To study the usefulness of common MRI perfusion parameters for identifying pseudoprogression in high grade astrocytomas.
Materials and methodsThis retrospective case–control study compared the relative cerebral blood volume (rCBV), the relative percentage of signal intensity recovery (rPSR), and the relative peak height (rPH) recorded in a sample of 17 cases of anaplastic astrocytomas and gliomas considered to be undergoing pseudoprogression by biopsy or follow-up with those recorded in a sample of histologically similar tumors that were treated and considered to be undergoing progression by histologic study or follow-up. We evaluated the accuracy of these parameters and the correlations among them. Statistical significance was set at p<.05.
ResultsThe rCBV, rPSR, and rPH were significantly different between the two groups (p=.001). The cutoff values rPH=1.37, rCBV=0.9, and rPSR=99% yielded sensitivity (S)=88% and specificity (Sp)=82.2% for rPH, S=100% and Sp=100% for rCBV, and S=100% and Sp=70.6% for rPSR, respectively. We found negative correlations between rPRS and rPH (−0.76) and between rPRS and rCBV (−0.81) and a high positive correlation between rPH and rCBV (0.87).
ConclusionThe variables rPH and rCBV were useful for differentiating between pseudoprogression and true progression in our sample. The variable rPRS was also very sensitive, although the overlap in the values between samples makes it less useful a priori.
Estudiar la utilidad de los parámetros de perfusión en RM para identificar la seudoprogresión tumoral en astrocitomas de alto grado.
Material y métodosEste estudio retrospectivo de casos y controles comparó el volumen sanguíneo cerebral relativo (VSCr), el porcentaje de recuperación de intensidad de señal relativo (PRSr) y la altura relativa del pico (relative Peak Height [rPH]) en una muestra de 17 casos de astrocitomas anaplásicos y glioblastomas diagnosticados de seudoprogresión (mediante biopsia o control evolutivo), con otra muestra de 17 tumores tratados, histológicamente parecidos, y diagnosticados anatomopatológica o evolutivamente de progresión. Se evaluó la precisión de tales parámetros y su correlación. La significación estadística se estableció con una p<0,05.
ResultadosEl VSCr, PRSr y rPH fueron estadísticamente distintos entre ambos grupos (p=0,001). La sensibilidad (S) y especificidad (E) para los puntos de corte 1,37 rPH; 0,9 VSCr y 99% PRSr fueron respectivamente del 88% (S) y 82,2% (E) rPH; 100% (S) y 100% (E) VSCr; y 100% (S) y 70,6% (E) PRSr. Las variables PRSr-rPH (−0,76) y PRSr-VSCr (−0,81) se correlacionaron negativamente. La correlación rPH-VSCr fue alta (0,87).
ConclusiónLa rPH y el VSCr fueron útiles en nuestra muestra para diferenciar los casos de seudoprogresión tumoral de los de progresión verdadera. El PRSr también fue un parámetro muy sensible aunque el solapamiento de valores entre las muestras lo hacen a priori menos útil.
Standard care of high-grade astrocytomas (anaplastic astrocytoma and gyoblastoma [GBM]) is usually based on a combination of surgery, radiotherapy (RT) and chemotherapy (QT). Temozolamide (TMZ) is the chemotherapeutic agent normally used.1–3
In 2005 it could be demonstrated that adding TMZ to the RT treatment prolonged average survival in 12.1–14.6 months.4,5 But it also triggered a phenomenon known as pseudoprogression,6 consisting of a secondary reaction to therapy with an increased enhanced area even edema similar to a tumor progression. Usually it occurs the first 2–3 months post-therapy.3,4,7 Without histological confirmation the progression was discarded by the stabilization or decrease of the size of the lesion during follow-up without a new anti-tumor therapy. This fact already present with the RT treatment alone made the combination of TR and TMZ6 more evident. The progression of pathological substrate has not been well defined yet.4,8,9
Finding this pseudoprogression has significant clinical implications since misdiagnosing progression would deprive patients of a therapy that is really being effective by changing it for other bailout therapies.
Another important consequence of false positives is the incorrect selection of patients with alleged tumor progression in clinical trials in such a way that the results can be misinterpreted as responses to therapies.10 The diagnosis of pseudoprogression has even been related to a better prognosis of patients who did not experience the phenomenon of pseudoprogression.11
The conventional magnetic resonance (MR) modality cannot prospectively distinguish between pseudoprogression and true tumor progression since both of them can debut with enhanced areas with perilesional edema.1–4,7,10,12 Other image modalities such as MR perfusion, electroscopy and positron emission tomography (PET) can help us distinguish between side effects to therapy and true progressions even though they need to be validated thoroughly before adding them to the Neuro-Oncology Working Group (RANO) response criteria for high-grade gliomas.7,13–15
Tumor enhancement at the contrast-enhanced MRI depends on the disruption of the blood–brain barrier (BBB) and vascular density; this is why it is not a reliable method to assess tumor growths particularly if patient is being treated with antiangiogenic agents or vascular patency-lowering agents.16–18
There are several MR-induced perfusion modalities, wherein the T2-weighted magnetic susceptibility-enhanced MR based on the paramagnetic effect of gadolinium being the most used of all which when passing through the brain’ vessels causes a T2 signal drop and basically T2*.19–22
Parameters that can be measured the most are the brain–blood volume (BBV), the brain–blood flow (BBF) and the average transit time (ATT). Other parameters that can be obtained are the percentage of signal intensity recovery (PSIR) and peak height (PH) through easy mathematical calculations.23–25
For this reason the goal of our work is to study the utility of MR perfusion especially its relative brain–blood volume (rBBV), its relative percentage of signal intensity recovery (rPSIR) and relative peak height (PH) to be able to establish tumor pseudoprogression in high-grade astrocytomes.
Materials and methodsWe did a retrospective analytical observational study of cases and controls comparing perfusion parameters with MR in a sample of patients with pseudoprogression with another similar sample consisting of patients with tumors treated with RT and TMZ with the same histological distribution. Despite the fact that it was retrospective the study was approved by the ethical committee of our hospital.
