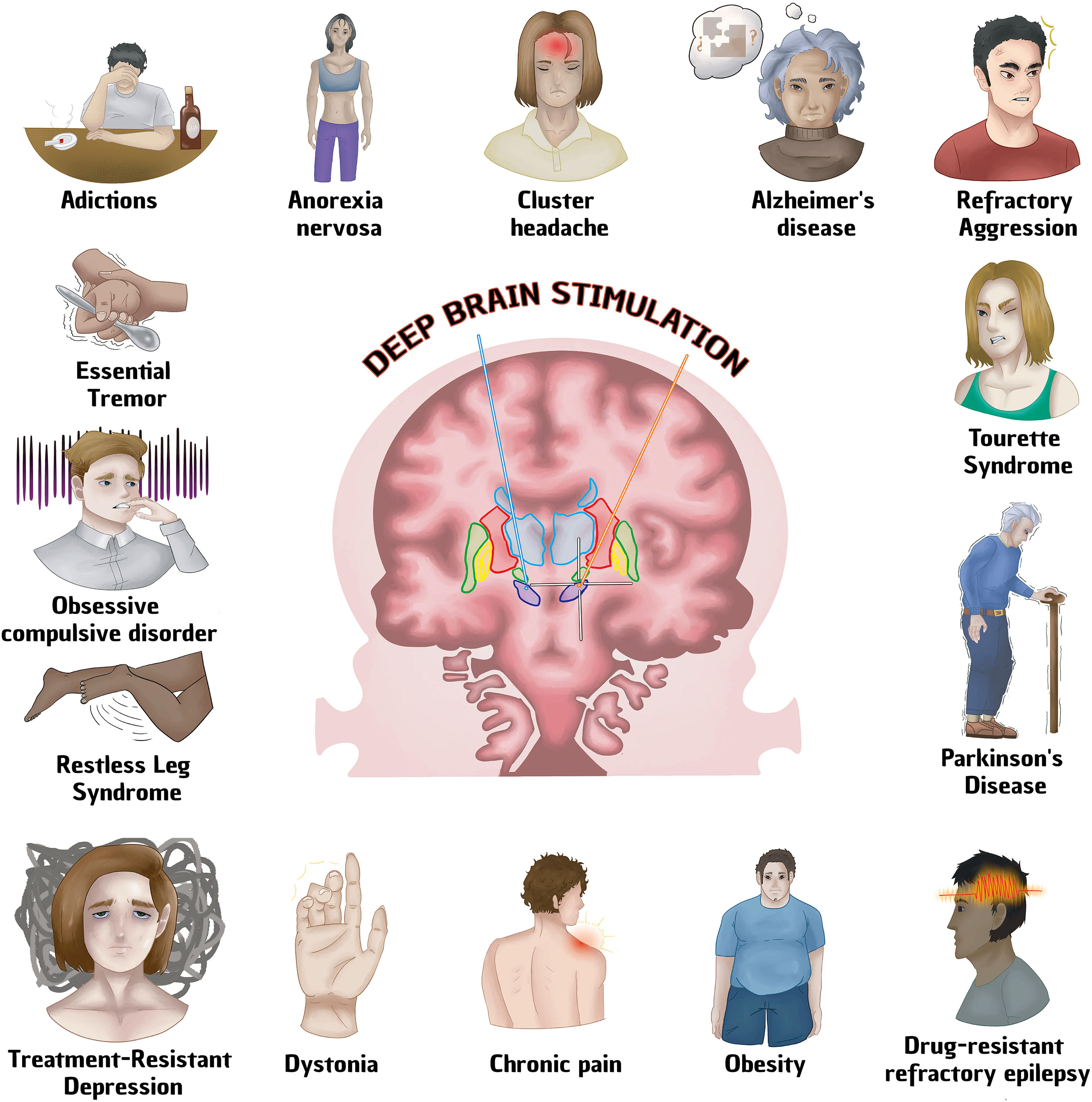
Deep brain stimulation (DBS) is a surgical procedure used to treat various neurological pathologies, being its greatest use in movement disorders. The FDA first approved deep brain stimulation in 1997 to treat essential tremor, in 2002 it was approved for Parkinson's disease, in 2003 for dystonia and in 2009 for obsessive compulsive disorder. However, until recently this technique began to be implemented for the treatment of other neurological diseases. To conduct research on the different neurological diseases where deep brain stimulation is used articles were chosen from Pubmed, Google Scholar, Redalyc and Scielo databases, references from the last 10 years to date were taken. The keywords that were written in the search engine were ECP/ECP + the required pathology. 75 references were found on the use of DBS in the following pathologies: Parkinson's disease, Alzheimer's, refractory epilepsy, depression, obsessive–compulsive disorder, Gilles Tourette syndrome, aggressiveness, addictions, anorexia nervosa, restless legs syndrome, headache, dystonia, essential tremor and obesity. The use of DBS is growing as technology advances, increasingly focused on neurological diseases, psychosurgery and even systemic diseases, however, its use is only approved by the FDA for some movement disorders, including Parkinson's disease, Dystonia, essential tremor and OCD.
