To compare the effectiveness, survival and cost in patients with unresectable hepatic cell carcinoma (HCC) treated with trans-arterial chemoembolization using doxorubicin-eluting beads (DEB-TACE) versus conventional TACE (cTACE) in clinical practice.
Materials and methodsThis single-centered retrospective observational study compared 60 consecutive HCC unresectable patients: 30 were treated with DEB-TACE and 30 used cTACE. Comparisons were with χ2 test, Student t-test, and Kaplan–Meier method.
ResultsOf the 60 patients with HCC in non-curative stage, baseline characteristics were similar for both groups of treatment, and of these we observed lower survival in male patients and those who had hepatitis C virus (p=0.014 and p=0.003, respectively). No statistically significant differences were observed as a function of treatment employed with respect to overall survival (OS) at 5 years (29.99 months; 95%CI: 21.38–38.60 versus 30.67 months; 95%CI: 22.65–38.70; p=0.626) and progression free survival (PFS) median of 11.57 months (95%CI: 0.97–22.18) versus 12.80 months (95%CI: 0.00–32.37; p=0.618). The median length of hospital admission was 2.6 and 5.4 days (p<0.001) for DEB-TACE and cTACE, respectively. Toxicities grade 2–4 were higher in cTACE group (54 versus 31; p<0.001). The cost of the treatment was 1581 € for DEB-TACE and 514.63 € for cTACE. The overall mean cost of intervention was 3134 € and 3694.35 €, respectively (p=0.173).
ConclusionsChemoembolization in patients with unresectable HCC achieved OS close to 30 months at 5 years, independent of the technique employed. Similar overall costs but better tolerance of the DEB-TACE justified the higher costs of the procedure.
Comparar la efectividad, supervivencia y coste de la quimioembolización transarterial con partículas liberadoras de doxorrubicina (DEB-TACE) y la quimioembolización convencional (cTACE) en pacientes con carcinoma hepatocelular (CHC) irresecable.
Material y métodosEstudio unicéntrico, observacional y retrospectivo que comparó 60 pacientes con CHC irresecable separados en dos grupos comparables de 30 pacientes tratados con DEB-TACE y otros 30 con cTACE. Se realizaron las pruebas de χ2 y t de Student, y se utilizó el método de Kaplan Meier.
ResultadosLa supervivencia fue menor en hombres y en pacientes con hepatitis C (p=0,014 y p=0,003, respectivamente). No hubo diferencias estadísticamente significativas en la supervivencia global a los 5 años (29,99 meses; IC del 95%: 21,38–38,60 y 30,67 meses; IC del 95%: 22,65–38,70; p=0,626) y la supervivencia libre de progresión (mediana: 11,57 meses; IC del 95%: 0,97–22,18 y 12,80 meses; IC del 95%: 0,00–32,37; p=0,618). El tiempo medio de ingreso fue de 2,6 y 5,4 días (p<0,001) para DEB-TACE y cTACE, respectivamente. La toxicidad grado 2–4 fue superior en el grupo cTACE (54 y 31; p<0,001). El coste del tratamiento fue de 1.581 € con DEB-TACE y de 514,63 € con cTACE. El coste total medio fue de 3.134 € y 3.694,35 €, respectivamente (p=0,173).
ConclusiónLa quimioembolización en pacientes con CHC irresecable tiene una supervivencia global cercana a 30 meses a los 5 años, independientemente de la técnica empleada. Los costes globales son similares, aunque la mejor tolerancia de la DEB-TACE justifica el mayor coste del procedimiento.
Hepatocellular carcinoma (HCC) represents 90% of the liver primary neoplasms. With approximately 500,000–1,000,000 deaths a year it accounts for one-fourth of all cancer deaths all around the world and the third most common cause of cancer death in men.1
The most important clinical risk factor to develop an HCC is liver cirrhosis, which is responsible for approximately 80% of the cases. The main cause for cirrhosis is chronic infection due to hepatitis B (VHB) or C (VHC) virus and chronic alcoholism.
