Cancer of the esophagus is a tumor with aggressive behavior that is usually diagnosed in advanced stages. The absence of serosa allows it to spread quickly to neighboring mediastinal structures, and an extensive lymphatic drainage network facilitates tumor spread even in early stages. The current TNM classification, harmonized with the classification for gastric cancer, provides new definitions for the anatomic classification, adds non-anatomic characteristics of the tumor, and includes tumors of the gastroesophageal junction. Combining endoscopic ultrasound, computed tomography, positron emission tomography, and magnetic resonance imaging provides greater accuracy in determining the initial clinical stage, and these imaging techniques play an essential role in the selection, planning, and evaluation of treatment. In this article, we review some particularities that explain the behavior of this tumor and we describe the current TNM staging system; furthermore, we discuss the different imaging tests available for its evaluation and include a diagnostic algorithm.
El cáncer de esófago es un tumor de comportamiento agresivo, que suele diagnosticarse en etapas avanzadas. La ausencia de serosa permite su rápida propagación a estructuras vecinas del mediastino, y una extensa red de drenaje linfático facilita la diseminación tumoral incluso en estadios precoces. La actual clasificación TNM, armonizada con la del cáncer gástrico, proporciona nuevas definiciones en la clasificación anatómica, añade características no anatómicas del tumor e incluye los tumores de la unión esofagogástrica. La mayor precisión en la determinación del estadio clínico inicial se obtiene con la combinación de ecoendoscopia, TC, PET-TC y RM, que desempeñan un papel esencial en la elección, la planificación y la evaluación del tratamiento. En este artículo repasamos algunas particularidades que explican el comportamiento de este tumor, revisamos la estadificación TNM actual y presentamos las distintas pruebas de imagen de que disponemos en la actualidad para su evaluación, incluyendo un algoritmo de diagnóstico.
Esophageal cancer is the eighth most common type of cancer worldwide and it ranks third among those of gastrointestinal origin. According to recent data by the Sociedad Española de Oncología Médica (Spanish Society of Medical Oncology) based on the 2012 GLOBOCAN project review the incidence in Spain amounts to 2090 cases, with an estimated five-year prevalence of 2238, and even though it represents 1% of all cancers, it accounts for 1.7% of all deaths for this cause. Unlike other tumors, it is expected that the incidence of esophageal cancer will continue to rise in the next few years.1–3
90% of the cases are epidermoid carcinomas or adenocarcinomas. Squamous cell cancer, associated with alcohol and tobacco consumption, is overall the most common worldwide, especially in Eastern countries, and its incidence is decreasing in Western countries. Today adenocarcinomas are the most common type of cancer in Western countries, due to a significant increase of its incidence during the last few years with respect to gastroesophageal reflux disease and obesity. While the former one is distributed similarly in different segments of the esophagus, the latter one fixes on the distal esophagus in three fourths of the cases.4,5 Other types of tumor such as lymphomas, fusiform cell carcinomas, neuroendocrine tumors and gastrointestinal stroma tumors (GIST), are extremely uncommon.6
Esophageal cancer is a tumor of aggressive behavior that is usually diagnosed in advanced stages, which is explained to a certain extent by some anatomical and lymphatic-drainage peculiarities of this organ. Despite recent breakthroughs in its diagnosis and treatment, prognosis continues to be poor and it represents the sixth cause of death worldwide, with a survival of less than 20% at 5 years.
Breakthroughs in multidisciplinary therapeutic approach during the last few years have allowed us to improve the median survival in these patients. In addition to surgery, today there are treatment options such as endoscopic resection or the use of neoadjuvant chemoradiotherapy followed by surgery or definitive chemoradiotherapy. Furthermore, chemotherapeutic treatments are being developed in the light of new receptors, such as HER-2 (human epidermal growth factor receptor 2) that appears to be overexpressed in esophageal adenocarcinoma, and growth factor inhibitors such as VEGF (vascular endothelial growth factor) and EGFR (epidermal growth factor receptor), which could extend the survival of patients with disseminated disease in the future.
Imaging modalities play an essential role in these patients’ integral assessment, both in the initial evaluation – determining resectability and in the most appropriate treatment and in the response assessment and follow-up.7
In this paper we review the anatomical and lymphatic-drainage peculiarities of the esophagus that explain the aggressive behavior of this tumor, we review the actual classification and its contributions to esophageal cancer staging, and we present the utility and limitations of each imaging modalities used in the initial assessment and during these patients’ therapeutic process.
Anatomic peculiarities in esophageal cancerThe esophagus is a muscular tube that connects the pharynx with the stomach, whose wall is made up of four layers: the mucosa, the submucosa, the muscular and the adventitia layers. The esophagus does not have serosa, a characteristic that facilitates the rapid dissemination of cancer to neighboring structures in the neck and mediastinum.8,9
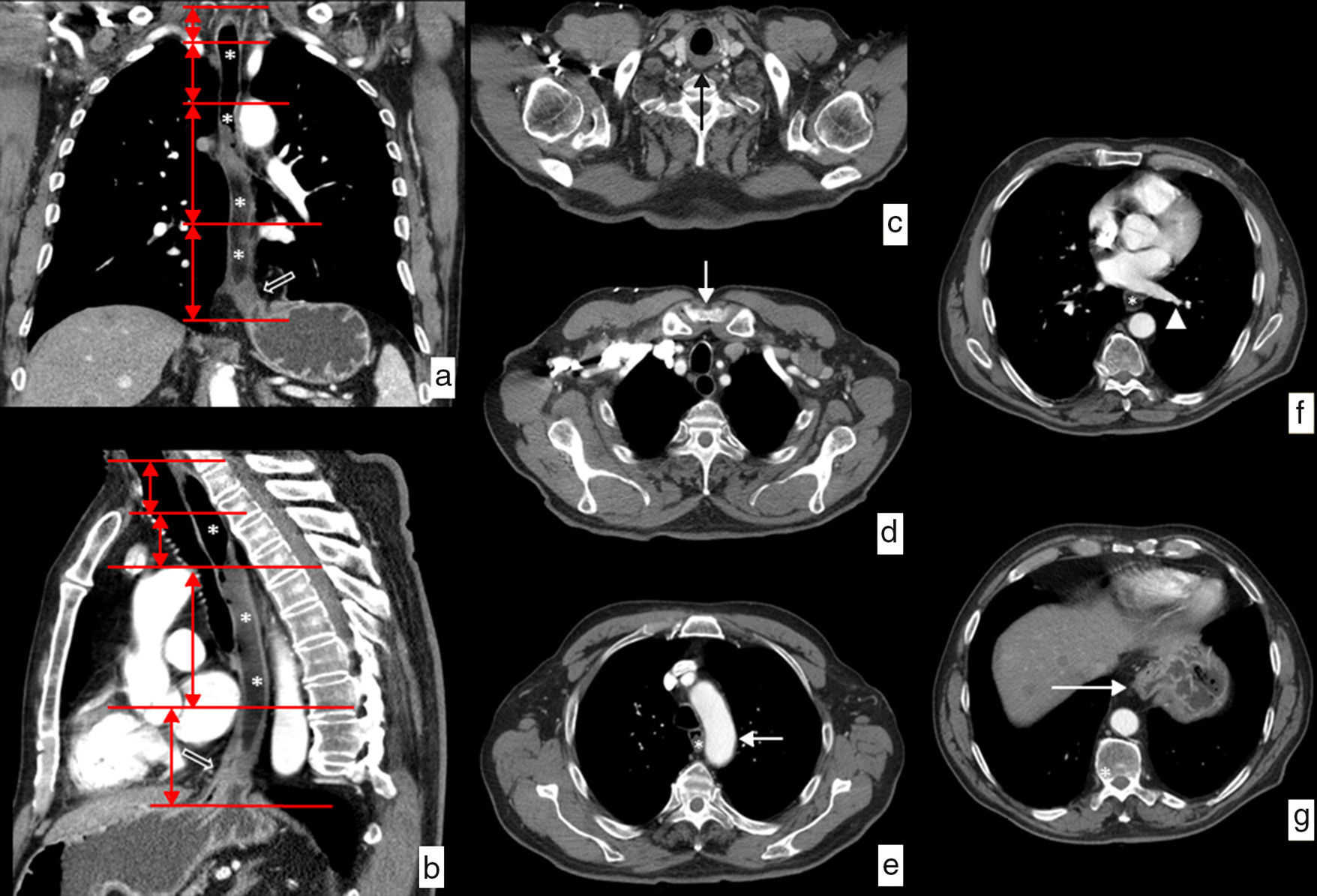
Although there is an endoscopic division based on the distance from the upper edge of the tumor to the incisors, we usually use a clinical division that divides the esophagus into cervical, upper, mid and lower thoracic regions, with well-defined relations and well-defined anatomic limits10–12 (Fig. 1):
- •
Cervical esophagus. Limits: from the cricopharyngeal muscle to the sternal notch. Anatomical relations: carotid arteries, thyroid gland, trachea and vertebral bodies.
- •
Upper thoracic esophagus. Limits: from the sternal notch to the aortic arch. Anatomical relations: the same ones.
- •
Mid thoracic esophagus. Limits: from the aortic arch to the lower pulmonary vein. Anatomical relations: trachea and main bronchi, aorta, pulmonary artery, vena cava superior, azygos vein, left atrium and vertebral bodies.
- •
Lower thoracic esophagus. Limits: from the lower pulmonary vein to the esophagogastric junction. Anatomical relations: aorta, vena cava inferior, azygos vein, left atrium, vertebral bodies and diaphragm.