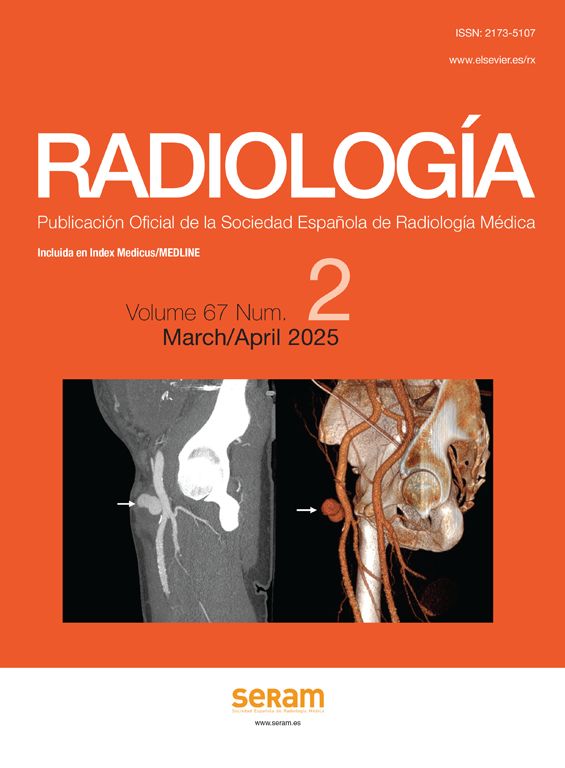
PatientsWe recovered all patients with histological diagnosis of anaplastic astrocytomes and bioblastomes diagnosed between March 2007 and September 2011 from our center’ coding statistical registry of new cases. There were 118 cases total (83 GBM and 29 anaplastic astrocytomes). Inclusion criteria for the group with pesudoprogression2,10,12,23 were: (a) patients followed in our center who would have undergone combined therapy RT+TMZ; (b) increase in size or appearance of new lesions on the contrast-enhanced MRI at the tumor bed within the irradiated field during the first 6 follow-up months after RT; (c) reduced size or disappearance of lesions in controls with no need for a new anti-tumor therapy, or histological confirmation of the absence of progression or tumor relapse (Fig. 1): and (d) optimal technical quality of MR-perfusion trials on the date of pseudoprogression.
Pseudoprogression. (A) SPGR T1-weighted MR with IV contrast in clinically asymptomatic patient with gioblastoma managed with TMZ+RT. At the left frontal lobe level (PS) we can see a peripheral enhanced area compatible with tumor persistence or relapse. (B) Evolutive control 3 months later without new antitumor drugs showing that the enhancement area is completely gone now. PS: pseudoprogression; RT: radiotherapy; SPGR: spoiled gradient recalled; TMZ: temozolomide.
Patients that were precluded from the sample: (a) patients whose perfusion studies were not available on the date of pseudoprogression; (b) patients without combined therapy RT-TMZ; and (c) patients undergoing antiangiogenic therapy during the follow-up who could alter the contrast-enhanced MR.
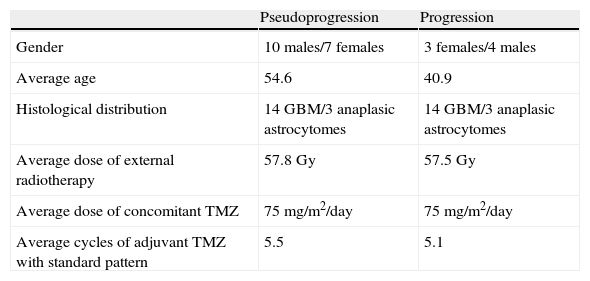
After precluding 2 trials technically unsatisfactory we collected 17 cases of pseudoprogression (14GMB and 3 anaplastic astrocytomes) that met all criteria. Average age was 54.6 years; 10 males and 7 females. This sample was compared to another sample obtained from our statistical archive of 17 tumors treated with RT and TMZ and confirmed progression through biopsy or evolutive control with the same histological distribution in 13 females and 4 males with an average age of 40.9 years (Table 1).
Demographic, clinical, histologic and therapeutic data of samples.
| Pseudoprogression | Progression | |
| Gender | 10 males/7 females | 3 females/4 males |
| Average age | 54.6 | 40.9 |
| Histological distribution | 14GBM/3 anaplasic astrocytomes | 14GBM/3 anaplasic astrocytomes |
| Average dose of external radiotherapy | 57.8Gy | 57.5Gy |
| Average dose of concomitant TMZ | 75mg/m2/day | 75mg/m2/day |
| Average cycles of adjuvant TMZ with standard pattern | 5.5 | 5.1 |
GBM: glioblastoma; Gy: gray; TMZ: temozolomide.
