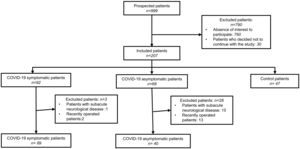
The severity of COVID-19 was assessed by considering previously published criteria.9 We excluded from the study patients with acute/subacute neurological or psychiatric pathologies, those in whom a neurosurgical procedure was performed during the study period or 6 months before, and those in whom a complete change in their therapeutic management between the 2 evaluated periods was performed. The patient inclusion flowchart is described in Fig. 1.
Several strategies were used to include patients who had symptoms of COVID-19: interviews with patients in the outpatient waiting room, an informational video shown in the outpatient waiting room, mailing to patients, and collaboration with consulting physicians at the institute. Asymptomatic COVID-19 patients were included if the presence of specific antibodies in their serum was observed, in samples collected at the INNNMVS laboratory for other purposes, before the start of vaccination in Mexico. Control patients were included by interviews in the outpatient waiting room.
Neurological/psychiatric diagnoses were confirmed by reviewing the patient's clinical record. By interview, the subjective impression on the evolution of their baseline neurological or psychiatric pathology was collected for each of the included subjects. We asked patients to compare their symptoms between the last 6 months of 2019 (before the onset of the COVID-19 pandemic) and different time periods according to their situation: 6 months after infection for patients with symptomatic COVID-19, 6 months after the determination of specific antibodies for asymptomatic COVID-19 patients, and 6 months before inclusion for control patients. Four categories of neurological/psychiatric evolution between the 2 study periods were considered. Improvement, if the patient refers to a decrease in symptoms due to the neurological/psychiatric condition treated at INNNMVS. Stability, if the patient indicates that his or her symptoms are similar or, in the case of degenerative pathologies, the intensity of the worsening is equal during the 2 periods evaluated. Worsening, if the patient refers to an increase in symptoms related to their basal pathology. New neurological/psychiatric symptoms, if new symptoms have appeared between the 2 periods.
Specific antibodies determinationEnzyme-linked immunosorbent assay (ELISA) was used for the semiquantitative determination of human IgG antibodies to the S1 domain of SARSCoV-2 (EUROINMMUN, Germany) in the sera of the included patients. Results were reported semi-quantitatively by calculation of a ratio of the extinction of the patient sample over the extinction of the calibrator. Following the manufacturer's recommendation a ratio ≤ 0.8 was considered negative; a ratio between 0.8 and ≤1.1 was considered borderline, and a ratio > 1.1 was considered positive.
Statistical analysisInformation was collated in an Excel database and statistical analyses were performed using SPSS 25.0 software [IBM Corp, Armonk, New York, USA]. Data were presented as numbers and percentages in the case of dichotomous variables, or as mean ± standard deviation in the case of continuous variables. Parametric or non-parametric analyses were performed according to the normality of the distribution (Kolmogorov–Smirnov test), using the chi-square test or Fisher exact test to compare proportions, and the T-student, Mann–Whitney, or ANOVA test to compare means. Correlation between continuous variables was assessed using Pearson or Spearman tests.