Anatomical limit and relations of the esophagus on CT. Water has been administered as a negative oral contrast filling the esophageal lumen (*) discretely dilated by an esophageal neoplasm on the distal third viewed in the images (hollowed arrows). (a) Coronal view of curve reconstruction following the axis of the esophagus. The horizontal red lines represent the limits of each of the segments in which the esophagus is divided. (b) Sagittal view of curve reconstruction following the axis of the esophagus showing the same information. (c) Axial image at the height of the upper limit of the cervical esophagus determined by the cricopharyngeal muscle (black arrow). (d) Axial image at the height of the limit between the cervical esophagus and the upper third of the thoracic esophagus determined by the sternal notch (white arrow). (e) Axial image at the height of the limit between the upper and the mid thirds of the thoracic esophagus determined by the aortic arch (white arrow). (f) Axial image at the height of the limit between the mid and lower thirds of the thoracic esophagus determined by lower pulmonary vein (arrowhead). (g) Axial image at the height of the lower limit of the lower inferior third of the thoracic esophagus determined by the esophagogastric junction (white arrow).
The upper limit of the lesion is the one that determines the location of neoplasm in each level of the esophagus.
Lymphatic drainageLymphatic dissemination is the most important individual prognostic factor in esophageal cancer and it is a good predictor of disease-free survival.13
The lymphatic drainage routes of the esophagus are complex and they show some peculiarities that need to be known to perform a correct diagnostic assessment and plan treatment adequately in patients with esophageal cancer.
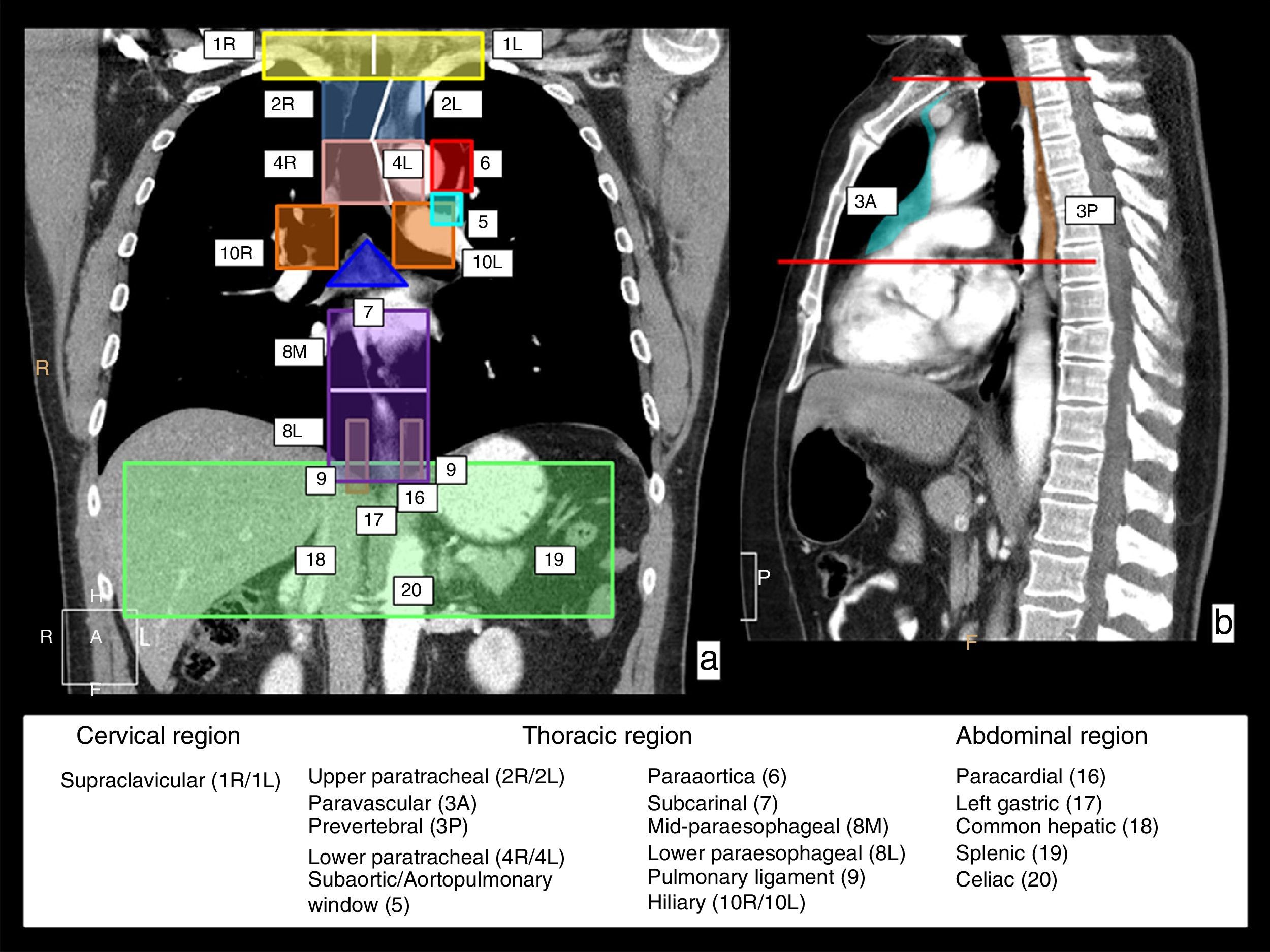
In the first place, lymphatic drainage of the esophagus involves lymphatic chains in three anatomical regions: the neck–in the supraclavicular chains; the thorax–in several mediastinal chains and the abdomen–in the upper abdomen chains. Fig. 2 shows a schematic representation of these lymphatic drainage stations on CT coronal and sagittal reconstruction images.
Lymphatic drainage chains of the esophagus. In a coronal (a) and sagittal (b) CT view, the chains in charge of the lymphatic drainage of the esophagus are represented with colors and numbers; they involve three anatomical regions. Cervical region–the right (1R) and left (1L) supraclavicular chains with the limit between both represented by the white vertical line. In the thoracic region, the upper mediastinum chains including the right (2R) and left (2L) paratracheal chains, and lower right (4R) and left (4L) paratracheal chains outlined by the left paratracheal line represented with a white line, and the prevascular (3A) and prevertebral (3P) chains that are represented in the sagittal view; the aortic chains include the subaortic chain or that of the aortopulmonary window (5) and paraaortic (6) chains; and the lower mediastinum chains include the subcarinal chain (7), the mid paraesophageal chain (8M), the lower paraesophageal chain (8L), the pulmonary ligament chain (9) and right hilar (10R) and left hilar chains (10L). In the upper abdominal region, the paracardial (16), left gastric (17), common hepatic (18), splenic (19) and celiac chains (20).
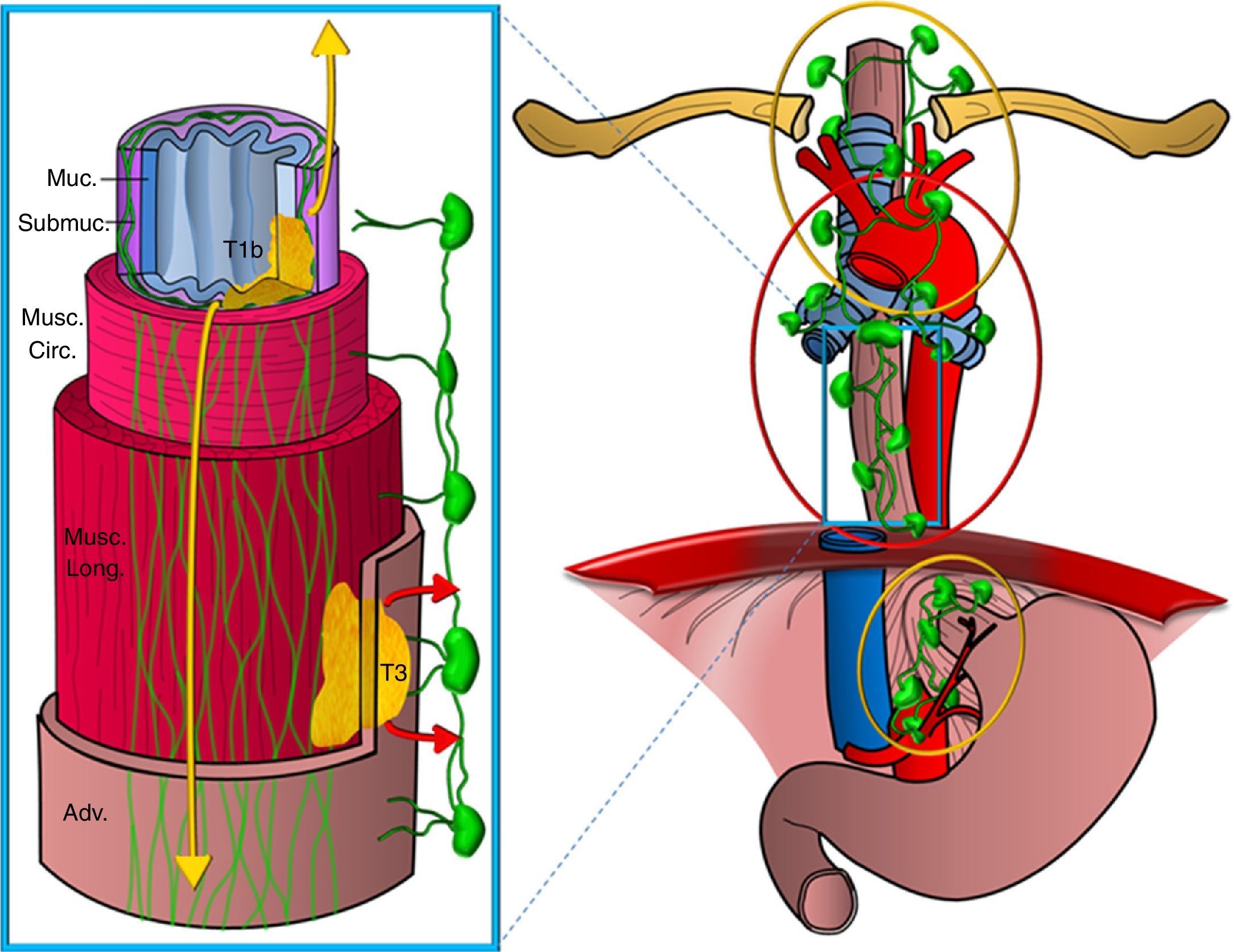
Secondly, the esophagus has two lymphatic drainage routes on its wall: one on the submucosa layer, with an extensive intramural network of lymphatic canals that stretches longitudinally and uninterruptedly from the hypopharinx to the stomach draining into the ganglia of the upper mediastinum and from there to supraclavicular ganglia and also into ganglia of the perigastric area, and another route on the muscular layer with a less developed network of lymphatic vessels draining in a segmentary manner into the periesophageal ganglia (Fig. 3). There is restricted communication between both networks and, in general, the two upper thirds of the esophagus drain in a cranial direction while the lower third drains in a caudal direction.14,15
Diagram showing the esophagus lymphatic drainage routes. Representation of a segment of the esophagus showing the different wall layers (Muc: mucosa; Submuc: submucosa; Musc. circ: muscular circular; Musc. long: muscular longitudinal; Adv: adventitia). Two tumors are represented infiltrating it, one superficially up to the submucosa (T1b) and another one that is more advanced (T3), as well as the lymphatic drainage networks, one on the submucosa layer, more extensive and on a longitudinal direction draining into the upper mediastinum and perigastric chains -tumor spread is represented in yellow colors and the other one on the muscular layer, less developed draining segmentary into the periesophageal chains (the spread is represented in red colors).
