The aim of this study is to assess the diagnostic value of magnetic resonance imaging (MRI), alone or combined with positron emission tomography (PET), for evaluation of mediastinal disease in lymphoma in comparison with PET/computed tomography (CT).
Material and methodsEighteen prospective studies with sample size ranging between 17 and 140 patients were included in this analysis. MRI, regardless of the sequence obtained, where used for the evaluation of lymphoma. Histopathology results and clinical or imaging follow-up were used as the reference standard. Studies were excluded if the sample size was less than 15 cases or if less than 15 lymph nodes assessment were presented. Papers not reporting sufficient data were also excluded.
ResultsAs compared to PET/CT, MRI proved to be an alternative to use in evaluation of mediastinal disease of lymphoma. MRI has high sensitivity and specificity for nodal and extranodal involvement in lymphoma and the agreement between MRI with the standard technique was high. The ALARA principle reinforces these findings because it is a radiation-free technique and its use is preferred in young population at risk of being subjected to several studies.
ConclusionMRI seems to be a promising and free-radiation alternative due to its high sensitivity and specificity, as well as its high level of agreement with the standard reference. This suggests that MRI could play a significant role in lymphoma study.
El objetivo de este estudio es evaluar el valor diagnóstico de la resonancia magnética (RM), sola o combinada con la tomografía por emisión de positrones (PET), para la valoración de la afectación mediastínica en el linfoma en comparación con la tomografía computarizada (TC)/PET.
Material y métodosSe incluyeron en el análisis 18 estudios prospectivos con un tamaño de muestra que oscilaba entre los 17 y los 140 pacientes. Se utilizó la resonancia magnética, independientemente de la secuencia obtenida para la evaluación del linfoma. Los resultados de histopatología y el seguimiento clínico o de imagen se utilizaron como estándar de referencia. Se excluyeron los estudios si el tamaño de la muestra era inferior a 15 casos, o si se presentaron evaluaciones de menos de 15 ganglios linfáticos. Los artículos que no proporcionaban datos suficientes también fueron excluidos.
ResultadosEn comparación con PET/TC, la RM demostró ser una alternativa útil en la evaluación de la afectación mediastínica del linfoma. La RM tiene una alta sensibilidad y especificidad para detectar afectación nodal y extranodal en el linfoma y la concordancia entre la RMy la técnica estándar fue alta. El principio ALARA refuerza estos hallazgos porque es una técnica libre de radiación y su uso se prefiere en la población joven en peligro de ser sometida a varios estudios.
ConclusiónLa RM parece ser una alternativa prometedora y libre de radiación debido a su alta sensibilidad y especificidad, así como a su alto nivel de concordancia con la referencia estándar. Esto sugiere que la RM podría desempeñar un papel importante en el estudio del linfoma.
Lymphomas represent a heterogeneous group of hematologic neoplasms with different behavior, treatment response and prognosis depending on the histological type, clinical factors and, more recently, molecular characteristics.1 The World Health Organization (WHO) classification of hematopoietic and lymphoid tumors, with the most recent edition published in 2016,2 represents nowadays the guidelines for the diagnostic of malignant lymphomas. WHO classifies lymphomas according to the cell in which they originate or from which they arise. In general, mature lymphoid neoplasms are comprised by non-Hodgkin lymphomas (NHLs), and Hodgkin lymphomas are considered separately.