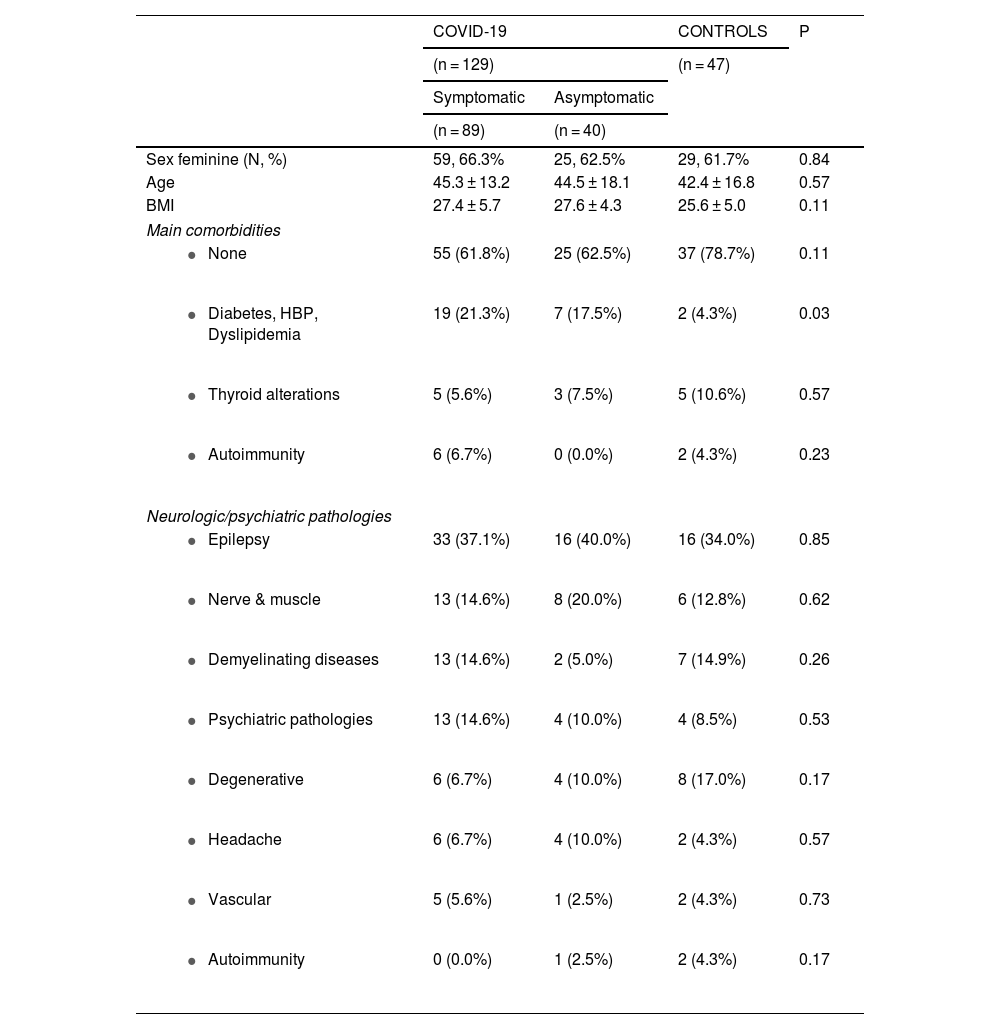
ResultsBetween August and October 2021, 176 neurological/psychiatric patients from the INNNMVS were included in the study, including 129 with a history of COVID-19 (89 symptomatic and 40 asymptomatic) and 47 patients as controls. The main characteristics of the included patients are presented in Table 1. Most were women (n: 113, 64.2%), with no significant difference between the 3 groups. Age and Body Mass Index (BMI) were not significantly different between the groups.
Main characteristics of the 3 groups of neurologic/psychiatric patients included in the study.
| COVID-19 | CONTROLS | P | ||
|---|---|---|---|---|
| (n = 129) | (n = 47) | |||
| Symptomatic | Asymptomatic | |||
| (n = 89) | (n = 40) | |||
| Sex feminine (N, %) | 59, 66.3% | 25, 62.5% | 29, 61.7% | 0.84 |
| Age | 45.3 ± 13.2 | 44.5 ± 18.1 | 42.4 ± 16.8 | 0.57 |
| BMI | 27.4 ± 5.7 | 27.6 ± 4.3 | 25.6 ± 5.0 | 0.11 |
| Main comorbidities | ||||
| 55 (61.8%) | 25 (62.5%) | 37 (78.7%) | 0.11 |
| 19 (21.3%) | 7 (17.5%) | 2 (4.3%) | 0.03 |
| 5 (5.6%) | 3 (7.5%) | 5 (10.6%) | 0.57 |
| 6 (6.7%) | 0 (0.0%) | 2 (4.3%) | 0.23 |
| Neurologic/psychiatric pathologies | ||||
| 33 (37.1%) | 16 (40.0%) | 16 (34.0%) | 0.85 |
| 13 (14.6%) | 8 (20.0%) | 6 (12.8%) | 0.62 |
| 13 (14.6%) | 2 (5.0%) | 7 (14.9%) | 0.26 |
| 13 (14.6%) | 4 (10.0%) | 4 (8.5%) | 0.53 |
| 6 (6.7%) | 4 (10.0%) | 8 (17.0%) | 0.17 |
| 6 (6.7%) | 4 (10.0%) | 2 (4.3%) | 0.57 |
| 5 (5.6%) | 1 (2.5%) | 2 (4.3%) | 0.73 |
| 0 (0.0%) | 1 (2.5%) | 2 (4.3%) | 0.17 |
*BMI: Body Mass Index. HBP: High Blood Pressure.