MR trials were conducted using a Signa® HDxt 1.5T scanner (GE Healthcare, Milwaukee, WI, USA) equipment. FLAIR T1-weighted conventional sequences on the sagittal axis, diffusion images on the axial axis, FSE T2-weighted and FLAIR T2-weighted images on the axial axis, and spoiled gradient recalled (SPGR) volumetric acquisitions in the T1-weighted axial axis were included with and without IV contrast. Perfusion trials using magnetic susceptibility were conducted through echo-planar T226-weighted sequences with the following parameters: TR/TE=2.000/80ms; NEX=1; size of matrix=256×256; thickness of section=8mm. Forty image volumes were acquired total. We made 14 contiguous cuts in which brain and cerebellar parenchyma were included. The echo-gradient T2-weighted method is widely used and accepted as it has proven to show a better relation signal/noise, more homogeneous curves of intensity signal/time and particularly greated CBV levels in high-grade astrocytomes than spin-echo methods.26,27
On the other hand spin-echo methods26 are less influenced by neighboring vessels and the effects of contrast extravasation. Thus its utility in lesions with rupture of BBB as in high-grade astrocytomes, metastasis and abscesses.17
We started infusing contrast material 8s after initiating the sequence to wait for the stabilization of signal intensity. Protocol consisted of infusing 10ml Gadovist® (Bayer Schering Pharma, Berlin, Germany) (Gadobutrol 1mmol/ml) and then 20ml saline solution both with a 5ml/s flow. A previous contrast dose capable of minimizing the effect T1 when extravasated was not used. Certain trials have suggested infusing gadilinium before the intensity of the steady signal is lower which makes rPSIR values be underestimated.23
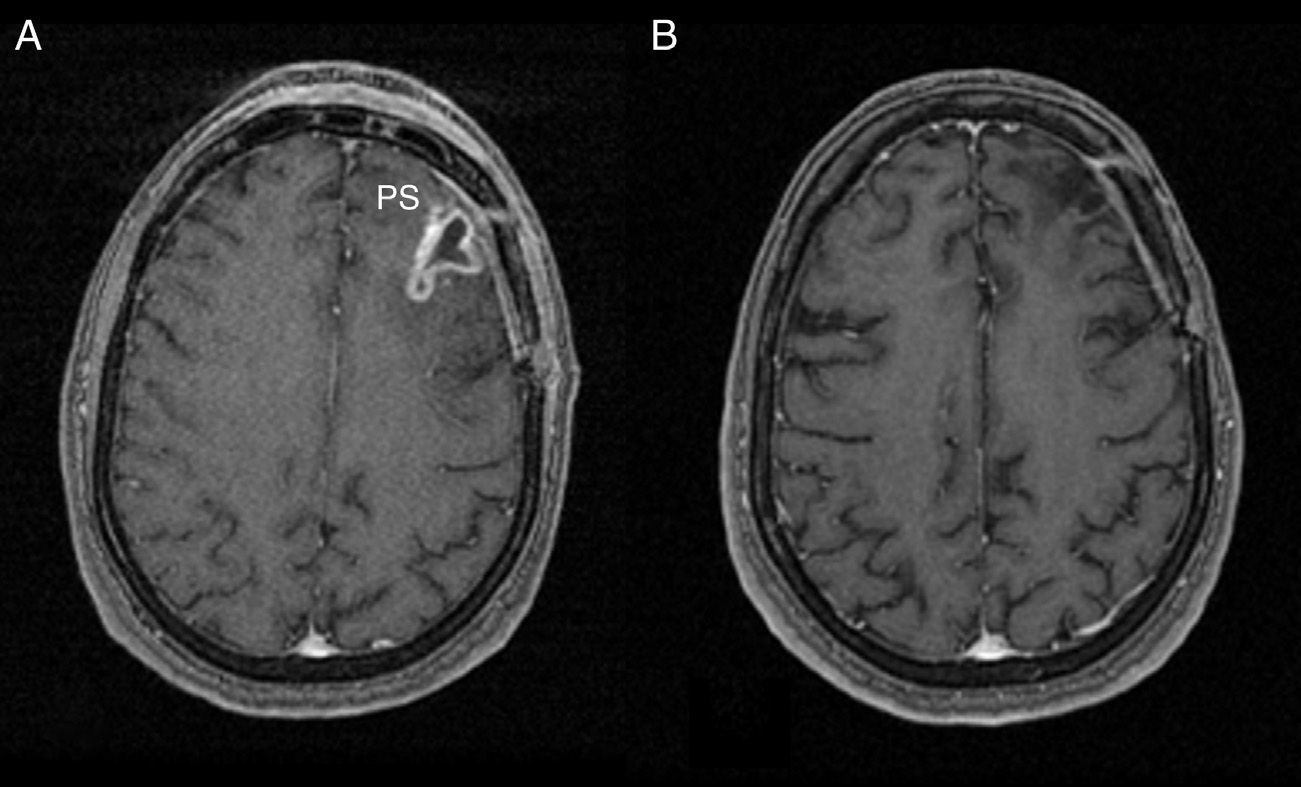
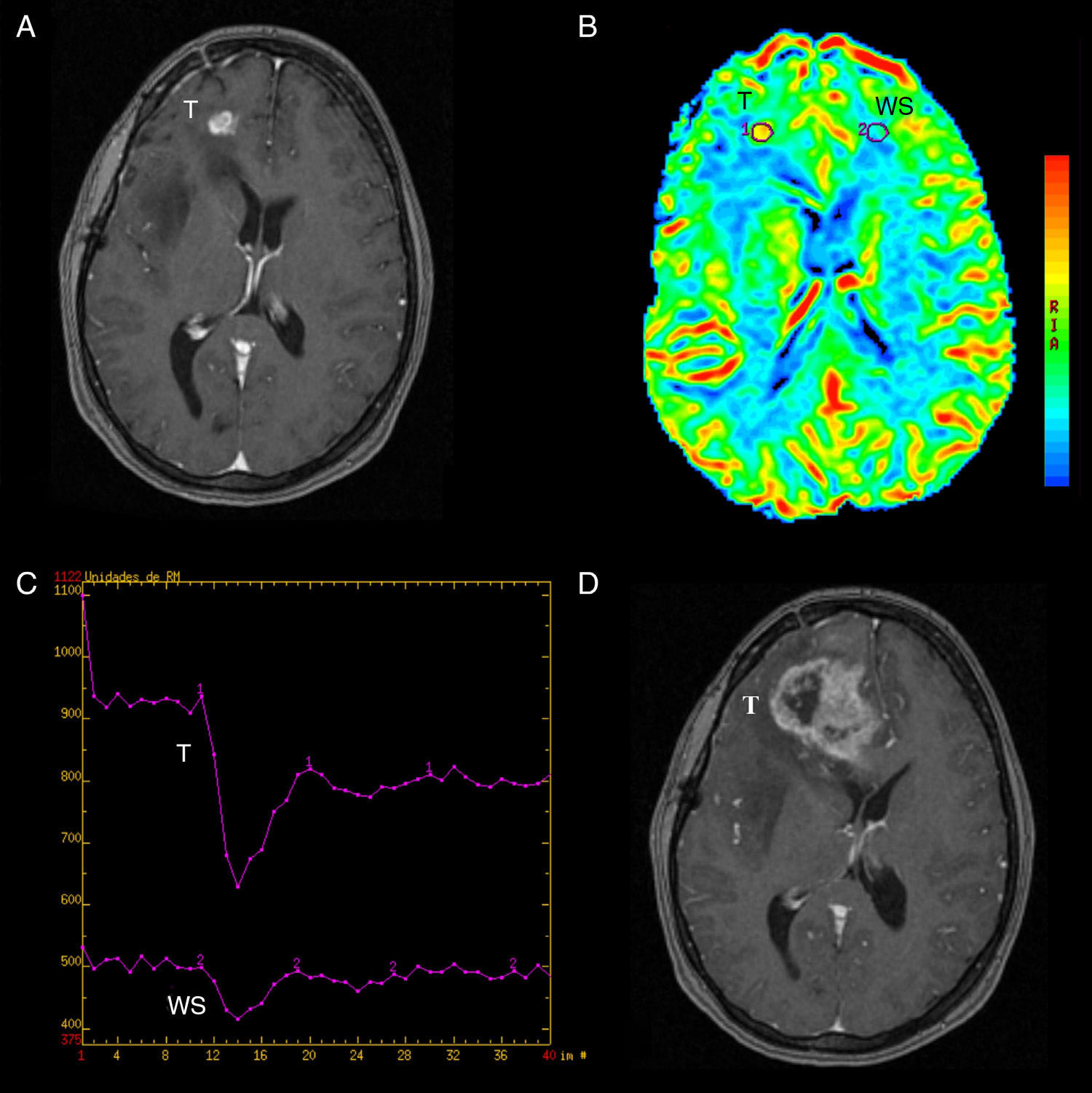
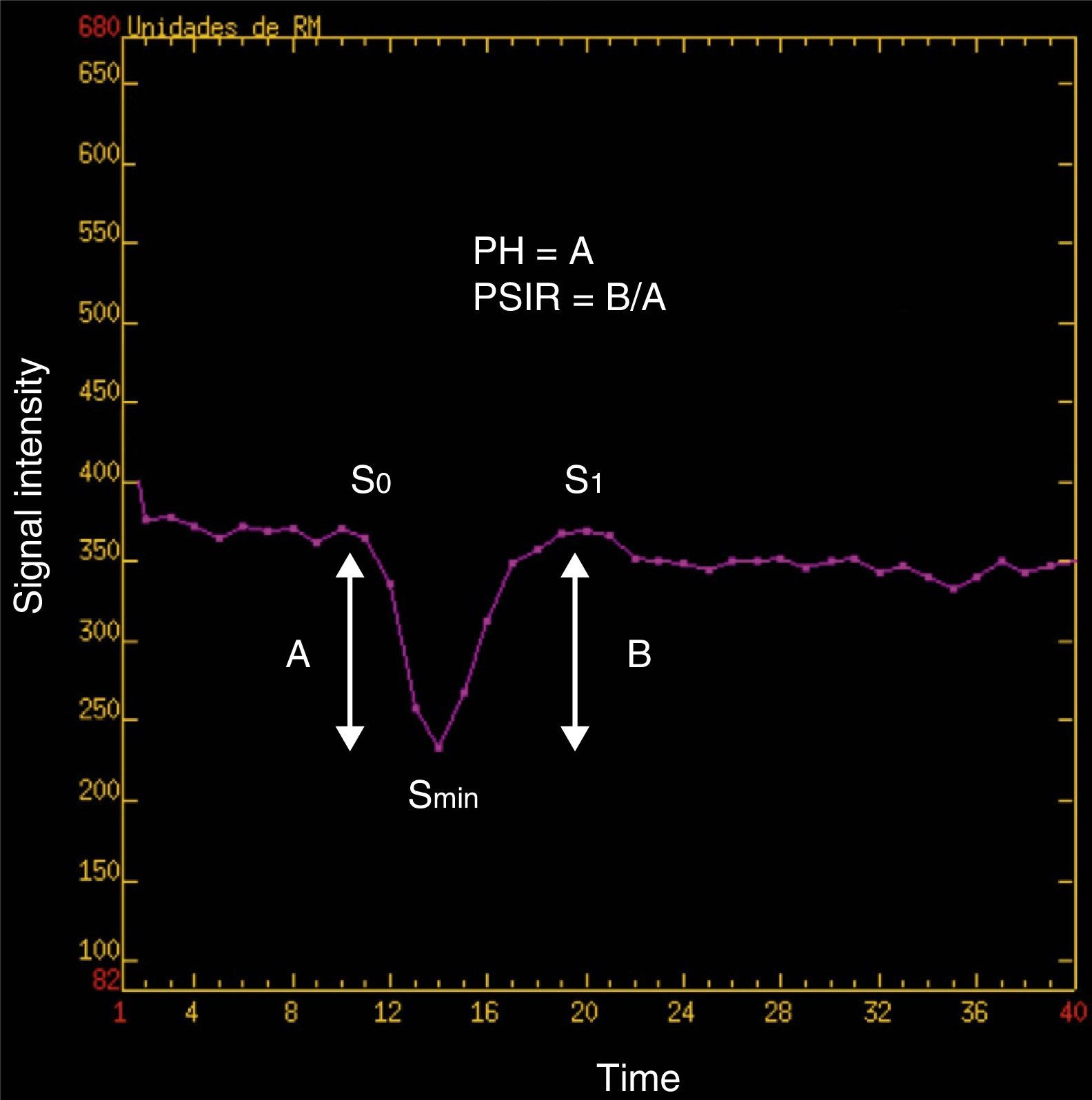