This is the reason why the tumors that stretch only to the submucosa layer can show expansion in ganglionic chains far from their location on the esophageal wall without the most proximal periesophageal chains being affected–which is called skip metastasis. Therefore, the presence of distant pathological ganglia from the primary tumor is not indicative of locally-advanced disease or leads to worse prognosis than tumoral adenopathies closer to the tumor (Fig. 4). However, the tumors that infiltrate at least the muscular layer, in addition to the above-mentioned chains, often affect the periesophageal ganglia or the rest of mediastinal chains.16,17
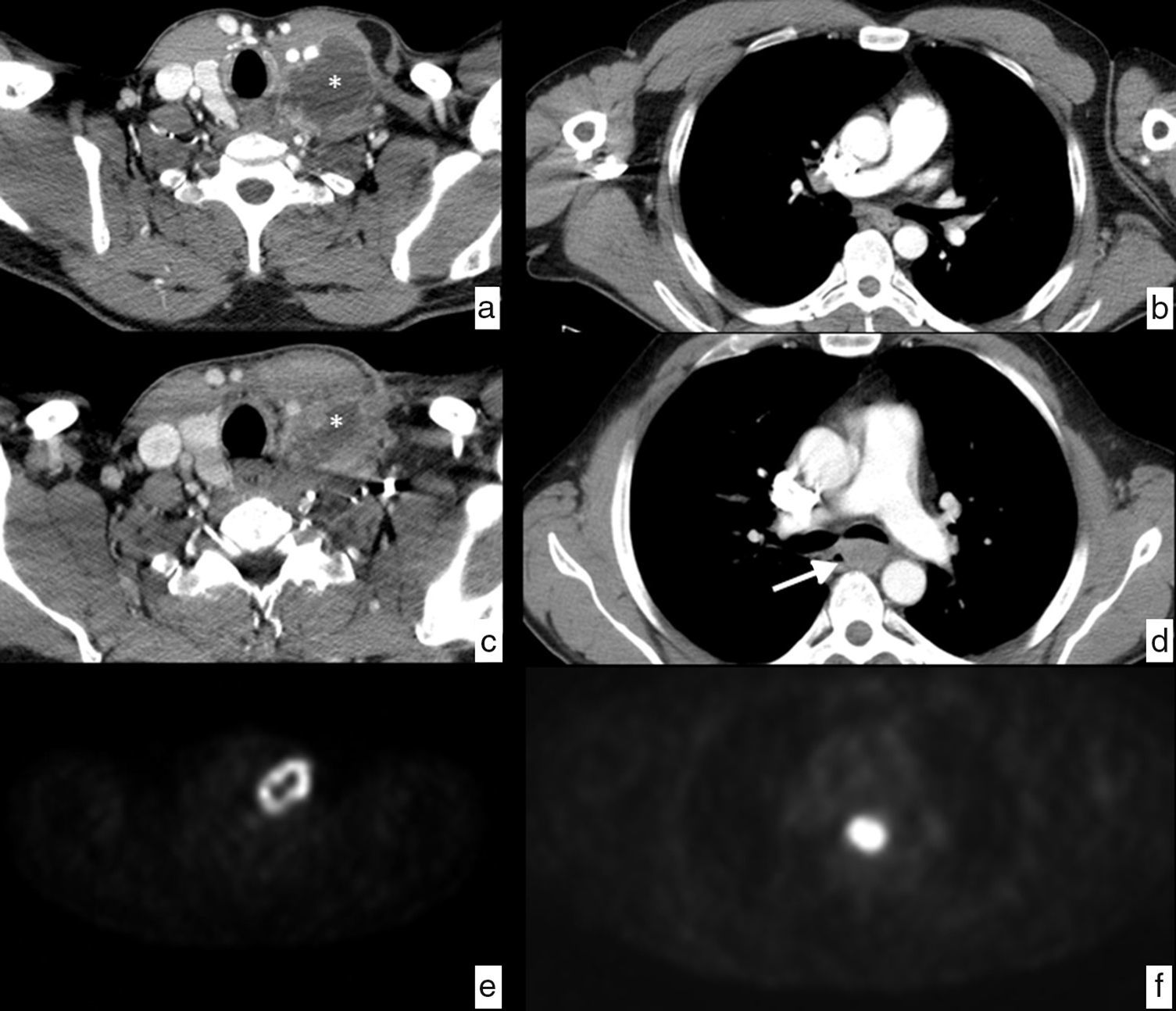
Patient with laterocervical adenopathy. Initial study, axial CT section in the cervical region (a), showing left laterocervical adenopathy (*) of heterogeneous density with peripheral enhancement and hypodense central area indicative of necrosis. Control study after treatment with antibiotics, CT cut in the cervical region at the same height (c) showing a size decrease of the pathological ganglion (*), but since the suspicion of neoplastic origin still remains a decision is made to perform a biopsy resulting in a lesion indicative of epidermoid carcinoma. To determine the primary origin, the study is completed with a PET showing pathological ring uptake of tumor origin in the left laterocervical region (e) and a pathological uptake in the mid third of the esophagus at the height of the carina, with SUV of 20.5, suspicious of primary neoplasm (f), in addition to mediastinal and retropancreatic adenopathies, and dubious osseous lesions in D1 and D4, not shown in the images. The endoscopic study confirms the existence of an esophageal neoplasm through the biopsy of the epidermoid carcinoma that is detected retrospectively (arrow) in the CT examination after the treatment (d) that goes unnoticed in the initial study (b). It is an esophageal cancer that was probably small in size at the beginning, but showed a distant pathological ganglion in the left laterocervical chain (skip metastasis). The final clinical diagnosis was epidermoid carcinoma of the mid third of the esophagus in stage IV, due to non-regional (retropancreatic) adenopathy and dubious osseous metastasis. It is decided to indicate treatment through definitive chemoradiotherapy.