There has been a significant breakthrough in therapies for the treatment of lymphomas in the last years. This is why it has become capital an update, standardization and universalization of staging and response assessment criteria.3 Since June 2011, we have the first recommendations for response assessment for NHLs (=) published in the International Conference on Malignant Lymphoma (ICML) in Lugano, Switzerland4 that was updated in 2015.3 The new Lugano classification includes recommendations on the use of computed tomography (CT) and the positron emission tomography (PET)-CT, the latter as the standard imaging study for staging of FDG(fluorodeoxyglucose)-avid lymphomas.3 Some patients with malignant lymphoma, depending on the histological type, are young and will require numerous examinations over the course of their treatment and postemission surveillance. For this reason it was considered the possibility of using magnetic resonance imaging (MRI) as an alternative test imaging offering an adequate contrast resolution in the evaluation of nodal and extranodal disease and being a radiation-free technique avoiding in that way secondary malignancies.5 MRI is characterized by high soft tissue contrast and high spatial resolution; even its image quality could be affected by susceptibility artifacts such as motion (breathing), pulsation artifacts or the alveolar architecture of the lung.6 Recent studies reported which whole-body MRI (Wb-MRI) and hybrid PET/MRI (technique that allows the simultaneous obtainment of PET and MRI data) were feasible for lymphoma evaluation of disease status with high sensitivity and specificity. Although MRI is currently not considered as the technique of choice, given its high diagnostic accuracy, it is considered as an alternative to conventional imaging methods. In general, the analyses of MRI images were made from quantitative and qualitative point of view. For the quantitative analysis, apparent diffusion coefficient (ADC)7 is calculated. Qualitative analysis depends on the consensus of two radiologists who according to the images obtained apply a five-point visual scoring system.8
The purpose of our study is to perform a systematic review to assess the diagnostic value of MRI, alone or combined with PET, for evaluation of mediastinal disease in lymphoma in comparison with PET/CT.
Material and methodsLiterature researchA literature research was performed in PubMed (Medline), EMBASE and Cochrane databases. To retrieve information the following search strategy was employed in PubMed using a combination of MeSH terms (“Magnetic Resonance Imaging” [MeSH] AND “Lymphoma” [MeSH]). The following limits were used: language (English or Spanish), humans and publications dating from 01/01/2003 to 31/12/2017. The reference lists of identified articles were also manually searched to obtain additional papers. The reports found to be eligible on the basis of their title and, subsequently, from the abstract, were then selected to further analysis to determine suitability for inclusion in the present study.