Most patients included in each of the 3 groups did not have comorbidities. Interestingly, the frequency of vascular risk factors (diabetes, hypertension, and dyslipidemia) was significantly different between the 3 groups (P = 0.03), with a higher frequency in symptomatic and asymptomatic COVID-19 patients compared with the control group (P = 0.01).
Epilepsy was the most frequent basal neurological/psychiatric pathology (n = 65, 36.9%) presented by the patients. Nerve or muscle pathologies occurred in 27 patients (15.3%). These pathologies were neuropathy (17 patients), radiculopathy (5), myopathy (3), myasthenia (1), and dystonia (1). Demyelinating diseases were present in 22 patients (12.5%): 10 with multiple sclerosis, 5 with optic neuritis, 5 with neuromyelitis optica, and 1 with demyelinating polyneuropathy. Psychiatric conditions occurred in 21 patients (11.9%); the pathologies were mixed anxiety and depressive disorders (6 patients), anxiety disorders (4), affective disorders (4), depressive disorders (3), schizophrenia (3), and 1 borderline personality disorder. Degenerative diseases were present in 18 patients (10.2%); 10 of them had Parkinson's disease, 7 had dementia, and 1 had essential tremor. Twelve patients (6.8%) consulted for headaches. Five of them had a primary headache (migraine) and the other 7 had a secondary headache, a sequela of a previous neurological condition (mainly treated benign tumors and inactive neurocysticercosis). Eight patients (4.5%) were followed for vascular diseases. These were cerebrovascular malformations (4), ischemic stroke (3), and aneurysm. The last 3 patients were followed for autoimmune encephalitis. As shown in Table 1, the frequency of each pathology was not different among the 3 groups of patients.
Of the 89 symptomatic COVID-19 patients, 57 (64.0%) had a confirmed diagnosis of COVID-19 because they had a positive PCR test and compatible symptomatology. The rest (32, 36.0%) had a probable diagnosis of COVID-19 according to WHO clinical criteria (all had symptoms compatible with COVID-19 after close contact with a confirmed COVID-19 case). Regarding the severity of COVID-19, most patients had mild infection (75, 84.3%), while 8 (9.0%) developed moderate disease, 4 (4.5%) severe disease, and 2 (2.2%) critical disease. The time from COVID-19 disease to inclusion ranged from 1 to 18 months (mean: 6.8 months, SD: 6.8), and 35 (39.3%) had received at least 1 dose of vaccine at inclusion. Specific antibodies were found in 69 of the 80 patients tested (86.2%), with a ratio of 5.62 (±3.3); the mean antibody ratio was significantly higher (P < 0.0001) in vaccinated patients (8.1 ± 2.0) compared with non-vaccinated (4.2 ± 3.1). Considering only unvaccinated patients, a significant negative correlation between antibody levels and time from infection to inclusion was observed (R = -0.37; P = 0.01). Comparing the evolution of their neurological/psychiatric symptoms between the 2 evaluated periods, 43 patients (48.3%) reported stability, 35 patients (39.3%) worsening, and 11 (12.3%) the appearance of new neurological/psychiatric symptoms (Table 2). The latter were: headache (n = 4), sleep disorders (n = 2), anxiety (n = 2), 1 case of vertigo, 1 case of cognitive impairment, and 1 case of tinnitus.
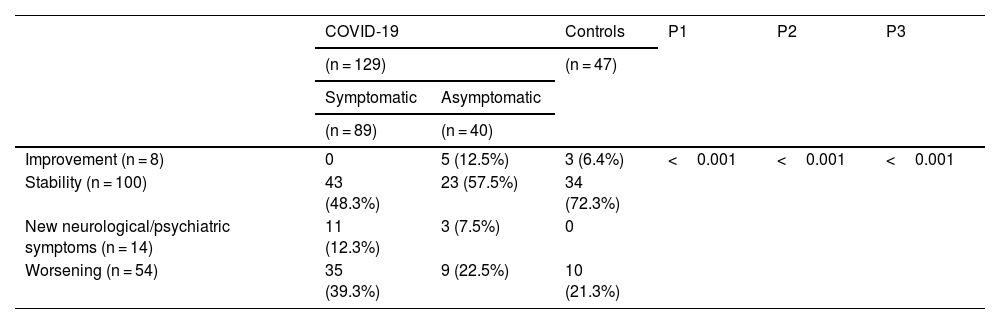
Evolution of neurological / psychiatric symptomatology in the 3 groups of evaluated patients.