La estimulación cerebral profunda (ECP) es un procedimiento quirúrgico utilizado para tratar diversas patologías neurológicas, siendo su mayor uso en los trastornos del movimiento. La FDA aprobó por primera vez la estimulación cerebral profunda en 1997 para tratar el temblor esencial, en 2002 fue aprobada para la enfermedad de Parkinson, en 2003 para la distonía y en 2009 para el trastorno obsesivo compulsivo. No obstante, hasta hace poco tiempo se empezó a implementar esta técnica para el tratamiento de otras enfermedades neurológicas. Para realizar esta investigación sobre las diferentes enfermedades neurológicas en las que se utiliza la estimulación cerebral profunda se eligieron artículos de las bases de datos Pubmed, Google Scholar, Redalyc y Scielo, se tomaron referencias de los últimos 10 años a la fecha. Las palabras claves que se escribieron en el buscador fueron ECP/ECP + + la patología requerida. Se encontraron 75 referencias sobre el uso de ECP en las siguientes patologías: enfermedad de Parkinson, Alzheimer, epilepsia refractaria, depresión, trastorno obsesivo-compulsivo, síndrome de Gilles Tourette, agresividad, adicciones, anorexia nerviosa, síndrome de piernas inquietas, cefalea, distonía, temblor esencial y obesidad. El uso de ECP crece a medida que avanza la tecnología, cada vez más enfocado en enfermedades neurológicas, psicocirugía e incluso enfermedades sistémicas, sin embargo, su uso solo está aprobado por la FDA para algunos trastornos del movimiento, incluida la enfermedad de Parkinson, la distonía, el temblor esencial y TOC.
According to the National Institute of Neurological Disorders and Stroke (NINDS), deep brain stimulation (DBS) is a surgical procedure used to treat multiple neurological diseases, especially movement disorders that do not adequately respond to medical treatment. It consists of a surgically implanted battery-powered device called a neurostimulator (similar to a cardiac pacemaker), which sends electrical stimulation to the specific area desired, blocking abnormal nerve signals.1 The FDA (Food and Drug Administration) approved deep brain stimulation for the first time in 1997 in order to treat essential tremor, in 2002 it was approved for Parkinson's disease, in 2003 for dystonia and in 2009 for obsessive–compulsive disorder. It is currently the Gold Standard in the treatment of drug-resistant Parkinson's disease, leaving behind ablative surgical procedures.2,3 DBS has also been used to treat mental disorders with promising results such as depression, obsessive–compulsive disorder, Gilles Tourette syndrome, aggressiveness, addictions, anorexia nervosa, restless legs syndrome.4 DBS has several advantages over surgical procedures: reversibility, programming option and the possibility of readjusting parameters according to the progression of the neurodegenerative disease. However, it also has disadvantages such as: high cost, longer availability of time from the patient compared to other techniques, technical complications that require specialized personnel.5 Although DBS has been used frequently in Parkinson's disease (including the advanced stage of the disease),5 the action mechanism in the brain is not known with full certainty. It is believed to act by inhibiting the neurons around the electrode and exciting the fiber tracts. The pathways involved are the cerebellar-thalamic (reducing tremor), nigrostriatal (increasing dopamine) and the zone of uncertainty (involved in any motor symptom). In general, the involvement of several factors that make the treatment efficient have been mentioned, such as: Treatment of the nosological entity with adequate stimulation parameters and stimulation of the cytoarchitecture of the brain structure (generally subcortical), in this way, they produce inhibition in structures that are rich in cell bodies or excitement where the nerve bundles meet, thus DBS performs synchronization of an abnormal pattern or on the other hand, it could create a “background noise” that would interrupt abnormal functioning patterns.6 Other authors report that clinical improvement is gradual, which suggests that brain neuroplasticity plays an important role in DBS.7–9 (Table 1, Fig. 1).