This extremely complex lymphatic drainage has caused the clinical guidelines to recommend extensive lymphadenectomies during the surgical act, with the intention of performing a curative procedure and reduce or avoid the possibility of local relapse. Nevertheless, it has always been a controversial practice and some recent papers put the need for this approach into question.18–23
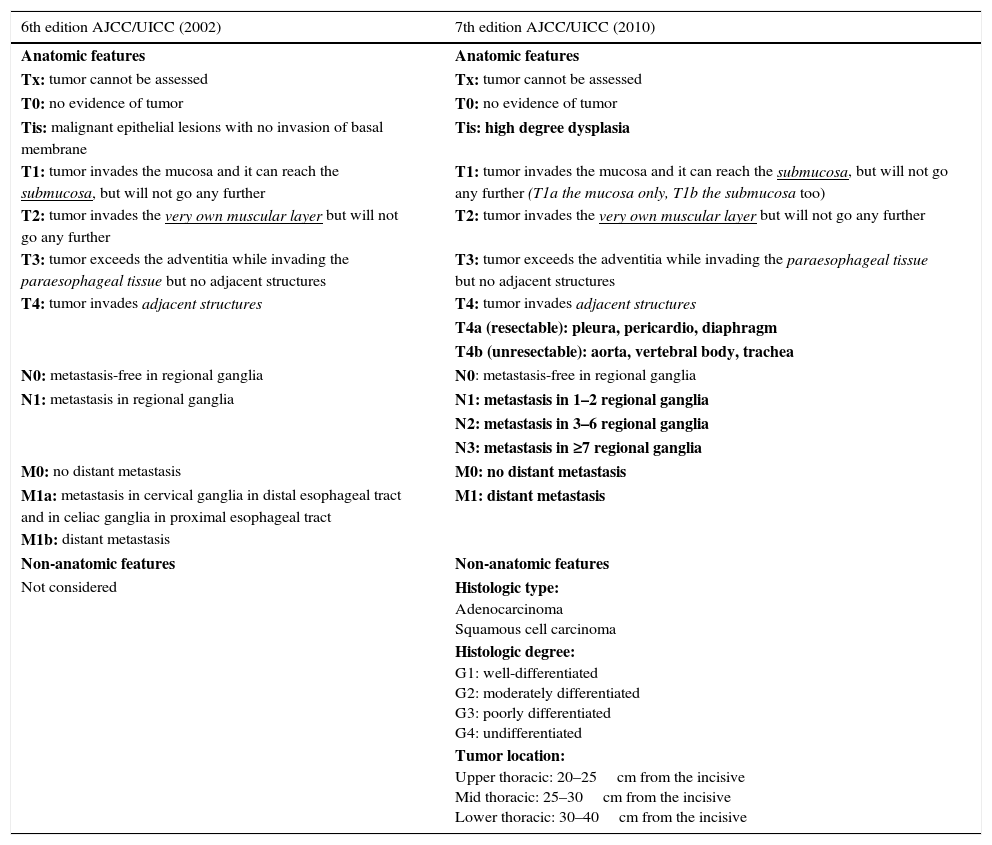
StratificationThe actual classification of esophageal cancer is in the 7th TNM edition of the American Joint Committee on Cancer,24 which was updated in 2010 and introduced important changes to the previous classification.25 It is the result of a collaborative project by several centers from five different countries in three continents, in which tumor characteristics were assessed as well as its relation with the survival rates in thousands of patients with esophageal and esophagogastric junction cancer who underwent esophagectomy. It also seeks harmonization with gastric cancer classification.26,27 It includes new definitions in the anatomical classification of the tumor in situ and of stage T4, which is divided into two: tumors that infiltrate the pleura, the pericardium or the diaphragm and are considered resectable (T4a), and tumors that infiltrate the aorta, the vertebral bodies or the trachea, which are considered non-resectable (T4b). It approaches a review of ganglionic spread classification (N), includes all the ganglia described before as regional ganglia, from the supraclavicular chains to the celiac trunk, and also considers the number of affected ganglia as a prognostic factor. Finally it reduces the category of metastatic extent (M) to two groups: absent (M0) or present (M1), including metastasis in non-regional ganglia. It also introduces non-anatomical characteristics, such as the type, the tumor degree and the location that were not taken into consideration in the previous classification (Table 1).
Comparison between the 6th and 7th editions of TNM classification. In bold on the right column the changes introduced in the actual categorization are shown.
| 6th edition AJCC/UICC (2002) | 7th edition AJCC/UICC (2010) |
|---|---|
| Anatomic features | Anatomic features |
| Tx: tumor cannot be assessed | Tx: tumor cannot be assessed |
| T0: no evidence of tumor | T0: no evidence of tumor |
| Tis: malignant epithelial lesions with no invasion of basal membrane | Tis: high degree dysplasia |
| T1: tumor invades the mucosa and it can reach the submucosa, but will not go any further | T1: tumor invades the mucosa and it can reach the submucosa, but will not go any further (T1a the mucosa only, T1b the submucosa too) |
| T2: tumor invades the very own muscular layer but will not go any further | T2: tumor invades the very own muscular layer but will not go any further |
| T3: tumor exceeds the adventitia while invading the paraesophageal tissue but no adjacent structures | T3: tumor exceeds the adventitia while invading the paraesophageal tissue but no adjacent structures |
| T4: tumor invades adjacent structures | T4: tumor invades adjacent structures |
| T4a (resectable): pleura, pericardio, diaphragm | |
| T4b (unresectable): aorta, vertebral body, trachea | |
| N0: metastasis-free in regional ganglia | N0: metastasis-free in regional ganglia |
| N1: metastasis in regional ganglia | N1: metastasis in 1–2 regional ganglia |
| N2: metastasis in 3–6 regional ganglia | |
| N3: metastasis in ≥7 regional ganglia | |
| M0: no distant metastasis | M0: no distant metastasis |
| M1a: metastasis in cervical ganglia in distal esophageal tract and in celiac ganglia in proximal esophageal tract | M1: distant metastasis |
| M1b: distant metastasis | |
| Non-anatomic features | Non-anatomic features |
| Not considered | Histologic type: Adenocarcinoma Squamous cell carcinoma |
| Histologic degree: G1: well-differentiated G2: moderately differentiated G3: poorly differentiated G4: undifferentiated | |
| Tumor location: Upper thoracic: 20–25cm from the incisive Mid thoracic: 25–30cm from the incisive Lower thoracic: 30–40cm from the incisive |
The actual edition includes esophagogastric junction tumors in the same group as esophageal tumors. They can be divided into three types based on Siewert's modified classification: type 1, with its epicenter in the distal esophagus some 5 and 1cm above the esophagogastric junction; type 2, with its epicenter some 1cm above and 2cm below the esophagogastric junction; and type 3, with its epicenter in the cardia some 2 and 5cm below the esophagogastric junction, in tumors that spread to the very esophagogastric junction.28
By applying this classification we can achieve group staging that are much more complex than those in the previous edition but they allow us to better stratification of the overall survival prognosis.29
A new review of the TNM classification (8th edition) has been announced for 2016, and will become effective on January 1st, 2017. The main limitation of the actual classification is that the data it is based on come exclusively from patients who underwent esophagectomy. The new classification is supposed to have a greater homogeneity among the different stages; an initial clinical classification and after the induction treatment; other non-anatomical characteristics of the tumor that can affect survival; survival data of patients that have not undergone esophagectomies, and a possible harmonization of cervical esophageal cancer with neck and head tumors.30,31
Diagnosis, clinical staging, response asesinen and image follow-upDetermination of non-anatomic featuresEsophagoscopy and biopsyThey are crucial tools to assess the non-anatomical characteristics of the tumor. Apart from defining tumor location based on the distance from the incisors to its upper limit, through the analysis of the sample obtained, they tell us its type and histologic degree.32
EsophagogramAlthough it was used in the past as the initial diagnostic modality before any suspicion of esophageal cancer prior to the endoscopic study, at present its systematic use has been abandoned. In concrete cases it can provide information about tumor location and extent of the tumor especially in stenosing neoplasms that prevent the passage of the endoscope or to prove the presence of tracheoesophageal fistula.33
Determination of the anatomic characteristicsEcoendoscopyIt is considered to be the most accurate image modality to assess the depth of tumoral infiltration on the wall (cT), with sensitivity and specificity values of 81.6% and 99.4% respectively for T1 tumors and, 92.4% and 97.4% for T4 tumors.34
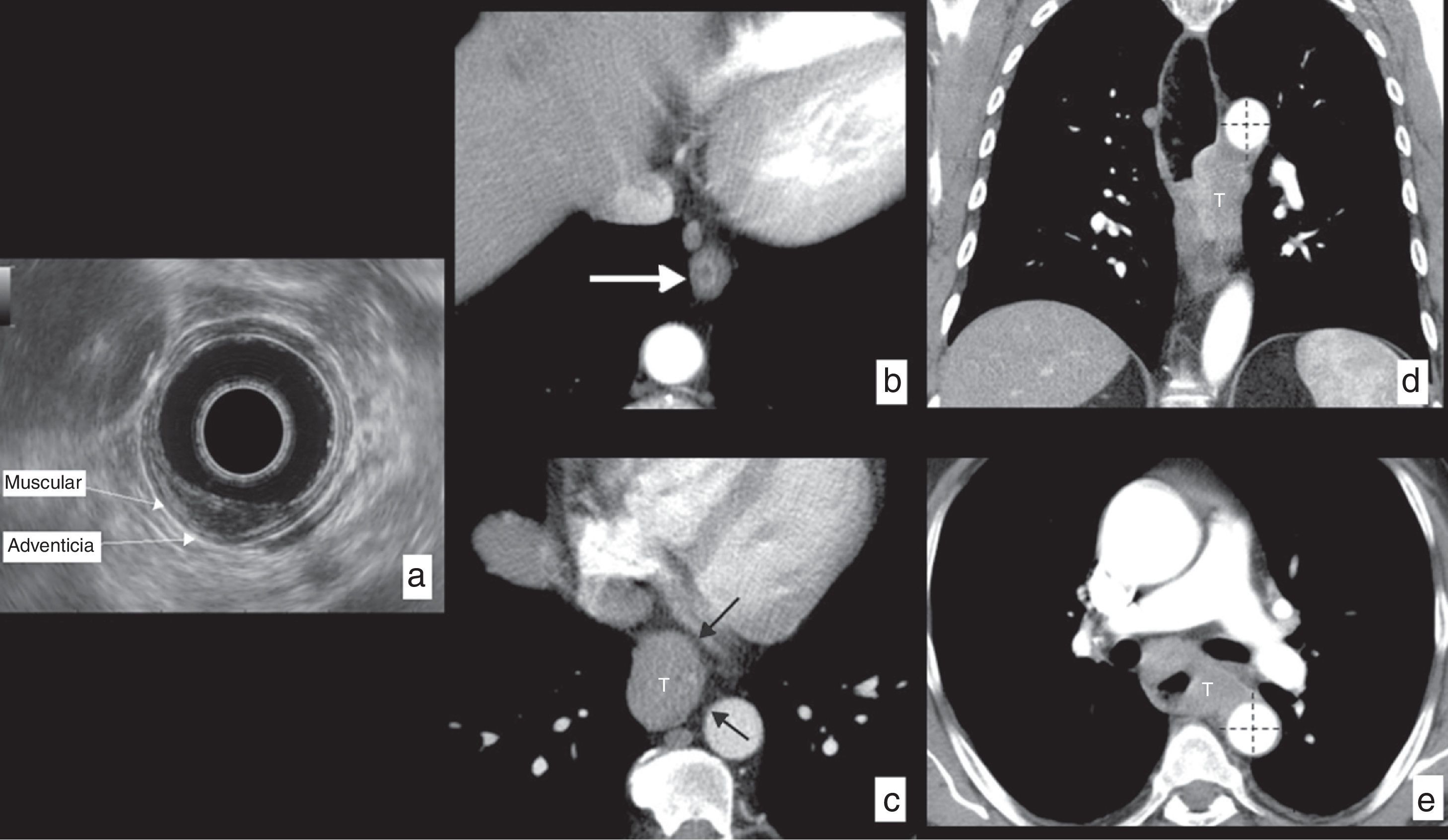
It is especially useful in the accurate assessment of tumors in their initial stages (T1–T2) in order to plan conservative endoscopic treatments (endoscopic resection) or initially surgical treatments with esophagectomy (Fig. 5), and it identifies patients in more advanced stages (T3–T4) that will receive neoadjuvant or definitive treatments through chemotherapy or chemoradiotherapy.