Post-process and perfusion measurementsPost-process of perfusion trials was carried out sharing the same view and simultaneously by 2 authors with 4 and 30 years of experience in neuroradiology, respectively. To assess the rBBV we used the perfusion software Lund University Perfusion Evaluation (LUPE) (software, Lund, Sweden)11,24 and to assess the rPH and the rPSIR the program FuncTool® v.2 (Advantage Windows, GE Healthcare, Milwaukee, WI, USA).23–25 In all cases the calculations were done by drawing manually the regions of interest (ROI) between the 2 operators. The 3 parameters were calculated in relative values. We got the rBBV parametric mapping in which 90–100 pixelate ROI were picked to make them coincide with the of SPGR T1-weighted enhanced-area-sequences while avoiding hemorrhage or the evident macroscopic vessel areas. The values obtained stabilized with the values of the symmetric contralateral ROI by inserting the corresponding software tool (Fig. 2). We used the perfusion software LUPE because it allows us to correct the effect of the contrast leak due to BBB rupture through mathematical algorithms. Both the rPSIR and the rPH were assessed using the signal/time intensity curve by drawing ROI of approximately 40–60mm2 in the negative integral enhancement mapping. ROI were drawn in the corresponding areas to the T1-weighed contrast-enhanced sequences excluding areas of evident macroscopic vessels or hemorrhages. Both the rPSIR and rPH values normalized with the contralateral white matter (Figs. 3 and 4). These are the formulas used to obtain them:
being S0(ROI) the intensity of the precontrast signal at the tumor ROI curve, Smin(ROI) the intensity of the signal at the tumor ROI curve peak, S0(SB) the intensity of the precontrast signal at the healthy contralateral white matter ROI curve, and Smin(SB) the intensity of the signal at the contralateral white matter ROI curve peak.being