Diseases treated with Deep Brain Stimulation. See text for more details.
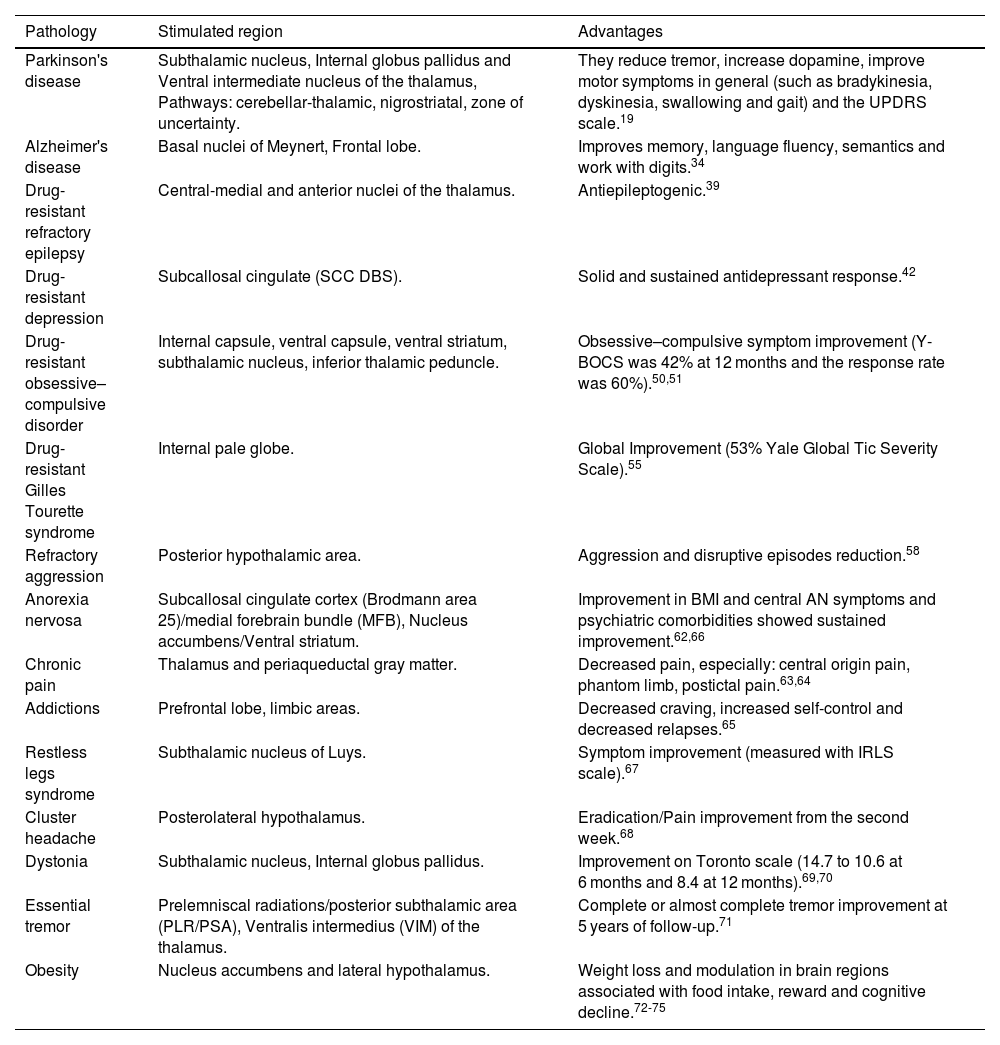
| Pathology | Stimulated region | Advantages |
|---|---|---|
| Parkinson's disease | Subthalamic nucleus, Internal globus pallidus and Ventral intermediate nucleus of the thalamus, Pathways: cerebellar-thalamic, nigrostriatal, zone of uncertainty. | They reduce tremor, increase dopamine, improve motor symptoms in general (such as bradykinesia, dyskinesia, swallowing and gait) and the UPDRS scale.19 |
| Alzheimer's disease | Basal nuclei of Meynert, Frontal lobe. | Improves memory, language fluency, semantics and work with digits.34 |
| Drug-resistant refractory epilepsy | Central-medial and anterior nuclei of the thalamus. | Antiepileptogenic.39 |
| Drug-resistant depression | Subcallosal cingulate (SCC DBS). | Solid and sustained antidepressant response.42 |
| Drug-resistant obsessive–compulsive disorder | Internal capsule, ventral capsule, ventral striatum, subthalamic nucleus, inferior thalamic peduncle. | Obsessive–compulsive symptom improvement (Y-BOCS was 42% at 12 months and the response rate was 60%).50,51 |
| Drug-resistant Gilles Tourette syndrome | Internal pale globe. | Global Improvement (53% Yale Global Tic Severity Scale).55 |
| Refractory aggression | Posterior hypothalamic area. | Aggression and disruptive episodes reduction.58 |
| Anorexia nervosa | Subcallosal cingulate cortex (Brodmann area 25)/medial forebrain bundle (MFB), Nucleus accumbens/Ventral striatum. | Improvement in BMI and central AN symptoms and psychiatric comorbidities showed sustained improvement.62,66 |