Esophageal cancer, assessment of wall infiltration (cT) in different patients. (a) Echoendoscopy in a patient with epidermoid carcinoma in the mid third of the esophagus invading the mucosa and the submucosa, but not exceeding it, respecting the muscular layer: T1b based on actual TNM classification. (b) Axial section CT in another patient showing a small polypoid lesion of endoluminal growth based on the esophageal wall (arrow). After surgical resection, the histological study confirmed that the tumor respected the muscular layer: T1 according to actual TNM classification. (c) Axial CT section showing a tumor (T) of the lower third of the esophagus infiltrating the periesophageal tissues, but at all times showing a fat plane with the neighboring structures (black arrows), ruling out their infiltration: T3 according to actual TNM classification. (d) CT in coronal reconstruction and axial section (e) of another patient with tumor (T) of the lower and mid thirds of the esophagus spreading beyond the wall and showing extensive contact with the descending aorta, in more than 90° (dotted lines), radiologically suggesting its infiltration: T4b (unresectable) based on the actual TNM classification.
The most important limitations of this modality are that it is operator-dependent and that the tumor is a stenosing tumor in a large number of cases and it cannot be evaluated.
It allows us to assess the mediastinal ganglia (cN), identifying as pathological those with a diameter >10mm, with rounded morphology, hypoechoic with poorly-defined contours, with sensitivity and specificity values based on these characteristics of 76–84% and 70–85%, respectively. In addition, in cases in which its determination conditions a change of the therapeutic strategy, it offers the possibility of performing a cytological analysis through a guided fine-needle puncture-aspiration (FNPA) that significantly increases sensitivity from 84.7% to 96.7% and specificity from 84.6% to 95.5% and its proven to be an independent prognosis predictor from survival.35 This way, it has become a very accurate tool to assess ganglionic local-regional spread (cN), though partially, since it cannot evaluate cervical ganglionic and mediastinal chains far from the esophageal wall.12,30,34,36,37
Even though the role of the echoendoscopy is well established in the initial assessment, it is not a useful tool for evaluation after neoadjuvance due to the high rate of overstaging as a consequence of inflammatory and fibrosis changes secondary to the treatment.38–40
Computerized tomographyIts main role in local tumor staging (cT) is ruling out invasion of adjacent organs (T4). It suggests tumor infiltration if the contact area between the tumor and the aorta is beyond 90° or if there is obliteration of the fat triangle between the esophagus, the aorta and the adjacent vertebral body; if there is displacement or deformation of the contour of the posterior wall of the trachea or the main bronchi, esophagotracheal or esophagobronchial fistula, or direct spread of the tumor into the airway lumen a bronchoscopy is indicated; and if an irregularity of the heart border contour is seen with loss of the adjacent fat planes. The presence of any of these findings leads to considering the possibility of an unresectable tumor (T4b). The sensitivity and specificity values of the technique to detect aortic or tracheobronchial infiltration are almost 100% and 52–97%, respectively.12 For the rest of the mediastinal structures, the image criterion used to rule out their infiltration is preservation of their fat planes with the lesion (Fig. 5).