S1(ROI) the intensity of the precontrast signal at the tumor ROI and S1(SB) the intensity of the precontrast signal at the healthy contralateral white matter (Fig. 5).PRSr=100%×[S1(ROI)−Smin(ROI)]/[S0(ROI)−Smin(ROI)][S1(SB)−Smin(SB)]/[S0(SB)−Smin(SB)]rBBV colored-map image done with the software LUPE in the same patient of Fig. 1. At the left frontal area (PS) we can see a predominant blue area compatible with the enhanced area of Fig. 1 which showed lower rBBV values than the analogous contralateral region. LUPE: lund university perfusion evaluation software; PS: pseudoprogression; rBBV: relative brain–blood volume.
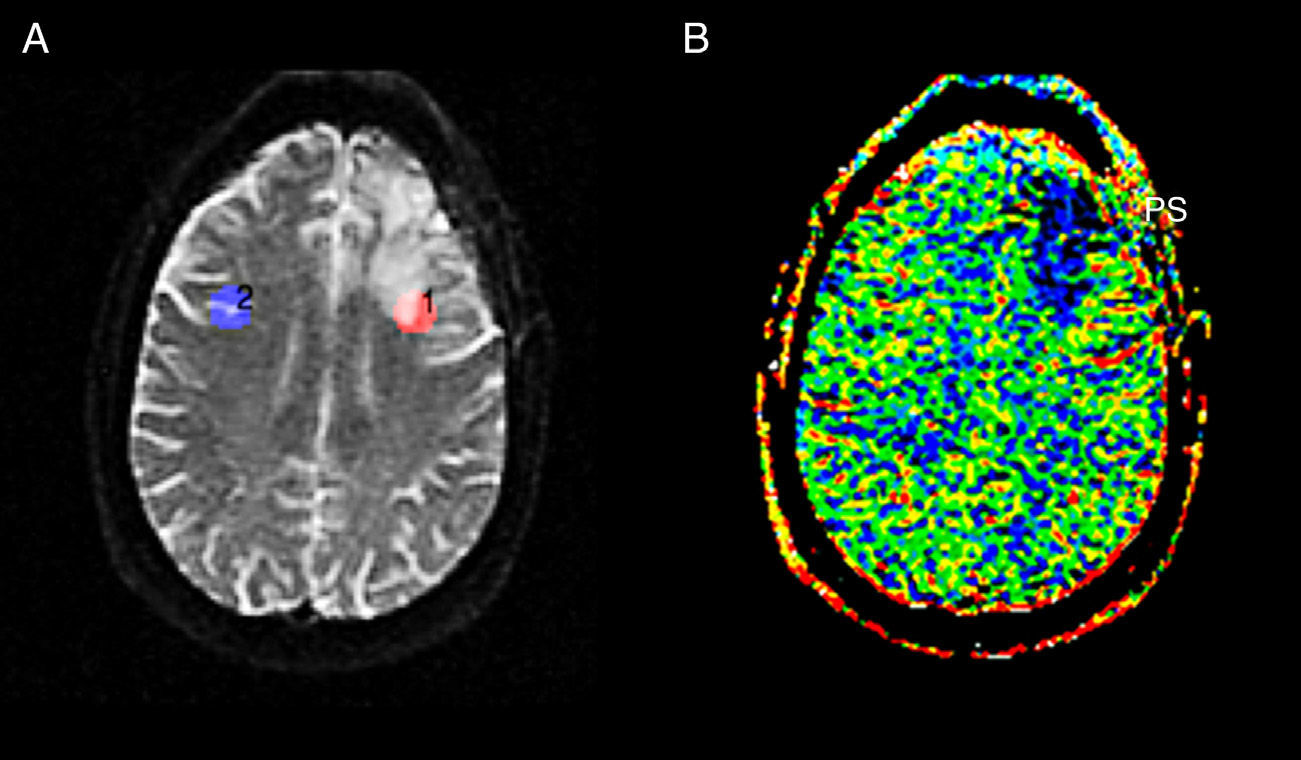
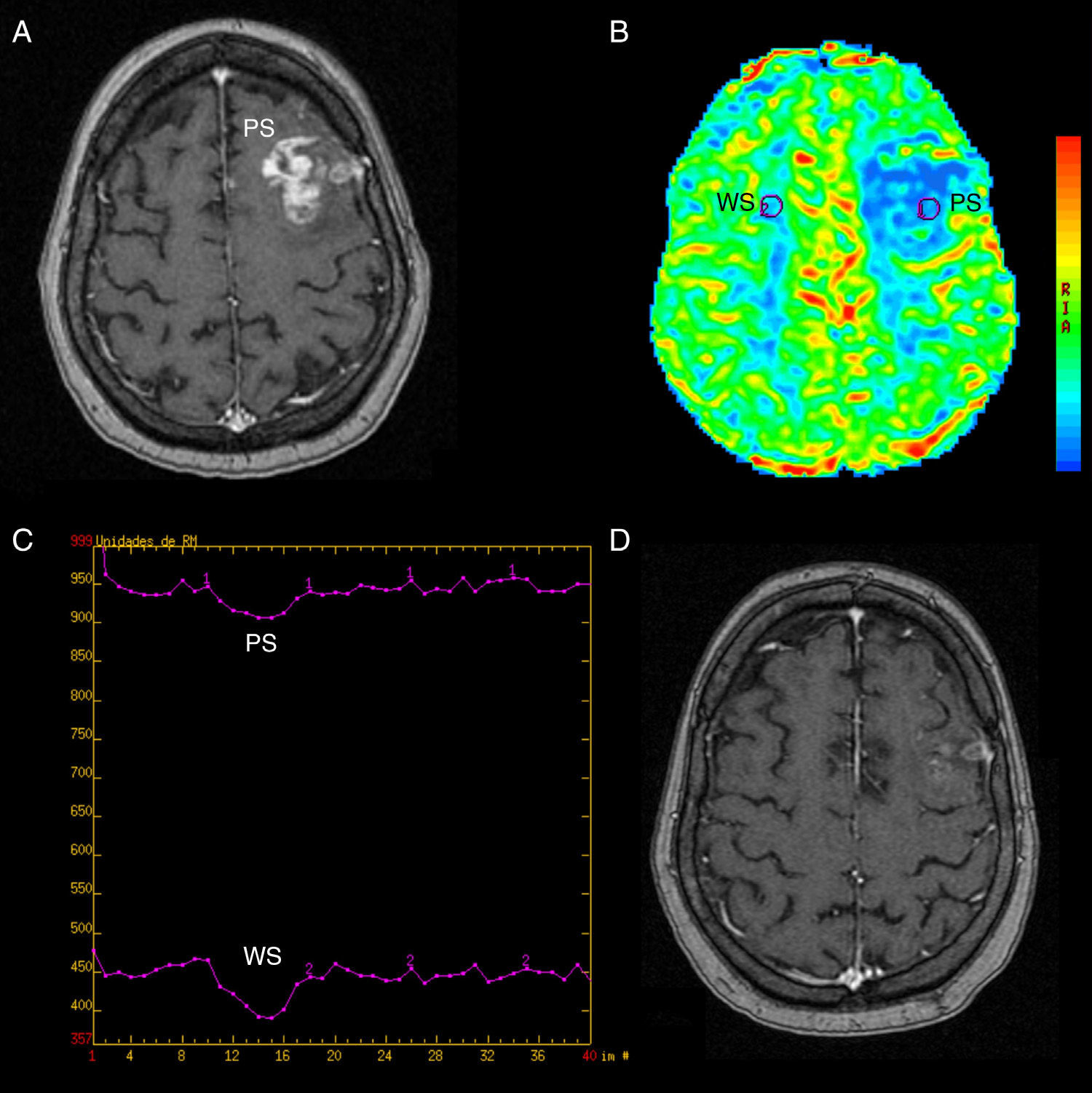
Pseudoprogression. (A) SPGR T1-weighted volumetric MRI with contrast agent. We can see a left frontal (PS) enhanced area in a follow-up patient due to gliobastome treated with surgery, RT and TMZ. (B) Colored-map of the negative enhancement integral done with FuncTool® v.2, with interesting regions in the lesion (PS) and the contralateral white substance (SB); reduced rBBV in the lesion. (C) signal/time intensity chart done with FuncTool® v.2 without significant differences in the fall of signal intensity and in the contralateral WS. (D) SPGR T1-weighted volumetric MRI with contrast agent 2 months later. Significantly reduced enhancement with no therapy at all. PS: pseudoprogression; MRI: magnetic resonance image; RT: radiotherapy; WS: white substance; SPGR: spoiled gradient recalled; TMZ: temozolomide; rBBV: relative brain–blood volume.
True progression. (A) SPGR T1-weighted volumetric MRI with contrast agent showing right frontal (T) enhanced area in a follow-up patient with gioblastoma treated with surgery, RT and TMZ. (B) Colored-map of the negative enhancement integral done with FuncTool® v.2, with interesting areas in the lesion (T) and the contralateral WS. In the lesion there is