Inclusion and exclusion criteriaEligible studies were reviewed and included in this systematic review according to the following inclusion criteria: MRI, either alone or combined with PET was used for the evaluation of disease status of lymphoma (initial staging, restaging, suspected recurrence or response assessment). Histopathology results and clinical or imaging follow-up were used as the reference standard. Studies were excluded if the sample size was less than 15 cases or if less than 15 lymph nodes assessment were presented. Papers not reporting sufficient data were also excluded.
Data extraction and quality assessmentSearch results were checked by one reviewer and if there were doubts regarding the inclusion or exclusion of a certain paper, this was solved by consensus by all authors. A quality scale has been used to score the quality of the included studies. Similar scales have been used previously by other group when performing other systematic reviews.9,10 Seven items have been taken into consideration to assess the quality of the included studies which can be seen in Table 1. Each item opted a score between 0 and 1–3 points (the higher the score the higher the quality).
Quality score to assess the included studies.
| Item assessed | Characteristic | Weight |
|---|---|---|
| Total sample size | 15–30 | 0 |
| 31–60 | 1 | |
| ≥60 | 2 | |
| Total nodal station | 0–50 | 0 |
| 51–100 | 1 | |
| ≥100 | 2 | |
| Radiologists | 1 | 0 |
| 2 | 1 | |
| 3 | 2 | |
| Type of MR | 1.5T | 0 |
| 3T | 1 | |
| Image analysis | Qualitative | 0 |
| Quantitative | 1 | |
| Both | 2 | |
| Use of the standard reference | Not used | 0 |
| Partially nodes confirmed | 1 | |
| All nodes confirmed | 2 | |
| Simultaneity of image testsa | No | 0 |
| Yes | 1 | |
| TOTAL | 12 | |
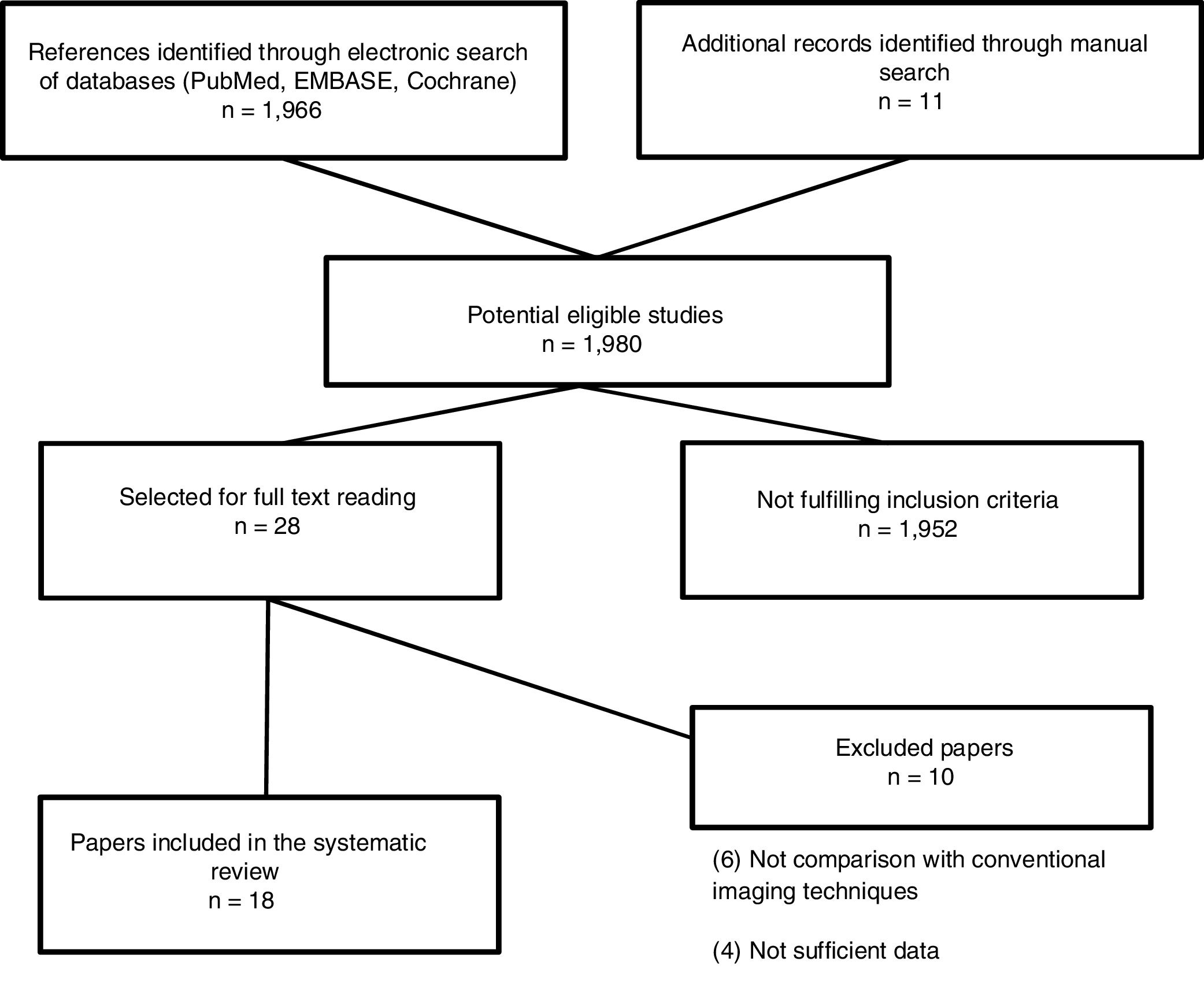
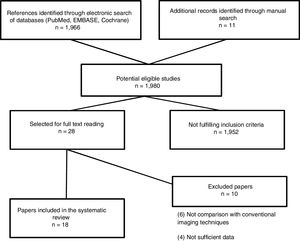
The search resulted in 1980 studies. Of these, 28 of these studies were selected for full text reading, and 18 prospective studies5,11–29 were finally included. Characteristics of the included studies are summarized in Table 2. The most frequent exclusion criterion was absence of sufficient data or no comparison with conventional imaging tests (PET/CT). The flowchart of the search is shown in Fig. 1. Sample size ranged between 17 and 140 patients and at least 2759 nodal stations were studied (not all studies specified the number of stations studied). We considered the presence of disease at the following nodal stations: Waldeyer ring, cervical right and left, supraclavicular right and left, axillary right and left, mediastinal, lung hilar, hepatic and splenic hilar, retroperitoneal periaortic and mesenteric, pelvic right and left, inguinal and femoral right and left. Extranodal sites such as lung, pleura, pericardium, chest wall, liver, spleen, kidney, stomach, bowel, pancreas and bone marrow were also valued. After careful reading of the studies, it could be observed that MRI has been studied in different combinations [whole-body magnetic resonance imaging (Wb-MRI), combined sometimes with diffusion-weighted (DWI) as whole-body diffusion-weighted magnetic resonance imaging (Wb-DWI-MRI), in its PET/MRI hybrid form with or without DWI and even some other more complex sequences as whole body short inversion time recovery (STIR) half fourier relaxation enhancement (RARE) MRI], has been compared with the standard reference PET/CT or only CT, as in the case of the first two studies found11,12 as well as other more recent13 and has been studied in adults and pediatric population.