The presence of tumoral tissue in mediastinal fat indicates T3 (extramural spread without infiltration of neighboring structures). CT offers a limited diagnostic precision in the early stages of the disease (T1–T2), being this case the lesion must appear confined to the esophageal wall10,12,32 (Fig. 5).
In the assessment of local-regional (cN) ganglionic spread, it is less accurate than the echoendoscopy, since the criterion size, normally used as a suspicious finding, is a poor predictor of tumor infiltration (there are reactive ganglia of increased size and tumor ganglia of normal size). The established sensitivity and specificity values of the technique are 59% and 81%, respectively.37
A ganglion is considered CT-suspicious when its smallest diameter is more than 10mm if it is located on the thoracic and abdominal chains, and 5mm in the supraclavicular chains. Other image characteristics should be taken into account, such as loss of normal morphology, hyper-uptake, heterogeneous density or border irregularity, to improve diagnostic precision (Fig. 6).
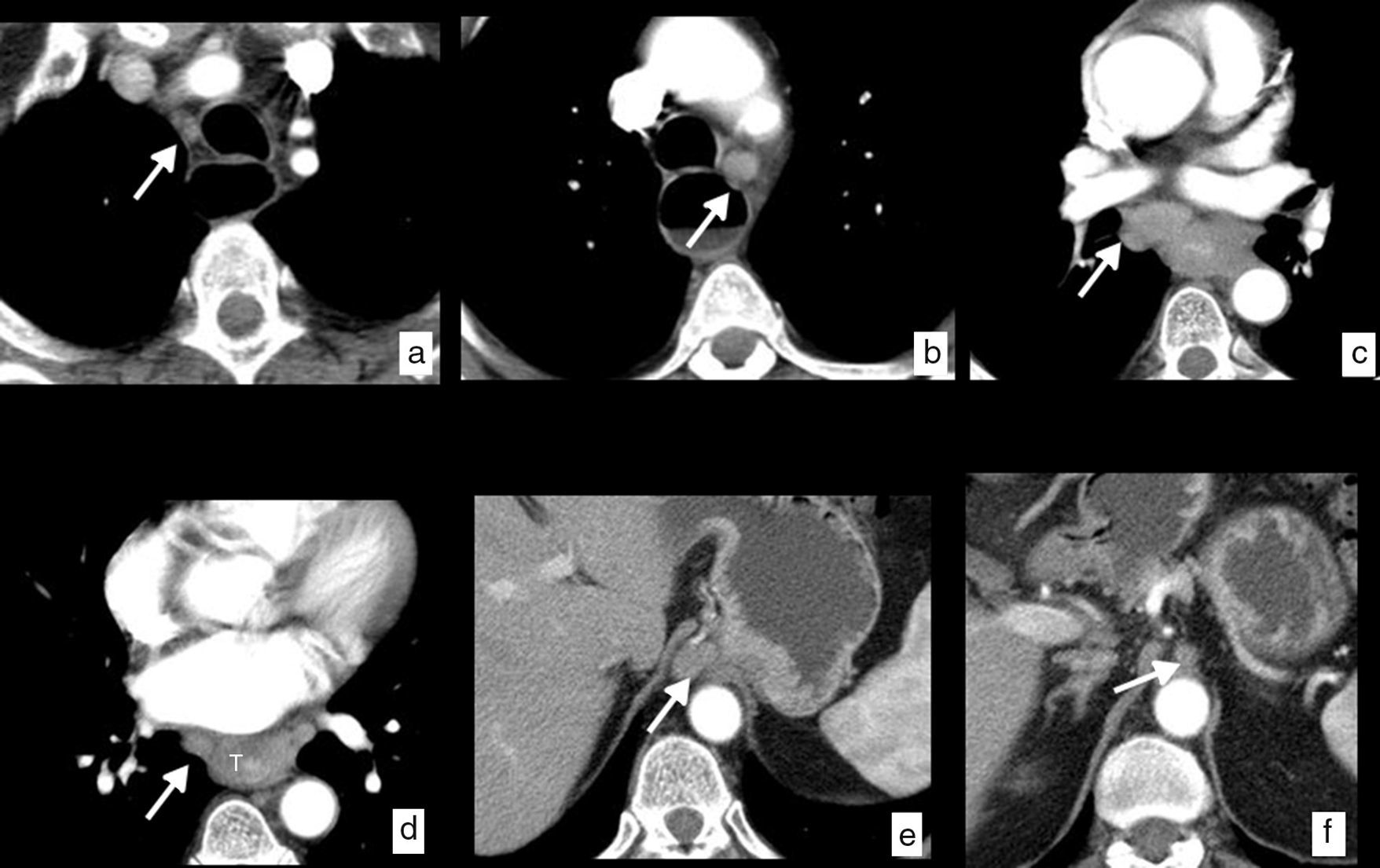
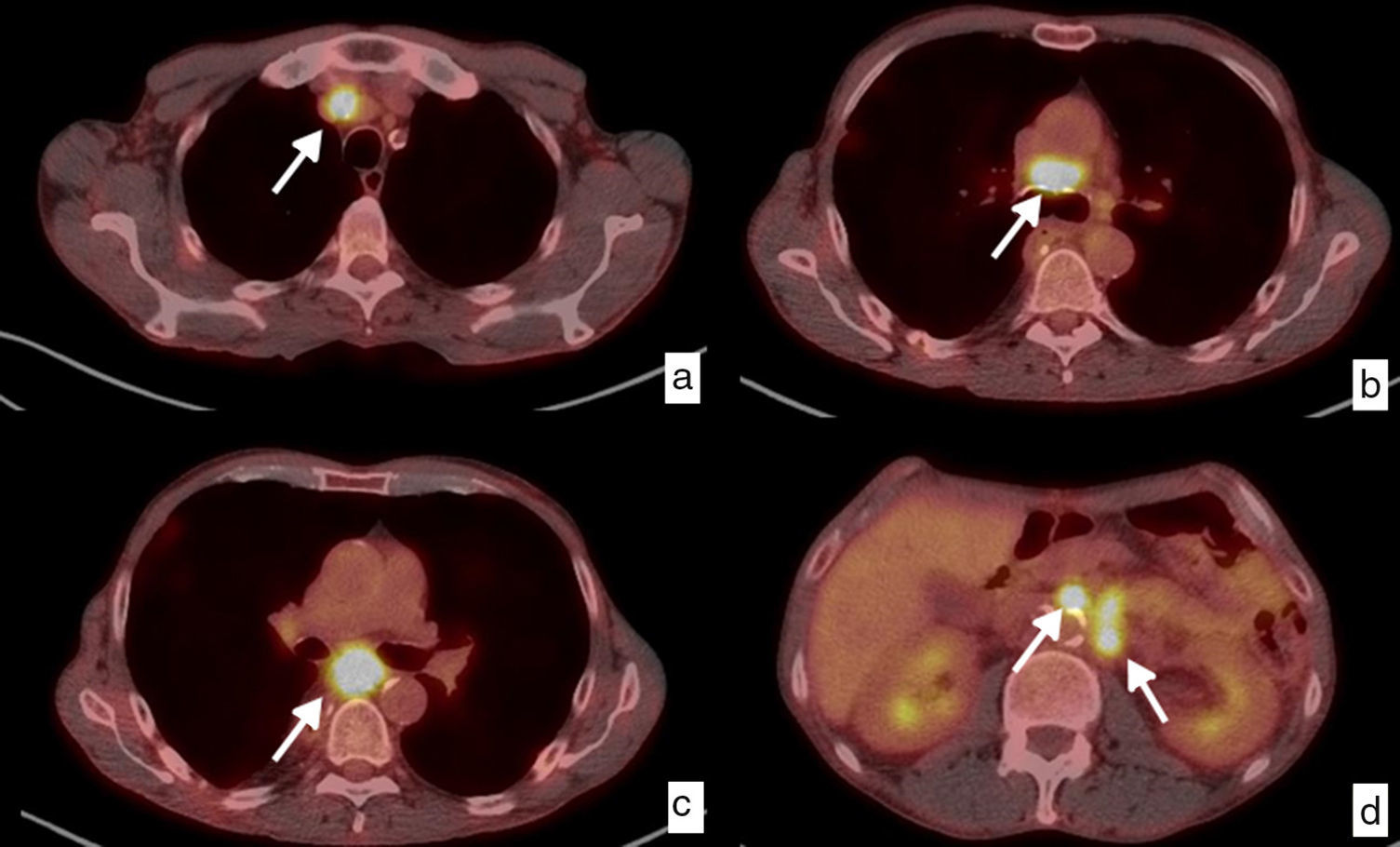
Esophageal cancer, with ganglionic dissemination on CT studies (cN). Axial images where it is possible to identify pathological ganglia (arrows) secondary to spread to ganglia in different loco-regional mediastinal and upper abdominal chains. (a) Right upper paratracheal chain (2R). (b) Left lower paratracheal chain (4L). (c) Subcarinal chain (7). (d) Mid paraesophageal chain (8M). (e) Paracardial chain (16). (f) Celiac chain (20). Thickening of the wall in the mid third of the esophagus (T) with respect to the primary tumor (d).
As an added value and unlike echoendoscopy, it allows us to assess all the esophagus lymphatic drainage chains.
Our study protocol in esophageal cancer includes a first helix that is acquired 30s after the beginning of the administration of IV iodine contrast and covers from the lower cervical region (due to the extent of the lymphatic drainage of the esophagus) to the costophrenic sinuses, to safely include tumors of lower segments, and a second helix with a 70-s delay from the diaphragmatic cupolas to the ischial tuberosities. Immediately before performing the study, the patient drinks four glasses of water as negative oral contrast.
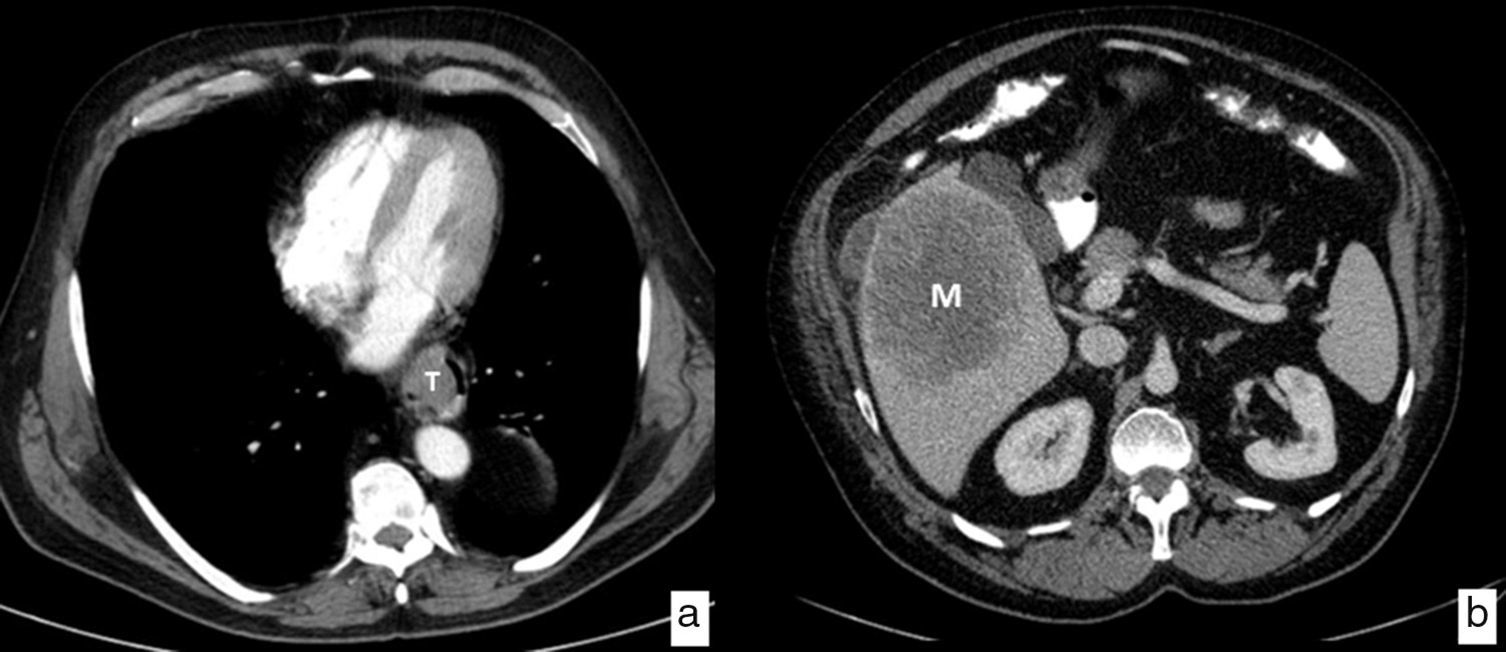
The CT is the image modality most widely used in the initial assessment of patients diagnosed with esophageal cancer to rule out distant metastasis (cM); the most frequently affected organs are the liver, the lungs, the bones and the suprarenal glands. It has a 90% sensitivity to detect liver metastasis>1cm (Fig. 7). Thirty percent of the patients have metastasis at the moment of diagnosis precluding them from the possibility of surgical treatment with curative intents.
It is usually the tool used to assess the response to neoadjuvant treatment, though it has important limitations due to the increase in density of the periesophageal fat planes resulting from it.