an increase of rBBV suggesting tumor progression. (C) Signal/time intensity chart done with FuncTool® v.2 showing evident greater fall of signal intensity at tumor area level with respect to contralateral WS. (D) SPGR T1-weighted volumetric MRI with contrast agent 3 months later after treatment with corticoids showing a greater enhanced area (T) compatible with tumor progression. RT: radiotherapy; WS: white substance; SPGR: spoiled gradient recalled; T: tumor; TMZ: temozolomide; rBBV: relative brain–blood volume.
Firstly the descriptive statistical values (average and 95% interval confidence interval) were assessed. Later we compared the averages of each parameter between both samples through Mann–Whitney’ non-parametric sample for independent samples U. Similarly we analyzed the received operating characteristic (ROC) of the three parameters to establish the value with the best possible sensibility and specificity relation to distinguish pseudoprogression from progression. The 3 variables correlated with the Spearman’ rank correlation test. Since the process was done simultaneously and the 2 authors shared the same view we did not calculate the interobserver concordance. For the statistical analysis we used the program SPSS® v.20.0 (IBM, Armonk, New York, USA). Statistical significance could be established around p<0.05.
ResultsThe cases of pseudoprogression showed a greater rPSIR, peak relative height, and a lower relative blood volume than that of the tumors in progression. The average values and 95% confidence intervals in the pseudoprogression group were 127.1% (110.5–148.4) for the rPSIR; 1.11 (0.98–1.26) for the rPH, and 0.41 (0.32–0.50) for the rBBV. In the group of true progressions the values were 92.2% (86.2–98.6) for the PSIR; 3.49 (2.61–4.47) for the rPH, and 3.07 (2.36–3.8) for the rBBV. The 3 parameters were statistically different among the groups (p=0.001).