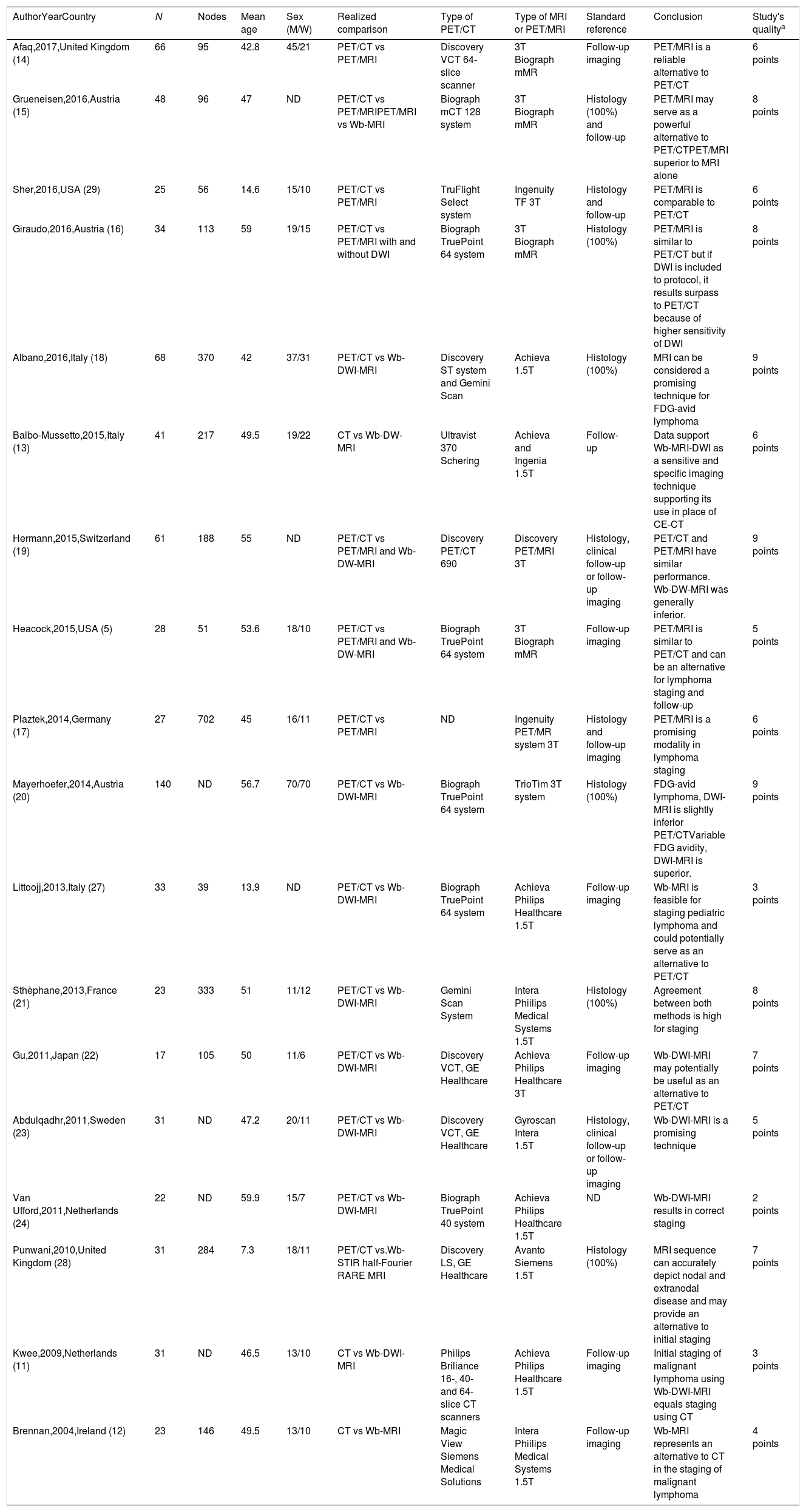
Characteristics of included studies.