| Chronic pain | Thalamus and periaqueductal gray matter. | Decreased pain, especially: central origin pain, phantom limb, postictal pain.63,64 |
| Addictions | Prefrontal lobe, limbic areas. | Decreased craving, increased self-control and decreased relapses.65 |
| Restless legs syndrome | Subthalamic nucleus of Luys. | Symptom improvement (measured with IRLS scale).67 |
| Cluster headache | Posterolateral hypothalamus. | Eradication/Pain improvement from the second week.68 |
| Dystonia | Subthalamic nucleus, Internal globus pallidus. | Improvement on Toronto scale (14.7 to 10.6 at 6 months and 8.4 at 12 months).69,70 |
| Essential tremor | Prelemniscal radiations/posterior subthalamic area (PLR/PSA), Ventralis intermedius (VIM) of the thalamus. | Complete or almost complete tremor improvement at 5 years of follow-up.71 |
| Obesity | Nucleus accumbens and lateral hypothalamus. | Weight loss and modulation in brain regions associated with food intake, reward and cognitive decline.72-75 |
Regardless of the cause of the improvement, our objective is to carry out an updated search of the main pathologies that are treated with DBS, their target brain structure for stimulation and what type of improvement is reported.
For this purpose, we have used the following search algorithm in the databases of PubMed, Google Scholar, Redalyc and Scielo (in that order): The keyword was “Deep Brain Stimulation” or “Deep Brain Stimulation + Pathology”, the search was performed only in the first 10 pages from the database search engine, the articles that provided a new pathology whose treatment was DBS and that had not been previously found were taken.