In located HCC (Barcelona Clinic Liver diseases, BCLC-A), the treatments with curative intention are partial hepatectomy, liver transplant or ablative techniques. The patients who do not meet the criteria for curative treatment (due to tumor spread, limited liver function or a poor physical condition) and who do not have disseminated or metastatic disease, would be candidates for non-curative therapies, such as transarterial chemoembolization (TACE), radiotherapy (RT), radioembolization or systemic treatments. TACE represents the standard therapy for patients with unresectable HCC on of an intermediate stage (BCLC-B).2 Two random studies3,4 and three meta-analyses5–7 have shown a significantly greater survival with TACE than with the “best support treatment”, whereas in other studies the procedure had insignificant results.8–10
A TACE variant uses drug-eluting bead (DEB-TACE). It is a new drug administration modality and it allows us to embolize the vessels of hypervascularized malignant tumors, and simultaneously, administer locally particles preloaded with a controlled and sustained dose of a chemotherapeutic substance, which implies a reduction of the systemic effect of the drug.6,11 The results of the clinical trials have shown that the drug concentration in the tumor is greater and the systemic one is lower than the intra-arterial injection and with cTACE. In addition, these studies have shown promising effectiveness with low toxicity.12–22 However, it is not clear that the effectiveness of DEB-TACE is greater than that of the cTACE,16,23–26 the costs of DEB-TACE are higher and the studies published have not proven if it is a cost-effective strategy.27–30
The goal of our study was to compare the effectiveness, survival and cost of DEB-TACE vs cTACE in patients with unresectable HCC.
Materials and methodsPatientsWe studied retrospectively data from a series of 30 patients, who had started treatment at our center with doxorubicin-eluting bead (DC BEAD; Biocompatibles, Farnham, United Kingdom) since 2009, and a series of 30 patients treated with cTACE with cisplatin since 2008; they had all been diagnosed with unresectable HCC, and had adequate liver function (Child–Pugh A–B). Our hospital used TACE with cisplatin until 2009, when it was replaced by DEB-TACE with doxorubicin. Patients treated with cTACE in 2008 and DEB-TACE in 2009 were included in the study. The follow-up period was 5 years since the beginning of each treatment. We have used survival at 5 years so that the follow-up in the cTACE subgroup was not any longer since this modality was used in our center before (for approximately 11.7 months).
Both treatments were approved by our hospital ethics committee and the committee for the study of liver tumors. The therapeutic procedure was decided in this liver tumor committee, once the surgical curative treatment or radiologic intervention had been ruled out based on the treatment algorithm of the BCLC classification.2 The study was conducted abiding by the Helsinki declaration. All patients signed their informed consent. The study was not evaluated by the ethical committee since it was a retrospective, observational series. The inclusion criteria were: (a) adult patients older than 18 years diagnosed with unresectable HCC complying with the criteria for chemoembolization based on the European guidelines2: Child–Pugh A–B Stages and BCLC A or B without portal invasion or extrahepatic dissemination, and (b) patients who had started the cTACE or DEB-TACE treatment between 2008 and 2009, and who had received at least one dose.
The exclusion criteria were: (a) patients who had received both cTACE and DEB-TACE; (b) patients with contraindication for chemoembolization (advanced liver cirrhosis – Child–Pugh C, low tumor vascularization (assessed through dynamic image study by angio-MRI or hepatic angio-CT in three phases), vascular invasion, extrahepatic dissemination or contraindication for cisplatin or doxorubicin administration).