| AuthorYearCountry | N | Nodes | Mean age | Sex (M/W) | Realized comparison | Type of PET/CT | Type of MRI or PET/MRI | Standard reference | Conclusion | Study's qualitya |
|---|---|---|---|---|---|---|---|---|---|---|
| Afaq,2017,United Kingdom (14) | 66 | 95 | 42.8 | 45/21 | PET/CT vs PET/MRI | Discovery VCT 64-slice scanner | 3T Biograph mMR | Follow-up imaging | PET/MRI is a reliable alternative to PET/CT | 6 points |
| Grueneisen,2016,Austria (15) | 48 | 96 | 47 | ND | PET/CT vs PET/MRIPET/MRI vs Wb-MRI | Biograph mCT 128 system | 3T Biograph mMR | Histology (100%) and follow-up | PET/MRI may serve as a powerful alternative to PET/CTPET/MRI superior to MRI alone | 8 points |
| Sher,2016,USA (29) | 25 | 56 | 14.6 | 15/10 | PET/CT vs PET/MRI | TruFlight Select system | Ingenuity TF 3T | Histology and follow-up | PET/MRI is comparable to PET/CT | 6 points |
| Giraudo,2016,Austria (16) | 34 | 113 | 59 | 19/15 | PET/CT vs PET/MRI with and without DWI | Biograph TruePoint 64 system | 3T Biograph mMR | Histology (100%) | PET/MRI is similar to PET/CT but if DWI is included to protocol, it results surpass to PET/CT because of higher sensitivity of DWI | 8 points |
| Albano,2016,Italy (18) | 68 | 370 | 42 | 37/31 | PET/CT vs Wb-DWI-MRI | Discovery ST system and Gemini Scan | Achieva 1.5T | Histology (100%) | MRI can be considered a promising technique for FDG-avid lymphoma | 9 points |
| Balbo-Mussetto,2015,Italy (13) | 41 | 217 | 49.5 | 19/22 | CT vs Wb-DW-MRI | Ultravist 370 Schering | Achieva and Ingenia 1.5T | Follow-up | Data support Wb-MRI-DWI as a sensitive and specific imaging technique supporting its use in place of CE-CT | 6 points |
| Hermann,2015,Switzerland (19) | 61 | 188 | 55 | ND | PET/CT vs PET/MRI and Wb-DW-MRI | Discovery PET/CT 690 | Discovery PET/MRI 3T | Histology, clinical follow-up or follow-up imaging | PET/CT and PET/MRI have similar performance. Wb-DW-MRI was generally inferior. | 9 points |
| Heacock,2015,USA (5) | 28 | 51 | 53.6 | 18/10 | PET/CT vs PET/MRI and Wb-DW-MRI | Biograph TruePoint 64 system | 3T Biograph mMR | Follow-up imaging | PET/MRI is similar to PET/CT and can be an alternative for lymphoma staging and follow-up | 5 points |
| Plaztek,2014,Germany (17) | 27 | 702 | 45 | 16/11 | PET/CT vs PET/MRI | ND | Ingenuity PET/MR system 3T | Histology and follow-up imaging | PET/MRI is a promising modality in lymphoma staging | 6 points |
| Mayerhoefer,2014,Austria (20) | 140 | ND | 56.7 | 70/70 | PET/CT vs Wb-DWI-MRI | Biograph TruePoint 64 system | TrioTim 3T system | Histology (100%) | FDG-avid lymphoma, DWI-MRI is slightly inferior PET/CTVariable FDG avidity, DWI-MRI is superior. | 9 points |
| Littoojj,2013,Italy (27) | 33 | 39 | 13.9 | ND | PET/CT vs Wb-DWI-MRI | Biograph TruePoint 64 system | Achieva Philips Healthcare 1.5T | Follow-up imaging | Wb-MRI is feasible for staging pediatric lymphoma and could potentially serve as an alternative to PET/CT | 3 points |
| Sthèphane,2013,France (21) | 23 | 333 | 51 | 11/12 | PET/CT vs Wb-DWI-MRI | Gemini Scan System | Intera Phiilips Medical Systems 1.5T | Histology (100%) | Agreement between both methods is high for staging | 8 points |