For the image follow-up of the patients treated for esophageal cancer with curative intention, it is recommended to perform a thoracic, abdominal and pelvic CT, every 6 months during the first 2 years, and subsequently every year for the next 3–4 years. It can be complemented with other examinations when in diagnostic doubt and based on the place where tumoral relapse is suspected, which more often appears as a distant disease. It is also the test indicated for the follow-up of patients in palliative treatment based on the clinical needs.41
PET-CTThe examination is usually performed from the cranial base to the thighs, with the arms above the head to avoid artifacts in the thorax. The dose used for a 70kg adult is 370MBq of IV 18F-FDG in slow infusion being images are acquired 60min later.42
The combined use of both modalities has proven to be superior to the use of each of them separately. The main utility of PET-CT with 18F-FDG lies in the identification of metastases (cM) undetected through other modalities – an estimate of up to 15% of patients, or synchronic tumors, not uncommon, in head and neck, lungs or stomach, associated with the use of alcohol and tobacco. For local-regional spread, limitations in the spatial resolution of the assessment of functional image conditions the degree of tumor infiltration on the wall (cT), and though it can detect tumoral ganglia more accurately than the CT and in territories far from the echoendoscopy, the intense uptake of the tracer by the primary tumor makes it hard to differentiate from peritumor ganglia (cN).12,35,43
Today, clinical guidelines do not recommend its systematic use in these patients’ initial assessment, except as an alternative to CT,44 though it is expected that it will be included in the next few years, given the availability of these machines. It allows us to indicate metabolic degree before the treatment, as a prognostic factor, while facilitating the subsequent follow-up after neoadjuvance or in case of a relapse.45
It has a well-established role in planning radiotherapy and it can be useful for the assessment of local response to neoadjuvant treatment, though contradictory results have been reported due to the tracer uptake persistence due to chemoradiotherapy-induced esophagitis and ulceration (false positives), so it necessary to have a larger number of studies backing up its utility.
It helps us identify interval metastasis (that appear in the course of the treatment prior to surgery), in up to 8% of the cases,46 and detect tumor relapse during follow-up, especially when there are diagnostic doubts with other modalities43,47–49 (Fig. 8).
Local and distant ganglionary relapse. Diagnosis through PET-CT. Patient with initial diagnosis of epidermoid carcinoma in the lower third of the thoracic esophagus who underwent surgery with pathologic staging pT3pN2. Axial images in follow-up study, 6 months after surgery. Pathologic ganglia (white arrows) that have increased in size, with an increase in the trace uptake, a maximum SUV ranging from 11.61 to 9.64, in the mediastinal chains indicative of local ganglionic relapse (a, b, c), and upper retroperitoneal, left pre- and para-aortic chains in relation to distant relapse (M1) (d).
Magnetic resonance images (MRI) is not used systematically in these patients’ assessment. However, the recent improvement in MRI protocols, with faster sequences, and the use of cardiac and respiratory synchronization, which has allowed us to improve image quality, and the valuable functional information provided by diffusion sequences, widely used in the assessment of other organs, makes it possible for MRIs to play a complementary role solving the limitations of the other modalities.49,50
The MRI has a greater tissue contrast resolution than CT and PET-CT, which in some cases can clarify doubts about any possible tumor infiltrations of neighboring structures (T4) when findings are not conclusive with those from other modalities (Fig. 9). The precision of the test reaches 81% in comparison with the histological findings in the assessment of tumor infiltration of the wall (cT).51
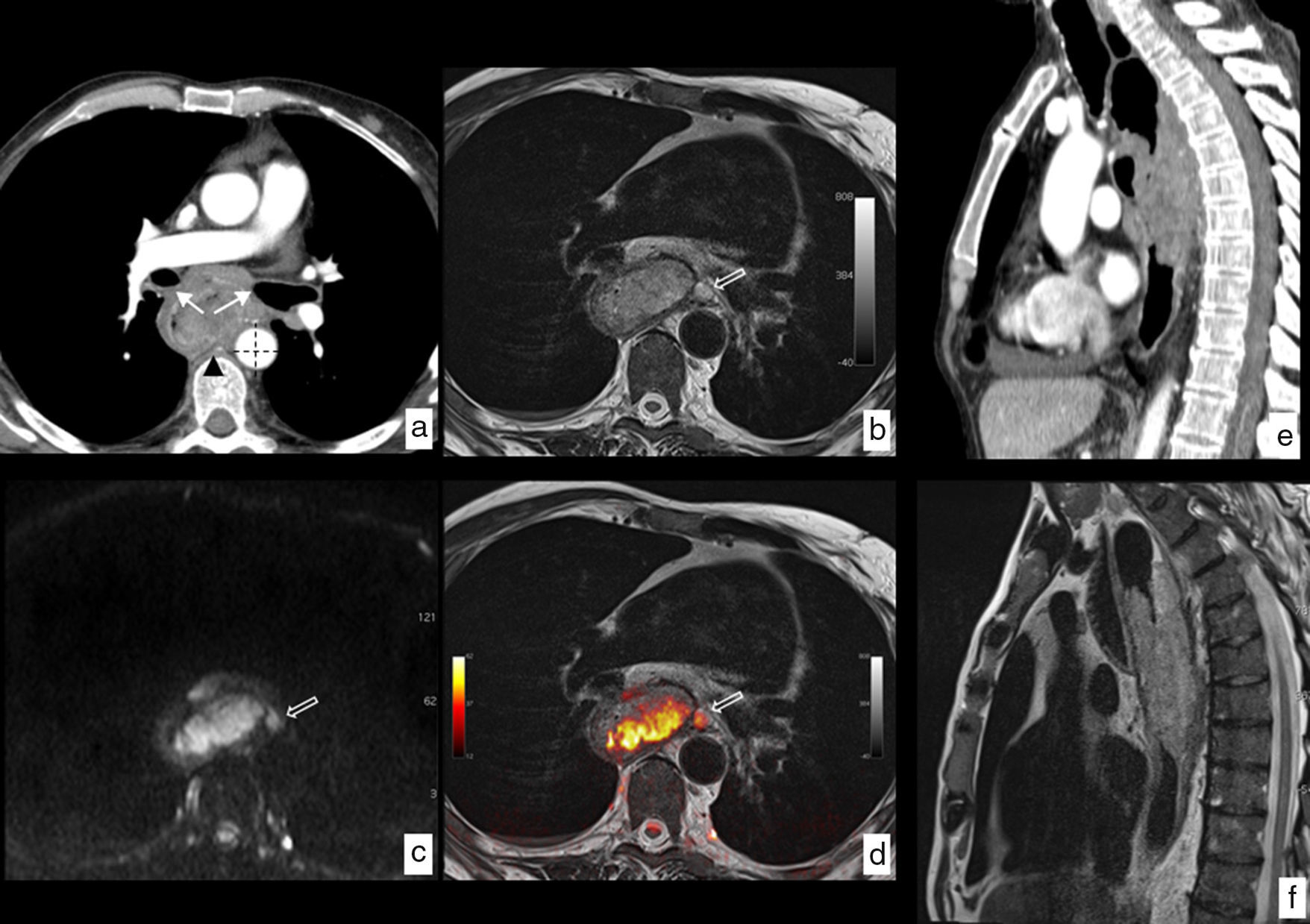
MRI of esophageal cancer with local infiltration. Neoplasm in tupper and mid third of the esophagus with a biopsy of epidermoid carcinoma. (a) Axial section CT where the esophageal lesion is identified contacting the posterior wall of both main bronchi (arrows), with the descending aorta in more than 90° (dotted lines) and with one of the vertebral bodies (arrowhead), no identification of fat plane with such structures, which radiologically indicates local infiltration being in this case unresectable. (b) Axial section T2-weighted MRI at the same height, showing the greatest tissue resolution of this modality which lets us see the absence of structure infiltration described and also shows a pathological ganglion (hollow arrow) unidentified in the CT study. (c) Diffusion-weighted MRI at the same height providing functional information, showing high signal with a b value b=1.000s/mm2, indicating restriction of water diffusion in the tumoral lesion and presence of the above-mentioned paraesophageal ganglion. (d) T2-diffusion fusion image including anatomical and functional information in a combined way. Comparison of the greatest tissue contrast resolution of the MRI (f) as opposed to the CT (e) on the sagittal plane ruling out infiltration of vertebral bodies. Final clinical staging T3 N2-3 M0 that was not considered for surgical treatment being the patient treated with definitive chemoradiotherapy.
Also STIR and diffusion sequences improve the detection of local-regional ganglia (cN) and allow us to distinguish them from tumor infiltration of the wall and periesophageal tissues. Values of up to a 81% sensitivity, a 95% specificity and a95% precision have been reported with the use of STIR sequences.52
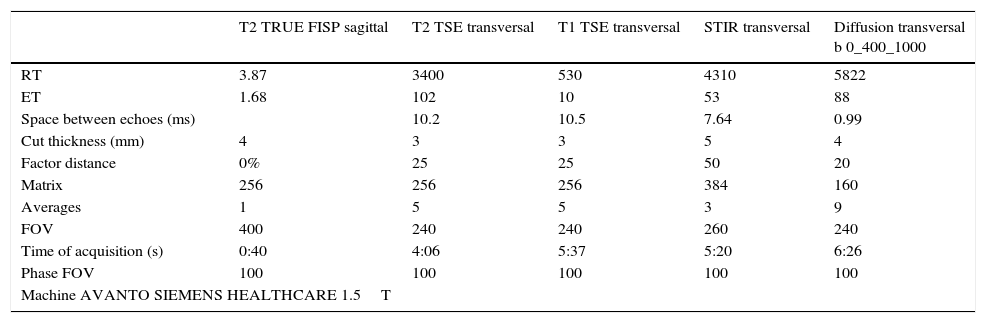
Even though the MRI study protocols should be adapted to the concrete reason why the examination is performed, in general it includes a fast T2-weighted sequence on the sagittal plane, which serves as locator; T1- and T2-weighted sequences in axial incidence, covering the entire lesion; and STIR and diffusion sequences, obtaining the corresponding apparent diffusion coefficient (ADC) map, also in axial incidence and covering the entire thorax53 (Table 2).