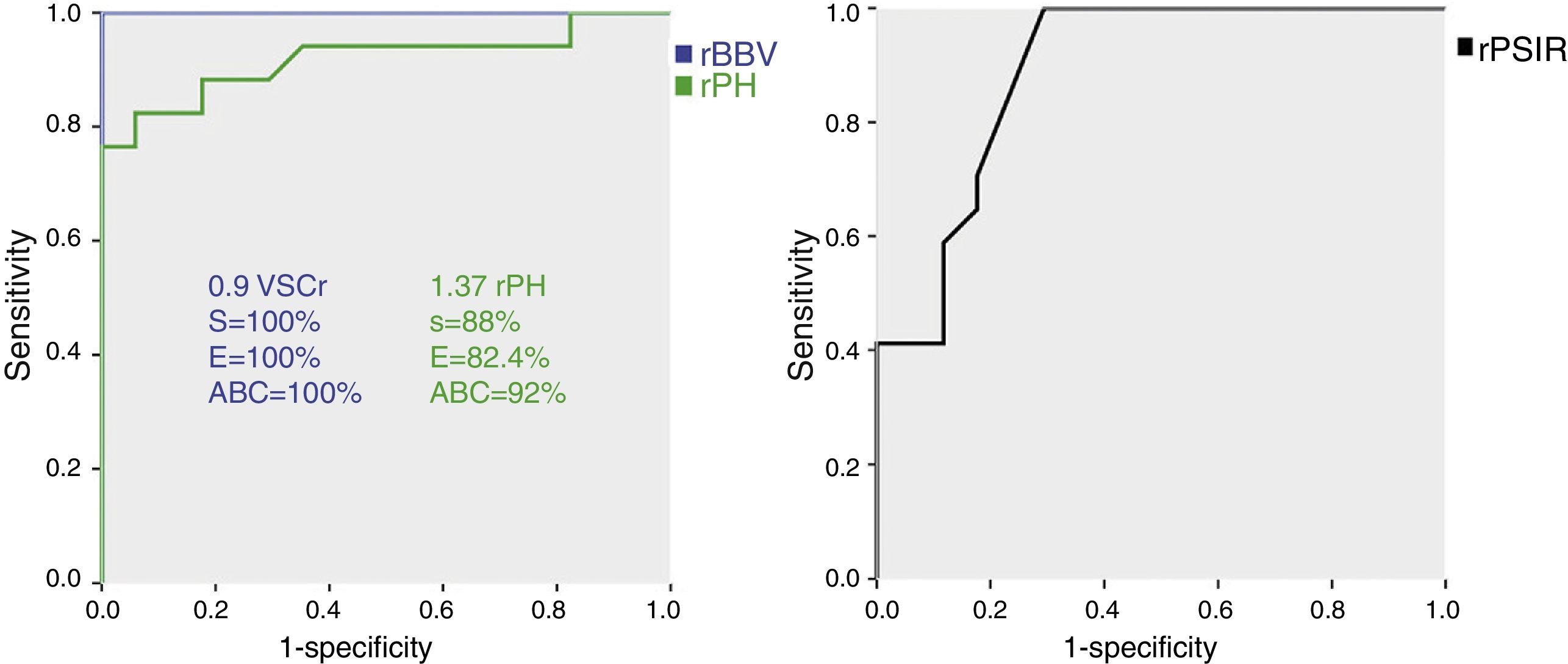
With the ROC analysis the rPH showed a sensibility of nearly 88% and a specificity of 82.4% for the value 1.37, area below the curve (ABC) 92%. When it comes to the rBBV, both the sensibility and specificity were around 100% taking 0.9 as the cut-off, ABC 100%. Finally for a rPSIR of 99% the sensibility was close 100% and specificity was 70.6%, ABC 89% (Fig. 6). These values were all statistically significant (p=0.001). The variables rPSIR-rPH and rPSIR-rBBV strongly correlated inversely (−0.76 and −0.81, respectively). The correlation of variables PSIR-rBBV was positive and high (0.87). Correlations were significant for all parameter valued p<0.01.
Receiver operating characteristic (ROC) curve of the relative brain–blood volume (rBBV), the relative peak height (rPH) and the relative percentage of signal intensity recovery (rPSIR). Both the corresponding sensibility and specificity values for the cut-off points picked and the area below the curve (ABC) are shown here.
Our trial showed that both progression and the phenomenon of pseudoprogression in treated high-grade astrocytomes have different rPSIR, rPH, and rBBV values. Of these it has been rBBV the one that has shown a higher value to be able to distinguish between both phenomena.
Several publications have shown that the existence of a correlation among the MR perfusion parameters, histopathological grade and the vascular density of gliomas is high,16,28–31 and that, they can probably be useful to distinguish lesions due to therapy from tumor relapses.1–4,22,23,32,33 Its application for such purpose has not been fully validated yet though.7 In our sample, the values of rBBV in cases of pseudoprogression were all inferior to those shown in cases of progression. Similarly the cases of pseudoprogression had an average lower rPH value and higher rPSIR than true progressions; rPH has turned out to be an indicator of tumor vasculature and in our series as it happens in others it is highly treated to the rBBV.7,8,31,34,35 Low rPH and rBBV values in the enhancement areas of the group of patients with pseudoprogression relate to those of other studies that have analyzed lesions due to therapy.1–4,21,23,32,33 Our cut-off points (rPH: 1.37, and rBBV: 0.9) are also similar to others published,23 in such a way that values over those cut-off points could relate to true tumor progressions.
There are very few trials that have investigated the utility of MR parameters in pseudoprogression. Barajas et al.23 used parameters similar to ours to distinguish between radionecrosis and GBM relapse. For them the parameter most likely to distinguish radionecrosis from relapse was rPH.23 Young et al.36 also examined the role of these MR parameters with echo-gradient T2-weighted magnetic susceptibility modalities. In their work rPH was the most accurate parameter of all to distinguish pseudoprogression from true GB progression showing sensibility and specificity next to 100% even though it was a small sample of 20 patients only (16 progressions and 4 pseudoprogressions). In our case the parameter with the best scores in sensibility and specificity values was rBBV. The software used to calculate it was LUPE which is capable of correcting the effect of contrast extravasation due to BBB rupture. We have not compared rBBV values calculated with FuncTool® and those assessed through LUPE. Various studies have warned about the possibility of underestimating rBBV in tumor progression due to contrast leak caused by BBB rupture.21,22 One of the strategies used to minimize this effect is giving a prior contrast dose before the perfusion sequence. In our study the fact of using the software LUPE allowed us to correct it.