Neurological diseasesParkinson's diseaseParkinson's disease (PD) is a neurodegenerative disease characterized by clinical manifestations that include bradykinesia (slowing of movements), tremor (at rest), rigidity (increased tone) and postural instability whose pathophysiology is related to the loss of dopaminergic neurons in the substantia nigra pars compacta.10 It is currently considered the second cause of neurodegenerative disease only after Alzheimer's11 and, on the other hand, Parkinsonism is considered to be more common.12 The average life of patients with PD is between 11 and 11.5 years,13,14 although currently there has been a gradual increase in mortality related to pneumonia, cardiovascular diseases and cancer.15,16 Similarly to any other neurodegenerative disease, Parkinson's disease progresses to the point of leaving the patient totally disabled, especially due to neuropsychiatric disorders, and the presence of a caregiver becomes necessary.17 When this happens, it is no longer possible to perform DBS, so it is essential that its natural history is taken into account at the early stages of the pathology in order to predict whether the patient is a candidate for DBS and perform the surgery in a timely manner. The characteristics of a good candidate are: 1. - Good response to dopaminergic treatment. 2. - Presence of on–off fluctuations. 3. - Dyskinesia. 4. - Tremor. 5. - Absence of dementia. 6. - Absence of co-morbidity diseases that reduce the quality of life. 7. - Adequate state of mind.18 DBS is primarily used in patients with refractory Parkinson's disease (which persists despite treatment); it is specifically used in the subthalamic nucleus (STN), Globus Pallidus Internus (GPI) and Ventral Intermediate (VIM) Nucleus of the Thalamus.19 In the subthalamic nucleus, activation of the indirect pathway decreases and the direct pathway of the basal ganglia increases, which allows increased communication between the basal ganglia–thalamus–premotor cortex, activating the latter. From this, there is improvement of sensory signals processing, initiation of movement, verbal skills, visual memory and face recognition, and increase in empathy and mood changes have also been reported. In the internal globus pallidus, it modulates the direct and indirect pathway of the basal ganglia in such a way that the basal ganglia–thalamus–cortex communication also increases, having similar effects to the previous technique, but with an emphasis on dystonia inhibition, and the improvement of swallowing and gait. Regarding the VIM Nucleus of the Thalamus, given that the fibers of the basal ganglia pass through the thalamus, there is decreased activity in the premotor, association and primary sensory-motor areas, yielding the clinical effect of reduced refractory tremor from therapy.20–23 Current evidence shows that DBS has demonstrated significant benefits when compared to pharmacology, where most of these studies are on the STN, including the following two outstanding references:
Weaver et al. compared the effect of DBS and Pharmacology in a sample of 255 patients. In 60 patients bilateral DBS of the STN was performed, 61 had DBS of the GPI and 134 only received pharmacological therapy. The STN group presented less on time with dyskinesias compared to the GPI and pharmacological therapy (between group mean difference of 4.5 h/day; 95% CI, 3.7–5.4; p < 0.001). On the other hand, when comparing the pharmacological therapy group vs DBS without medication after 6 months, it was observed that the latter had an improvement of 12.3 points on the UPDRS scale in motor function and small declines in neurocognitive tests, compared to pharmacological therapy that had an improvement, possibly be due to the fact that the decrease in dopamine is compensated by the treatment, unlike DBS, which only regulates certain structures.24
Deushl et al. compared DBS of the STN plus pharmacology (DBS-F) versus Pharmacology (F) without DBS, through a randomized clinical trial that included 156 patients with advanced PD and severe motor symptoms. The severity of symptoms was evaluated without pharmacological treatment using the UPDRS-III scale with an improvement of 19.6 points. After neurostimulation, there was a significant increase in mobility (p < 0.001), daily living activities (p < 0.001) and emotional well-being (0.001). On the other hand, adverse events were higher (p < 0.05) with DBS-F as expected after surgery, including fatal intracerebral hemorrhage.25
In general, the most frequent complications are Staphylococcus aureus infections with a prevalence of 0–15%.26 After that, there is intraventricular/intracerebral hemorrhage in the implant trajectory with a 10% frequency, which is generally self-limiting and asymptomatic.27 Neurological adverse effects include cerebrovascular events, rigidity (paradox), partial seizures and confusion, as well as manic, hypomanic or depressive events.28–30 Other adverse effects found were deep vein thrombosis, increased body mass, skin erosion in the pulse generator area, device migration, device cable breakage, adhesions and death. The implant cost at the moment ranges from $4184 to $29,178 dollars in the first year, after which it increases to an average of $1490 dollars per year.31