| Gu,2011,Japan (22) | 17 | 105 | 50 | 11/6 | PET/CT vs Wb-DWI-MRI | Discovery VCT, GE Healthcare | Achieva Philips Healthcare 3T | Follow-up imaging | Wb-DWI-MRI may potentially be useful as an alternative to PET/CT | 7 points |
| Abdulqadhr,2011,Sweden (23) | 31 | ND | 47.2 | 20/11 | PET/CT vs Wb-DWI-MRI | Discovery VCT, GE Healthcare | Gyroscan Intera 1.5T | Histology, clinical follow-up or follow-up imaging | Wb-DWI-MRI is a promising technique | 5 points |
| Van Ufford,2011,Netherlands (24) | 22 | ND | 59.9 | 15/7 | PET/CT vs Wb-DWI-MRI | Biograph TruePoint 40 system | Achieva Philips Healthcare 1.5T | ND | Wb-DWI-MRI results in correct staging | 2 points |
| Punwani,2010,United Kingdom (28) | 31 | 284 | 7.3 | 18/11 | PET/CT vs.Wb- STIR half-Fourier RARE MRI | Discovery LS, GE Healthcare | Avanto Siemens 1.5T | Histology (100%) | MRI sequence can accurately depict nodal and extranodal disease and may provide an alternative to initial staging | 7 points |
| Kwee,2009,Netherlands (11) | 31 | ND | 46.5 | 13/10 | CT vs Wb-DWI-MRI | Philips Briliance 16-, 40- and 64-slice CT scanners | Achieva Philips Healthcare 1.5T | Follow-up imaging | Initial staging of malignant lymphoma using Wb-DWI-MRI equals staging using CT | 3 points |
| Brennan,2004,Ireland (12) | 23 | 146 | 49.5 | 13/10 | CT vs Wb-MRI | Magic View Siemens Medical Solutions | Intera Phiilips Medical Systems 1.5T | Follow-up imaging | Wb-MRI represents an alternative to CT in the staging of malignant lymphoma | 4 points |
CT: computed tomography. M: male. MRI: magnetic resonance imaging. ND: not documented. PET: positron emission tomography. RARE: relaxation enhancement. STIR: short inversion time inversion recovery. T: tesla. W: women. Wb-MRI: whole-body magnetic resonance imaging. Wb-DWI-MRI: whole body diffusion-weighted magnetic resonance imaging.
Fifteen studies used MRI to study lymphoma in adults. Four20–23 compared the diagnostic performance of PET/MRI with or without DWI with the current standard of care, PET/CT. In Afaq et al. study14 intermodality agreement between PET/MRI and PET/CT was very good (κ=0.97) and SUVmax from PET/CT and PET/MRI correlated significantly (p<0.001). Grueneisen et al.15 studied 48 consecutive lymphoma patients with PET/CT an PET/MRI. SUVmax and SUVmean of lymphoma lesions were significantly higher in PET/MRI than in PET/CT (p<0.05). The SUVmax exhibited a strong and positive correlation in both imaging modalities (R=0.91; p<0.001). The SUVmean also revealed a highly and significant correlation (R=0.87; p<0.001). Giraudo el al.16 assessed nodal and extranodal involvement in 34 patients with histologically proven lymphoma. Sensitivities for PET/CT, PET/MRI and PET/MRI with DWI were 82.1%, 85.7% and 100%, respectively. Specificity was 100% for all 3 techniques, and accuracy was 87.5%, 90% and 100%, respectively. Agreement between PET/MRI and PET/MRI with DWI according to the standard technique was high (κ=0.95 and κ=0.96, respectively). No significant correlation between ADC and SUVmax was observed (r=0.46, p=0.65). Similar results were observed by Platzek et al.17 who evaluated sensitivity and specificity of PET/MR for nodal involvement in lymphoma (93.8% and 99.4%, respectively).