| COVID-19 | Controls | P1 | P2 | P3 | ||
|---|---|---|---|---|---|---|
| (n = 129) | (n = 47) | |||||
| Symptomatic | Asymptomatic | |||||
| (n = 89) | (n = 40) | |||||
| Improvement (n = 8) | 0 | 5 (12.5%) | 3 (6.4%) | <0.001 | <0.001 | <0.001 |
| Stability (n = 100) | 43 (48.3%) | 23 (57.5%) | 34 (72.3%) | |||
| New neurological/psychiatric symptoms (n = 14) | 11 (12.3%) | 3 (7.5%) | 0 | |||
| Worsening (n = 54) | 35 (39.3%) | 9 (22.5%) | 10 (21.3%) | |||
P1: Evaluation of differences between the 3 groups of patients and the 4 variables / P2: Evaluation of differences between symptomatic COVID-19 patients versus asymptomatic COVID-19 patients + controls, on the 4 variables / P3: Assessment of differences between symptomatic COVID-19 patients and asymptomatic COVID-19 patients + controls with respect to symptom stability and improvement versus worsening and new symptoms.
All 40 asymptomatic COVID-19 patients were included on the basis of the presence of specific antibodies in their serum before any vaccination. The mean ratio was 5.1, SD 2.8, which was not significantly different from the symptomatic COVID-19 patients (P = 0.34). Although most asymptomatic COVID-19 patients were surprised by the notification of previous SARS-Cov2 infection, 25 (62.5%) acknowledged contact with a COVID-19 patient or activities that could promote infection (e.g., use of public transportation). Comparing the evolution of their symptoms between the 2 evaluated periods, most patients considered their neurological/psychiatric symptoms to be stable (23, 57.5%), 9 (22.5%) considered them to be worsening, 5 (12.5%) to be improving, and 3 (7.5%) reported the appearance of new symptoms (Table 2). The latter were 1 case of anxiety, 1 case of depression, and 1 case of alteration in facial sensitivity.
Regarding the patients included in the control group, most of them (34; 72.3%) considered that their neurological/psychiatric symptoms remained stable, 10 (21.3%) reported worsening, and 3 (6.4%) improving. (Table 2).
When comparing between the 3 groups of patients the 4 possibilities of evolution of their symptoms (improvement, stability, development of new symptoms, and worsening), clear significant differences were found (P < 0.001) (Table 2). The difference was also significant if we compared the 4 evolutions between 2 groups of patients, symptomatic COVID-19 versus asymptomatic and controls (P < 0.001). The same trends were obtained when comparing both improved and stable patients to patients with new symptoms and worsened patients (P < 0.001), and when comparing worsened patients alone to the 3 other categories (P = 0.01).
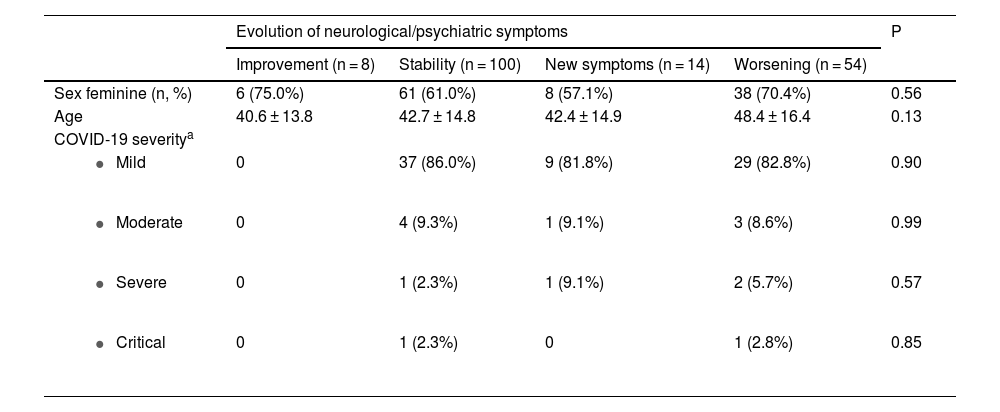
Patient age and sex were similar in the 4 patient groups defined by type of neurological/psychiatric symptom progression (Table 3). There was no significant relationship between COVID-19 severity and type of neurological/psychiatric symptom progression (Table 3).