Speech and language disorders of Parkinson's diseaseAs shown in the previous section, deep brain stimulation is already well established in common clinical manifestations of Parkinson's disease (tremor, rigidity)32; however, PD is not limited to motor functions, so different frequencies have been explored. To improve language disorders, which is paradoxical, since according to Zhang et al. (2019), deep brain stimulation of the subthalamic nucleus (STN-DBS) at a fixed high frequency (>100 Hz), improves primary motor symptoms, although this stimulation has no effect or it may even exacerbate symptoms of advanced PD such as gait or speech problems. In turn, a fixed lower frequency (<100 Hz) may improve speech and gait but worsen PD tremor; whose problem is resolved with the application of variable frequency stimulation that contains a combination of high frequencies only, which they observed in only one patient.33
Alzheimer's diseaseAs a definition, Alzheimer's disease (AD) has different approaches, where its first definition from 1984 designates it as an acquired and progressive amnesic dementia that is clinically diagnosed and whose etiology is unknown. Another more complete clinical–pathological definition would be: chronic-degenerative disease linked to amnesic dementia and to the neuropathology of neuritic plaques containing β-amyloid and neurofibrillary tangles.34 It is currently considered the most common cause of dementia.35 Of course, being the most prevalent disease, it could not be left aside, at least that was possibly what Kuhn et al. thought in 2015 when stimulating the basal nucleus of Meynert, which have been related to memory in this pathology. In said article, data were collected from patients with moderate to severe cognitive impairment, aged 57–79 years, for a total of 6 patients, 2 men and 4 women, who underwent bilateral DBS, whose procedure consisted of inserting electrodes in the basal nucleus of Meynert for subsequent stimulation for one year. In the first month, three patients received DBS for the first 2 weeks and the other two remained without stimulation, the other three patients received inverse DBS. The remaining 11 months continuous DBS was performed with low frequency in all patients, four of six patients had a positive response to treatment as they experienced improvements in neuropsychological tests such as language fluency, semantics and work with digits. In other tests, such as those for depression, apraxia or dementia, there do not seem to be differences between the presence or absence of treatment. However, when the patients began to become dependent, there were no responses even with DBS.34 In contrast to this article, Scharre et al. in 2017 evaluated the efficacy of DBS in the ventral capsule and ventral striatum to specifically modulate frontal lobe cognitive and behavioral networks as a new treatment approach for Alzheimer's disease (AD), despite being limited in the number of patients they used, they concluded that stimulation of frontal behavior and cognitive neural networks in AD patients is a promising treatment modality that should be further studied.36,37
Drug-resistant refractory epilepsyAccording to the WHO, epilepsy is defined as a chronic brain disease characterized by two or more unprovoked seizures. These seizures are involuntary movements that affect a part of the body (partial seizures), or the whole body (generalized seizures), this may be accompanied by loss of consciousness and/or loss of sphincter control.38 Refractory Epilepsy refers to when a patient has used at least two anticonvulsants with correct indications according to the type of seizure and adequate doses in monotherapy or polytherapy without reaching a seizure-free state.39 The prevalence of epilepsy worldwide ranges between 1 and 2% of the population; approximately 8–17 epileptics per 1000 inhabitants. 80% of all epileptic patients are controlled with medical treatment. The other 20% are medically untreatable and, of these, 5–10% are eligible for epilepsy surgery, which is why epileptic patients may be a good target for DBS treatment. Torres et al. (2011), in a review article on DBS in the thalamus for refractory epilepsy, specifically in the central-medial and anterior nuclei of the thalamus, conclude that, at least in animal models, there has been a favorable result. However, more randomized double-blind studies are needed in humans with a larger sample.40–45