MR study acquisition protocol used in our center for esophageal cancer.
| T2 TRUE FISP sagittal | T2 TSE transversal | T1 TSE transversal | STIR transversal | Diffusion transversal b 0_400_1000 | |
|---|---|---|---|---|---|
| RT | 3.87 | 3400 | 530 | 4310 | 5822 |
| ET | 1.68 | 102 | 10 | 53 | 88 |
| Space between echoes (ms) | 10.2 | 10.5 | 7.64 | 0.99 | |
| Cut thickness (mm) | 4 | 3 | 3 | 5 | 4 |
| Factor distance | 0% | 25 | 25 | 50 | 20 |
| Matrix | 256 | 256 | 256 | 384 | 160 |
| Averages | 1 | 5 | 5 | 3 | 9 |
| FOV | 400 | 240 | 240 | 260 | 240 |
| Time of acquisition (s) | 0:40 | 4:06 | 5:37 | 5:20 | 6:26 |
| Phase FOV | 100 | 100 | 100 | 100 | 100 |
| Machine AVANTO SIEMENS HEALTHCARE 1.5T | |||||
RT: repetition time; ET: echo time; FOV: field of view.
The role of the MRIs in planning treatment with radiotherapy is well-established in other tumors, such as head and neck, prostate, rectum and cervix tumors, and based on the above-mentioned better tissue contrast resolution; this modality can be of a significant utility in the future, allowing us to optimize the doses and minimization of the volume of radiation of neighboring structures.54
Recent studies indicate that MRIs can play a significant role in the assessment of neoadjuvant treatment response especially when the results of CT and PET-CT are contradictory. It could even predict tumor response before treatment using functional images through MRI (diffusion sequence).55–57
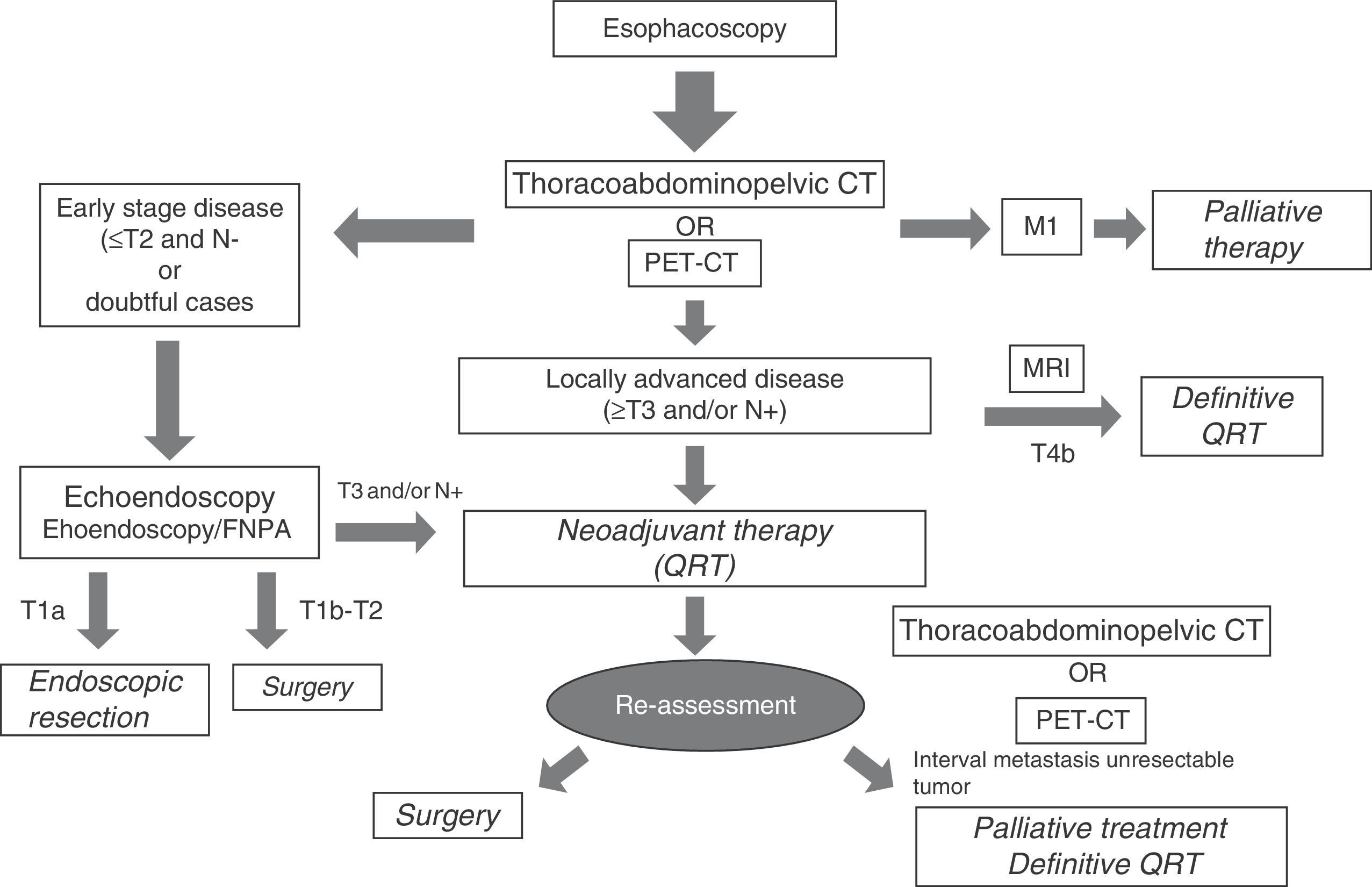
Selecting diagnostic testsTable 3 summarizes the strengths and weaknesses of each image modality used in the clinical staging of esophageal cancer. The greatest diagnostic precision is obtained with an adequate combination of them, which is presented in a diagnostic algorithm that allows us to properly classify patients to receive the most appropriate treatment based on the characteristics of the tumor, on each and every one of the stages of the therapeutic process (Fig. 10).
This way, after the endoscopy and the histological confirmation of esophageal cancer, the initial image modality test in most centers is a thoracoabdominopelvic CT, including the supraclavicular region. Based on availability, it could be replaced by a PET/CT. in those cases in which an early-stage disease is suspected, which are unfortunately few, it would be indicated to perform an echoendoscopy to select those patients in stage T1a (only mucosa infiltration, respecting the submucosa), on whom an endoscopic resection of the tumor could be performed. If the T1a clinical stage is confirmed in the histology, definitive treatment would be considered, whereas if incomplete resection or infiltration of the submucosa layer (T1b) is confirmed, the treatment would be insufficient and it would have to be completed with an esophagectomy and radical lymphadenectomy, given the risk of ganglionic dissemination through the extensive lymphatic network in this layer of the esophageal wall. In the cases in which infiltration of the muscular layer (T2) is confirmed, the initial approach is surgery. To perform an initially surgical treatment, in addition to the degree of infiltration on the wall, a possible ganglionic local-regional (N0) spread must be ruled out using the different imaging modalities available and, eventually, through an echoendoscopy-guided fine-needle aspiration when in the presence of suspicious ganglia. If metastatic disease (M1) or unresectable tumor (T4b) is diagnosed on the initial CT or PET-CT, surgical treatment is ruled out and the patient will be referred to palliative treatment with chemotherapy or chemoradiotherapy, therefore, on occasion it is advised to confirm the stage through histology or MRI. Locally-advanced resectable tumors (T3, T4a and N+) will receive neoadjuvant treatment with chemoradiotherapy and subsequent reevaluation with a new CT or PET/CT, followed by surgery and adjuvant chemotherapy, unless interval metastasis appears or the tumor becomes unresectable due to local progression. At some centers the use of radical chemoradiotherapy is being considered in this group of patients, especially in those whose particular conditions lead to an expected greater surgical morbimortality. Management of esophageal cancer must be approached by multidisciplinary committees, and it will be individualized to the specific circumstances of each case.5,7,12,21,28,44,58,59
In sum, as radiologists we must be acquainted with the special anatomical characteristics and the actual classification of esophageal cancer, and know about the utility of each image diagnostic tools available to provide relevant information in the clinical management of these patients.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments with human beings or animals have been performed while conducting this investigation.
Confidentiality of dataThe authors confirm that in this article there are no data from patients.
Right to privacy and informed consentThe authors confirm that in this article there are no data from patients.
Authors’ contribution- 1.
Manager of the integrity of the study: JEI.
- 2.
Study idea: JEI.
- 3.
Study design: JEI, MACC.
- 4.
Data mining: JEI, RRP, AAD.
- 5.
Data analysis and interpretation: JEI, MACC, GCFP.
- 6.
Statistical analysis: N/A.
- 7.
Reference: JEI, MACC.
- 8.
Writing: JEI, MACC, GCFP, RRP.
- 9.
Critical review of the manuscript with intellectually relevant remarks: JEI, MACC, GCFP, RRP, AAD.
- 10.
Approval of final version: JEI, MACC, GCFP, RRP, AAD.
The authors declare no conflict of interests associated with this article.
Please cite this article as: Encinas de la Iglesia J, Corral de la Calle MA, Fernández Pérez GC, Ruano Pérez R, Álvarez Delgado A. Cáncer de esófago: particularidades anatómicas, estadificación y técnicas de imagen. Radiología. 2016;58:352–365.