According to prior outcomes with MR perfusion through T2*-weighted magnetic susceptibility modalities rPSIR can be a more sensitive parameter than rBBV to distinguish gliobastome metastasis from lymphome mestastasis.24 It consists of the initial PSIR recovering after administering the first step of the contrast media and it also displays as we have been able to show in our series a strong negative correlation with rPH and rBBV. It is those cases of pseudoprogression which show higher PSIR values and lower rPH and rBBV values. The degree of recovery depends on various factors such as the contrast extravascular leak (which makes the T2-weighted signal intensity go lower due to interstitial accumulation of contrast media), the features of the extravascular space or the blood flow rate. This produces different T1 and T2 effects which eventually will determine the recovery of signal intensity at the intensity/time curve. Some authors have suggested that the rPSIR is lower in metastasis than in the GBM and above all than in lymphomes.24 We have found only one study where its utility to diagnose tumor pseudoprogression with a sample smaller than ours36 has been studied. In our series rPSIR was greater at pseudoprogression (127.12%) than in astrocytomes III and IV with true progression (94%). This difference was statistically significant even though rPSIR values discretely overlapped between both samples. Overlapping can be justified for the endothelial disruption and laxity of extravascular space that happens during pseudoprogression,24 which in turn would facilitate extravasation and accumulation of contrast media. The coexistence of small areas of true progression, pseudoprogression and gliosis in cases of pseudoprogression can also justify it.24 In the study of Barajas et al.23, rPSIR seen in GBM recurrences (80.2±10.3%) was lower than in cases of radionecrosis (89.3±12.4%). However these results cannot be compared to ours since the MRI perfusion was done through T2*-weighted magnetic susceptibility modalities. With these modalities the variation of signal is more sensitive to contrast extravasation and thus after the fall in the signal/time intensity chart recovery is shorter. This fact can explain why the rPSIR values in such study have always been lower than ours.
It is widely known that perfusion modalities by magnetic susceptibility especially parameters like rBBV can be biomarkers that determine the tumor degree estimating angiogenesis that coupled with cellularity, mitosis, pleomorphism and necrosis is one of the most important histological criteria used to determine the degree of astrocytomes.37,38 Another potential utility of MR perfusion is monitoring the response to therapy through the appearance of effects such as radionecrosis, pseudoprogression and pseudoremission.4,6,7 When it comes to pseudoprogression several authors have suggested that the rBBV is useful to be able to distinguish it from true progressions so it can be a biomarker of the course of the disease.39 As it has already been stated here the utility of rPSIR has been evaluated to distinguish recurrent giobastomes from radionecrosis with good results even though as it happened to us the values have overlapped among the different samples; rPSIR also shows superiority over parameters such as Ktrans, that estimate patency in T1-weighted perfusion trials.23 The idea that the T1-weighted intensity signal is proportional to contrast concentration does not seem to be valid and without a prior estimation Ktrans values can be imprecise. Such estimations are not necessary to calculate the rPSIR as an estimator of capillary patency. The greater limitation in the use of T1-weighted perfusion is the impossibility to calculate rBBV and rPH simultaneously–the two (2) widely-known biomarkers for their capacity to estimate angiogenesis.35,37
Our study has several limitations: (1) it is a small sample retrospective study; (2) another important limitation is that in none of our patients there was histological confirmation; (3) in our analysis we did not consider therapy with corticoids which can modify perfusion parameters even though they are not an antiangiogenic therapy; (4) we could not compare perfusion in pseudoprogression to perfusion prior to therapy to avoid interindividual variability. However we built two similar samples when it comes to tumor degree and therapy received; (5) the fact that ROI were drawn by 2 neuroradiologists and the fact that calculations were done jointly makes it impossible to determine inter-observer variability for a better generalization of results; (6) Post-process was done defining normalized ROI with contralateral white matter values in the case of rPSIR and rPH and with a homologous symmetrical ROI in the case of rBBV. We did not carry out a voxel by voxel analysis with mathematical tools which could be done in future research; and (7) in this trial we have not calculated something that could contribute to our goal the estimation of perfusion parameters associated with a bicompartment pharmacokinetic model more widely used in T1-weighted perfusion modalities such as Ktrans (pass of contrast from intravascular compartment to the interstitium through the endothelium or patency), Kep (return to vascular space or extraction ratio), Ve (interstitial volume) and Vp (vascular volume).40
In summary our data suggest that both rPH and rBBV parameters can be useful to distinguish tumor pseudoprogression from progression in patients treated with RT and TMZ; initially the rPSIR seems to be a less useful parameter.
Ethical responsibilitiesProtection of humans and animalsAuthors confirm that no experiments have been done with humans or animals during this research.
Confidentiality of dataAuthors confirm that in this report there are no personal data from patients.
Right to privacy and informed consentAuthors confirm that in this report there are no personal data from patients.
Authors- 1.
Manager of the integrity of the study: AMM.
- 2.
Original idea of the study: AMM and JMB.
- 3.
Study design: AMM and JMB.
- 4.
Data mining: AMM.
- 5.
Data analysis and interpretation: AMM.
- 6.
Statistical analysis: AMM.
- 7.
Reference search: AMM and JMB.
- 8.
Writing: AMM.
- 9.
Manuscript critical review with intellectually relevant contributions: AMM and JMB.
- 10.
Final version approval: AMM and JMB.
Authors reported no conflicts of interests.
Please cite this article as: Martínez-Martínez A, Martínez-Bosch J. Resonancia magnética de perfusión en astrocitomas de alto grado: el volumen sanguíneo cerebral, la altura del pico y el porcentaje de recuperación de intensidad de señal ¿pueden discriminar entre progresión y seudoprogresión? Radiología. 2014;56:35–43.