Main characteristics of the patients considering the type of evolution of their basal neurological/psychiatric diseases.
| Evolution of neurological/psychiatric symptoms | P | ||||
|---|---|---|---|---|---|
| Improvement (n = 8) | Stability (n = 100) | New symptoms (n = 14) | Worsening (n = 54) | ||
| Sex feminine (n, %) | 6 (75.0%) | 61 (61.0%) | 8 (57.1%) | 38 (70.4%) | 0.56 |
| Age | 40.6 ± 13.8 | 42.7 ± 14.8 | 42.4 ± 14.9 | 48.4 ± 16.4 | 0.13 |
| COVID-19 severitya | |||||
| 0 | 37 (86.0%) | 9 (81.8%) | 29 (82.8%) | 0.90 |
| 0 | 4 (9.3%) | 1 (9.1%) | 3 (8.6%) | 0.99 |
| 0 | 1 (2.3%) | 1 (9.1%) | 2 (5.7%) | 0.57 |
| 0 | 1 (2.3%) | 0 | 1 (2.8%) | 0.85 |
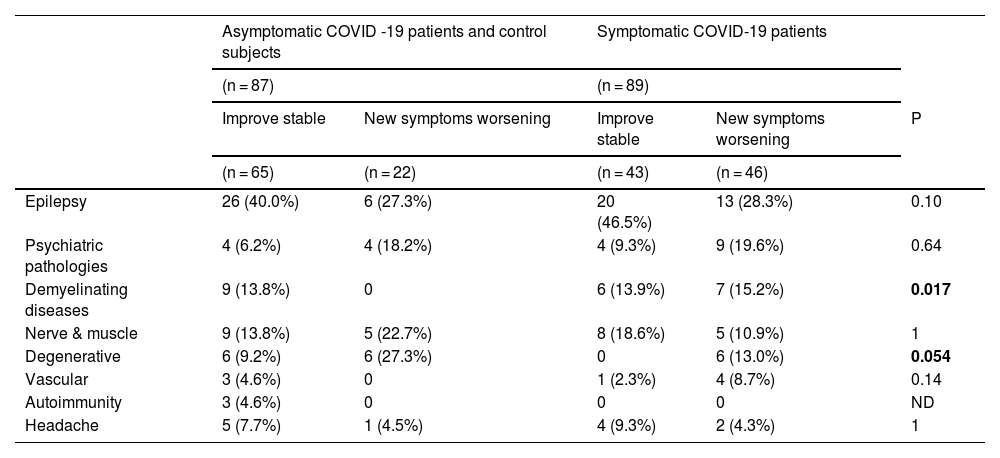
When comparing symptomatic COVID-19 patients with asymptomatic COVID-19 patients and control patients together, patients with demyelinating diseases showed significantly greater worsening or development of new symptoms (P = 0.017); a similar trend was obtained for patients with degenerative diseases (P = 0.054) (Table 4). The same comparison did not reveal significant differences for other pathologies (Table 4).
Comparison of the course of different neurological/psychiatric diseases between the group of patients with a history of symptomatic COVID-19 and the group of patients composed by those with a history of asymptomatic COVID-19 and the controls.
| Asymptomatic COVID -19 patients and control subjects | Symptomatic COVID-19 patients | ||||
|---|---|---|---|---|---|
| (n = 87) | (n = 89) | ||||
| Improve stable | New symptoms worsening | Improve stable | New symptoms worsening | P | |
| (n = 65) | (n = 22) | (n = 43) | (n = 46) | ||
| Epilepsy | 26 (40.0%) | 6 (27.3%) | 20 (46.5%) | 13 (28.3%) | 0.10 |
| Psychiatric pathologies | 4 (6.2%) | 4 (18.2%) | 4 (9.3%) | 9 (19.6%) | 0.64 |
| Demyelinating diseases | 9 (13.8%) | 0 | 6 (13.9%) | 7 (15.2%) | 0.017 |
| Nerve & muscle | 9 (13.8%) | 5 (22.7%) | 8 (18.6%) | 5 (10.9%) | 1 |
| Degenerative | 6 (9.2%) | 6 (27.3%) | 0 | 6 (13.0%) | 0.054 |
| Vascular | 3 (4.6%) | 0 | 1 (2.3%) | 4 (8.7%) | 0.14 |
| Autoimmunity | 3 (4.6%) | 0 | 0 | 0 | ND |
| Headache | 5 (7.7%) | 1 (4.5%) | 4 (9.3%) | 2 (4.3%) | 1 |
Neurological complications associated with COVID-19 have been reported in most patient series. This is now well recognized, although the importance of the different mechanisms possibly involved is not yet precisely known.