Technique of trans-arterial chemoembolizationGiven the hypervascular nature of the HCC, TACE consisted of the intrarterial infusion of chemotherapeutic and embolizing drugs at high doses and selectively in the tumor to cause cellular death and reduce the tumor volume. All patients received antiemetics during the procedure (IV Ondansetron 8mg), proton pump inhibitors (oral Omeprazol 20mg) and sedation (propofol at maintenance IV doses 10–50mg/kg/min).
cTACEWe used Seldinger's technique to puncture the common right femoral artery with an 18G needle, hydrophilic guide and introducer sheath 5F (Terumo-Europe, Leuven, Belgium). The selective angiography of the hepatic vascular territory was performed using Cobra-2 or Simmons-2 catheters (Terumo-Europe, Leuven, Belgium). Once the hepatic vascular map had been obtained, the afferent artery to the tumor was catheterized selectively using a microcatheter 2.7 F-catheter (Progreat; Terumo-Europe, Leuven, Belgium). The cytostatic agents were administered combined with ethiodol (Lipiodol Ultra-fluide; Guerbet S.A., France) in a 1:1 ratio. In the reference the most widely used were doxorubicin (20–50mg), mitomycin C (10mg) or the combination of doxorubicin (60mg) with cisplatin (50–100mg). At our center we use cisplatin (2mg/kg) in monotherapy with the same volume of Lipiodol (1ml in 1mg of cisplatin). Subsequently, the 300–500μm polyvinyl alcohol embolizing particles (Bead Block; Biocompatibles, United Kingdom) were administered. In the case of bilobar treatment, it was carried out in two different sessions in a 4 week-interval.31,32
The DEB-TACE techniqueThe interventional technique was the same as that of cTACE. To administer the cytostatics, the hydrogel beads vials (100–300 and 300–500μm) were preloaded with 75mg of doxorubicin and released selectively. The procedure started with the smaller beads and based on the volume and vascularization of the tumor larger beads followed up to a maximum dose of 150mg of the cytostatic drug.33 Both therapies were controlled through a conic beam CT immediately after the transarterial procedure. After the DEB-TACE, the uptake of contrast or Lipiodol in the target tumor confirms the embolization which improves the operator's confidence when it comes to the complete coverage of treatment.34
Variable results- -
Main variable. The main result was overall survival of both procedures at 5 years. We used the Kaplan–Meier curves to evaluate the survival of each therapy. The follow-up covered from the first TACE treatment to all-cause mortality. The patients were selected so that both groups of treatment were as close to each other as possible in order to avoid biases due to the fact that at our hospital the cTACE was used considerably prior to DEB-TACE.
- -
Secondary variable. We studied the relations between survival and age, weight, basal condition and the development of the chemoembolization procedure.
With respect to the procedure, we analyzed the number of doses administered, the number of complete doses, the percentage of successful treatments (complete treatment and without serious post-intervention complications), the length of hospital stay and the postoperative adverse effects that required additional treatment. Based on version 4.03 of the Common Terminology Criteria for Adverse Events (CTCAE), toxicity was grouped as binary variables (negative: grade 0–1; positive: grade 2–4), taking into account the number of adverse effects and the percentage of patients affected in each TACE group.
Progression-free survival was also evaluated, understanding as progression the intrahepatic or extrahepatic dissemination of the disease, vascular invasion, clinical intolerance to TACE or the development of liver failure. Progression and tumor response were measured according to the modified EASL response criteria2 obtained through image modalities (MRI or CT) performed periodically at one month and at 3, 6, 9 and 12 months during the first year, and at 6 months from the first year onwards.
We analyzed the direct costs of each type of treatment together with the costs of the hospital stay. In order to quantify the direct costs, the costs of buying the chemotherapy, the contrast material and the particles for each of the procedures for each patient were calculated. We used the official prices established by the National Health System35 and the EPIMED36 study in order to calculate the daily hospitalization cost. These official studies determined the mean hospital stay for the different groups related by the diagnosis (GRD) and their mean hospital cost including all the drugs used in each admission. This analysis did not include the costs of preparing and bottling the cytostatic though it is estimated that it could be the same for both procedures.