Eight studies5,18–24 compared the diagnostic performance of whole-body MRI (Wb-MRI) with or without DWI with the current standard of care, PET/CT. The largest study in this group was performed by Mayerhoefer et al.20 in 140 patients who were prospectively studied to determine the value of Wb-DWI-MRI for pretherapeutic assessment and staging of lymphoma. Patients were divided into two groups depending on fluorodeoxyglucose (FDG)-avid lymphoma. FDG-avid lymphoma group showed a high sensitivity and specificity (97.8% and 100%, respectively) of Wb-DWI-MRI and significant agreement with the reference standard (κ=0.92, p<0.0001) for nodal and extranodal regions. These results did not significantly differ from PET/CT (p=0.096). Nevertheless in variable FDG-avidity group, Wb-DWI-MRI seemed to be superior to PET/CT and agreement with the reference standard was clearly superior to PET/CT (κ=0.89, p<0.0001 and κ=0.52, p<0.0001, respectively). The second largest study published by Albano et al.18 included 68 patients with histologically confirmed lymphoma and concluded an excellent agreement between two techniques (κ=0.88) as well as a high sensitivity and specificity for Wb-DWI-MRI. Remaining studies5,19,21–24 showed similar results for Wb-MRI with or without DWI, proposing it as an alternative to conventional techniques in the evaluation of lymphoma disease.
Finally, three studies11–13 compared the diagnostic performance of whole-body MRI (Wb-MRI) with or without DWI with CT although the guidelines consider the PET/CT as the most sensitive and specific technique considered for the study of lymphomas.25,26 Studies support that Wb-DWI-MRI is a sensitive and specificity technique for lymphoma evaluation, supporting it as an alternative technique to use in place of CT.
Pediatric populationIn the 3 included studies, the standard reference, PET/CT, was compared with three different forms of resonance imaging, one in each study. The largest study published on lymphoma assessment in children was published by Littooij et al.27 in 2014 including 36 patients with newly diagnosed lymphoma who underwent both PET/CT and Wb-DWI-MRI. In qualitative lymphoma assessment (ADC measurements were not used for disease detection), Wb-DWI-MRI showed that interobserver agreement of Wb-DWI-MRI was good for all nodal sites (κ=0.79) and all extranodal sites together (κ=0.69), that was a very good consensus between Wb-DWI-MRI and PET/CT for nodal sites (κ=0.91) and extranodal (κ=0.94) and yielded sensitivity and specificity data for Wb-DWI-MRI of 93% and 98%, respectively. Punwani et al.28 compared Wb-STIR half-fourier RARE MRI with PET/CT for initial lymphoma staging in 31 children with histologically proved lymphoma. The study showed a very good agreement between reported MRI and PET/CT reference standard for nodal and extranodal staging (κ=0.96 and 0.86, respectively) and sensitivity and specificity of MRI were 98% and 99%, respectively, for nodal disease and, 91% and 99%, respectively for extranodal disease. Sher et al.29 compared the diagnostic performance of PET/MRI and PET/CT in 25 pediatric patients with lymphoma for lesion detection, lesion classification, and disease staging. There was substantial agreement between compared techniques (κ=0.61) and sensitivity and specificity of PET/MRI were 92% and 61%, respectively. No statistically significant differences between PET/MRI and PET/CT were observed. These studies, individually, concluded that MRI was a potential alternative for PET/CT in the pediatric population in the evaluation of lymphoma.
Study qualityThe scoring of the included studies ranged between 0 and 12 points. The highest quality was for three studies with 68.61 and 140 patients, respectively.18–20 The mean scoring of the included studies was 6.2 points. Table 2 presents in detail the quality of each study.