However, the consequences of COVID-19 on previous neurological/psychiatric diseases have not been clearly evaluated.
In our study, different interesting results were obtained. First, we found that a significant proportion (30.7%) of neurological and psychiatric patients, regardless of their COVID-19 status, considered that their pathology had worsened and 7.5% had developed new symptoms. This feeling was significantly more frequent in symptomatic COVID-19 patients (39.3% for worsening and 12.3% for developing new symptoms) than in the asymptomatic COVID-19 groups and controls (21.8% for worsening and 3.4% for new symptoms; P < 0.001). Furthermore, we found that this worsening was not related to the severity of COVID-19 infection, and that the neurological conditions that worsened most frequently, when comparing symptomatic COVID-19 patients with asymptomatic COVID-19 patients and controls, were demyelinating and degenerative diseases.
The impact of the COVID-19 pandemic, independently from COVID-19 infection, on patients with neurological/psychiatric pathologies has been observed in different studies. This has been well seen in patients affected by dementia,19,23,24 multiple sclerosis,25 chronic pain,26 Parkinson's disease,20 cerebellar ataxia,21 neuropsychiatric disorders,22 headache,27 and epilepsy.28 The 2 main factors implicated in the worsening of neurological/psychiatric illnesses are lockdown and the associated social isolation, and lack of comprehensive treatment. Experiencing the COVID-19 pandemic has been shown to affect the mood of all individuals in this condition, whether or not they have presented COVID-19.29 Anxiety, depression, and distress have increased, and in this sense, it has been shown that post-COVID-19 neurological symptoms can be affected by non-neurological factors such as diagnosis threat.30 The worsening, in our study, of neurological/psychiatric conditions reported by controls and patients affected by asymptomatic COVID-19 is most likely related to these factors.
On the other hand, we found that patients who had symptomatic COVID-19 had a significant increase in neurological symptoms or the development of new neurological symptoms, compared with the asymptomatic COVID-19 group or the control group. Anxiety and depression levels have been found to be elevated in patients who were affected by COVID-19,31 and a study comparing these symptoms in infected and uninfected health care workers showed that these symptoms were significantly higher in infected individuals.32 This excess of mood affectation in symptomatic COVID-19 patients could explain our results, although we cannot rule out a direct affectation of the nervous system by the pathogenesis of COVID-19. Viral particles have been found to persist in patients long after infection33 and the neuroinflammatory process can become chronic.34 The findings of no association between COVID-19 severity and frequency of worsening that we observed in our study are contrary to this hypothesis, but the fact that the patients who worsened the most were patients with demyelinating and degenerative diseases, 2 conditions in which neuroinflammation is clearly linked to pathology, could be a sign of the validity of this hypothesis.
In conclusion, neurological patients, like all people who have experienced the COVID-19 pandemic, were affected by the situation. Social isolation, stress, and lack of adequate care are obviously involved in the worsening of the situation. Although these factors are probably also involved in the worsening of the condition of patients who presented COVID-19, we cannot rule out the involvement of a direct neurological affectation by SARS-CoV2. Long-term follow-up of these patients is needed to better understand the factors involved.
Ethical considerationsThe authors declare that they have followed their center's protocols on the publication of patient data and have obtained the corresponding permissions.
Conflict of interestThe authors declare no competing interests.
FundingThis work was funded by the Programa de Apoyo a Proyectos de Investigación e Innovación Tecnológica (PAPIIT, DGAPA, UNAM), Project number: IN206921.
We gratefully acknowledge the neurologists and psychiatrists at INNNMVS who assisted us in including patients in this study by referring their patients with a history of SARS-Cov-2 infection. We acknowledge Mario Contreras Fleury for English copyediting.