Drug-resistant depressionDepression is defined as a mental disorder characterized by loss of interest or pleasure, feelings of guilt or lack of self-esteem, the presence of sadness, a feeling of tiredness, sleep or appetite disorders, and lack of concentration.41 The prevalence rate of depressive disorders is 300 million people worldwide, affecting more than 17 million people in the United States, these disorders are a major cause of years lived with disability. Among patients with major depression, an estimated one-third have treatment resistance. Drug-resistant depression is usually defined as the persistence of symptoms when treated with two or more antidepressants. Subcallosal cingulate deep brain stimulation (SCC DBS) has been studied as a potential treatment for severe and refractory (drug-resistant) major depressive disorder since 2005. The authors of one study used an open-label, long-term follow-up design to examine participants enrolled in a clinical trial of SCC DBS for treatment-resistant depression. Response and remission rates were maintained at ≥50% and ≥30%, respectively, during the 2–8 year follow-up period, where treatment was generally safe and well tolerated, and there were no side effects from acute or chronic stimulation. The authors concluded that, over 8 years of observation, most participants experienced a robust and sustained antidepressant response to SCC DBS.42,46,47
Drug-resistant obsessive–compulsive disorderObsessive–compulsive disorder (OCD) is defined as a neurological disease characterized by obsessions in the form of recurrent thoughts, in response to which they have behaviors called compulsions due to the need to repeat an action.48 According to the World Health Organization, OCD is among the top 20 disease-related causes of disability in people ages 15–44.49 Despite extensive use of optimal cognitive behavioral therapy and pharmacological treatment algorithms, it is estimated that 10% of OCD patients do not optimally respond to therapies and suffer severe symptoms leading to marked functional impairment, and for this group of patients, DBS has been tested on targets such as: ventral capsule, ventral striatum (nucleus accumbens septi, deep portions of the olfactory tubercle similar to the striatum and ventral parts of the caudate nucleus and putamen), subthalamic nucleus, inferior thalamic peduncle. However, the FDA-approved indication is stimulation of the inferior portion of the internal capsule.50,51 On the other hand, in a prospective, interventional open-label, multicenter study aimed at monitoring the safety and efficacy of electrical stimulation of the AIC in patients with chronic, severe and treatment-resistant OCD where safety, efficacy and functionality at 3, 6 and 12 months after implant concluded that as far as efficacy measures, the Y-BOCS reduction was 42% at 12 months and the response rate was 60%. Although some serious adverse effects occurred, most adverse effects were mild or moderate, transient and related to programming/stimulation and tended to be resolved with stimulation adjustment. They concluded that, in a severely treatment-resistant population, the open-label study supports that the potential benefits outweigh the potential risks of DBS.52
Drug-resistant Gilles Tourette syndromeTourette syndrome is defined as a neurological disease characterized by repetitive, involuntary and stereotyped movements which are accompanied by tics (the most notable being the emission of vocal sounds).53 Although DBS is not approved for Tourette syndrome in the United States and other countries, multiple single reports and a series of studies have collectively demonstrated that DBS could be a potentially valuable therapy for select cases of drug-resistant Tourette syndrome. A recent systematic review and meta-analysis of 57 studies including 156 cases of DBS for Tourette syndrome reported an overall improvement of 53%, as measured by the Yale Global Tic Severity Scale (YGTSS) total score.54 Stimulation has been practiced on the internal globus pallidus.55 In another study, where the international deep brain stimulation database and registry enrolled 185 patients (out of whom 171 have available data, 37 women and 134 men; age mean [SD] at surgery, 29.1 [10.8] years [range, 13–58 years]) concluded that deep brain stimulation was associated with symptomatic improvement in patients with Tourette syndrome but also with important adverse events.56