DiscussionThe results of this systematic review point out that MRI, regardless of how it was done (combined with PET as PET/MRI, with or without DWI or Wb-MRI with or without MRI), is an alternative to use in the evaluation of disease status of lymphoma. MRI has high sensitivity and specificity for nodal and extranodal involvement in lymphoma and the agreement between MRI with the standard technique was high.
In addition to the promising results obtained in the different studies, the ALARA principle (concept of As Low As Reasonably Achievable) is capital in order to accommodate MRI in neoplasm's staging. The ALARA principle consists in the reduction of the exposure to radiation in image studies in order to obtain certain clinical information.30 Using radiation free techniques such as resonance, especially in the young or pediatric population in which lymphomas are typically diagnosed with the need for multiple follow-up examinations, a reduction in radiation exposure is achieved and consequently, the risk of radiation-induced secondary malignancies.31 Potential advantages of MRI over CT and PET/CT could be considered as reduced radiation exposure, as high contrast soft tissues and the possibility of using DWI sequences which provide complementary information to PET. Although there have been recent advances and improvements which have allowed to reduce radiation in CT, MRI completely eliminates this risk.32
MRI employment has some additional advantages: (1) patients do not have to fast before examinations; (2) the cost is significantly lower; (3) total examination is shorter (25–30min vs. 60min)33; (4) it is not necessary the administration of contrast medium so it can be used in patients with renal disease34; and, (5) there is easier accessibility to MRI because not all hospitals have PET/CT.13 The first study analyzing patient experience with Wb-MRI and CT for staging newly diagnosed lymphoma concluded that Wb-MRI was a more friendly technique compared to CT.35
Methodological shortcomings of the available literatureDifferent methodological shortcomings continue to hinder MRI use in clinical practice. Radiologists and nuclear medicine physicians have more experience and availability with PET/CT images than MRI,36 probably because their use has not been sufficiently encouraged or protocols development have somewhat been neglected. Patient cohorts available in recent studies are relatively small21,23 and heterogeneous,5,15 as well as, the objective of these studies (staging, restaging, follow-up, treatment response, etc.). Most lesions detected were not histologically analyzed,11,19,21,23 though it would have been desirable in order to analyze the additional value of MRI. Nevertheless, obtaining histological samples is sometimes unpractical or even unethical. Although MRI provides reliable data on the size and functional tissue information there are no validated ADC criteria yet for discriminating involved from non-involved sites. It is necessary to develop a standard protocol acquisition as a routine clinical application, but its absence makes it difficult to make reliable ADC measurements.27
ConclusionIn conclusion, the available evidence about the use of MRI in the evaluation of mediastinal disease in lymphoma in comparison to conventional imaging techniques (PET/CT or CT) seems to propose it as a promising and free-radiation alternative due to its high sensitivity and specificity, as well as its high level of agreement with the standard reference. It would be advisable to carry out studies with a greater number of patients, with more homogeneous populations, ideally with histological verification of all lesions, as well as to validate a resonance protocol, the standardization of ADC values to differentiate involved and non-involved sites and to develop more studies to find out where the most useful role of MRI in lymphoma may be (staging, restaging, follow-up, response to treatment, etc.). Additional prospective and multicenter studies are warranted to confirm the clinical role of MRI in lymphoma evaluation.
Authors’ contributionTara Pereiro-Brea. Conception of the work. Data collection. Data analysis and interpretation. Drafting the article. Critical revision of the article. Final approval of the version to be published.
Alberto Ruano-Raviña. Conception of the work. Drafting the article. Critical revision of the article. Final approval of the version to be published.
Antonio Golpe-Gómez. Critical revision of the article. Final approval of the version to be published.
Anxo Martíne de Alegría Critical revision of the article. Final approval of the version to be published.
José Martín Carreira-Villamor. Critical revision of the article. Final approval of the version to be published.
Aitor Abuín-Blanco. Critical revision of the article. Final approval of the version to be published.
Luis Valdés. Conception of the work. Drafting the article. Critical revision of the article. Final approval of the version to be published.
ScholarshipsThis work has been carried out without scholarships.
Conflict of interestNone.