Refractory aggressionAggressiveness is related to different intensity attack patterns that may involve physical, verbal, facial, indirect and sexual attacks, it is usually innate and it is regulated by the limbic system. In human beings, it is considered pathological when the response to the triggering stimulus of said behavior does not match and it is magnified. This may be the most important pathology to be treated in psychiatric patients, since it leads to self-inflicted harm and/or to the people around them. Until now, the handling of pathological aggressiveness has been conducted through psychiatry with the use of drugs; however, there are patients who are resistant to any therapy (refractoriness) and/or who develop adverse metabolic effects.57 In the study by Franzini et al., an attempt was made to describe deep brain stimulation used in the treatment of aggressive and disruptive behavior refractory to conservative treatment, with stereotactic methodology and under general anesthesia, where seven patients (from 2002 to 2010) received DBS in the posterior hypothalamic region, bilaterally, and with the aid of intraoperative microrecording. Six of the seven patients presented a clear reduction in the aggression and disruptive bouts, with subsequent simplification of familiar management, concluding that DBS is an effective treatment.58
Anorexia nervosaAnorexia nervosa (AN) is defined as a restriction of energy intake relative to the body's requirements, leading to low body weight associated with distorted body image and anxiety regarding weight gain. This has the highest mortality rate among all psychiatric disorders.59–61 Current treatment options include physiological therapies, nutritional support and various psychopharmacological treatments. Due to the low success rate of long-term treatment, new treatment options such as DBS are needed. Alterations in cortico–striatal–thalamic loop circuit activity and its components that comprise the excellent response-based pathway found in OCD and other compulsive disorders are commonly present in AN. Brain areas associated with sensory response and processing may be effective DBS targets in individuals with treatment-refractory AN. Modulating these brain areas will not only regulate the patient's weight, but also influence positive body image and reduce psychiatric comorbidities. The most common stereotactic targets include the subcallosal cingulate cortex (Brodmann area 25)/medial forebrain bundle (MFB) for AN and comorbid major depressive disorders (MDD) and nucleus accumbens (NAc)/ventral striatum for AN and obsessive–compulsive disorder (comorbid OCD). In most cases, bilateral DBS of various reward system structures achieved good BMI results, with core AN symptoms and psychiatric comorbidities showing sustained improvement. DBS is a promising treatment modality for AN and comorbid OCD or MDD. However, further studies with larger patient populations are needed to shed light on the long-term results of DBS and its effects in the treatment of AN.62,66
Other pathologiesIn addition to neurological disorders, with the exponential growth and acceptance of DBS, it has also been used to treat other neural diseases such as: chronic drug-resistant pain63,64 addictions,65 restless legs syndrome resistant to medical treatment,67 cluster headache,68 movement disorders: dystonia69,70 essential tremor71 and even metabolic diseases such as obesity and psychiatric disorders.72–75
The management of DBS is growing exponentially, increasingly used in neural diseases, psychosurgery and even systemic diseases. However, in the latter two cases it will be necessary to do a lot of basic and applied research in the coming years, before seeing results and being approved by the corresponding institutions, since the results are not yet conclusive; however, the use of the DBS technique will be key to understanding many of the enigmas that the brain and its diseases contain. On the other hand, the use of the technique is usually focused on “alleviating” structures adjacent to the etiological one, that is, in Parkinson's disease the subthalamic nucleus is treated with DBS, instead of the substantia nigra pars compacta. Consequently, by not treating the etiology, we have an incomplete “cure”, which causes different adverse effects in patients. It is necessary to determine an adequate procedure in the brain to be able to interpret those specific synaptic signals in order to create neurogenesis or plasticity on demand and make them functional. With this, we will be able to focus our efforts on regenerative medicine based on the etiology.
ConclusionDuring the review, 15 pathologies were found that have DBS as their target treatment. Of these, practically all are drug-resistant, and DBS is increasingly accepted for the treatment of multiple diseases; nevertheless, the FDA has only approved Parkinson's disease, essential tremor, dystonia and OCD to be treated with this technique. As for the other pathologies, most of them have demonstrated the cost–benefit of their praxis through multiple studies; perhaps it is only a matter of time before they are accepted by the FDA, meanwhile, it is imperative that the different therapeutic alternatives offered by DBS for drug-resistant treatments of nervous system pathologies be made known.
Special thanks to Brain Research Institute and CONACyT for the Mexico doctoral scholarship (scholarship 893764, to JRGP). To Diego Castañeda Marín, thanks for all your support in this article, a great future awaits you in your career.