Statistical analysisThe quantitative variables were described using the medians and the standard deviation or, in the case of asymmetric distributions using medians and percentiles (p25, p75). The qualitative variables were studied using frequencies and percentages. The quantitative variables with normal distributions demonstrated by the Kolmogorov–Smirnov test were analyzed with the Student t test for independent samples. The qualitative variables were compared using the χ2 test or Fisher's exact test. To evaluate the overall survival and the survival free progression at 5 years, the Kaplan–Meier curve and the log-rank test were used. The whole analysis of data was carried out using the statistic software SPSS (version 19.0), p<0.05 values being considered statistically significant.
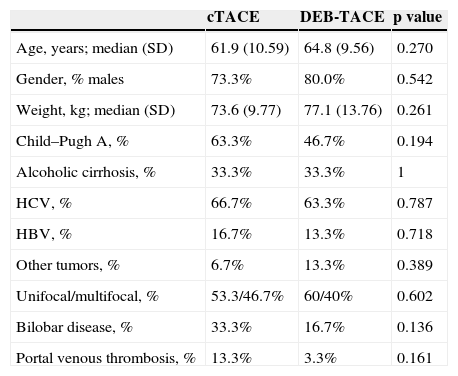
ResultsBasal characteristics of the populationWe treated 190 patients with cTACE and 303 with DEB-TACE between 2005 and 2012. After excluding the patients who received treatment with both techniques, the groups were reduced to 164 and 278 patients, respectively. Focusing on the 2008–2009 period, 34 and 70 patients were treated, but only 34 and 41 patients had a five-year follow-up, respectively. Lastly, 30 patients were selected per group closer in time. Three (3) patients were excluded because the procedure was contraindicated due to scarce vascularization of the tumor during the intervention (1 with cTACE and 2 with DEB-TACE). The study sample included 60 patients with unresectable HCC, 55% of whom were on the Child–Pugh A Stage and 45% on Child–Pugh B Stage. There were no significant demographic differences between the groups or in the tumor volume or their health condition (Table 1). Men were more prevalent (76.7%) as well as HCV infections (65.5%) as the main cause for HCC. Other underlying diseases detected though with low frequency (3.3%) were HIV (one case in each group), hepatorenal polycystic disease (1.1% in the cTACE group), cryptogenic cirrhosis and non-alcoholic steatohepatitis (1.1% and 1.1%, respectively, in the DEB-TACE group). Overall survival was statistically lower in men (p=0.014) and the HCV group (p=0.003), and greater in patients with HBV (p=0.022). With cTACE survival was lower in men (p=0.001) and in overweight patients (p=0.016). Finally with DEB-TACE survival was lower in patients with HCV (p=0.008) (Supplementary material, Table 1).
Basal characteristics of the study population. Comparison between cTACE and DEB-TACE using the χ2 test and the Student t-test.
| cTACE | DEB-TACE | p value | |
|---|---|---|---|
| Age, years; median (SD) | 61.9 (10.59) | 64.8 (9.56) | 0.270 |
| Gender, % males | 73.3% | 80.0% | 0.542 |
| Weight, kg; median (SD) | 73.6 (9.77) | 77.1 (13.76) | 0.261 |
| Child–Pugh A, % | 63.3% | 46.7% | 0.194 |
| Alcoholic cirrhosis, % | 33.3% | 33.3% | 1 |
| HCV, % | 66.7% | 63.3% | 0.787 |
| HBV, % | 16.7% | 13.3% | 0.718 |
| Other tumors, % | 6.7% | 13.3% | 0.389 |
| Unifocal/multifocal, % | 53.3/46.7% | 60/40% | 0.602 |
| Bilobar disease, % | 33.3% | 16.7% | 0.136 |
| Portal venous thrombosis, % | 13.3% | 3.3% | 0.161 |
SD: standard deviation; HBV: Hepatitis B virus; HCV: Hepatitis C virus.
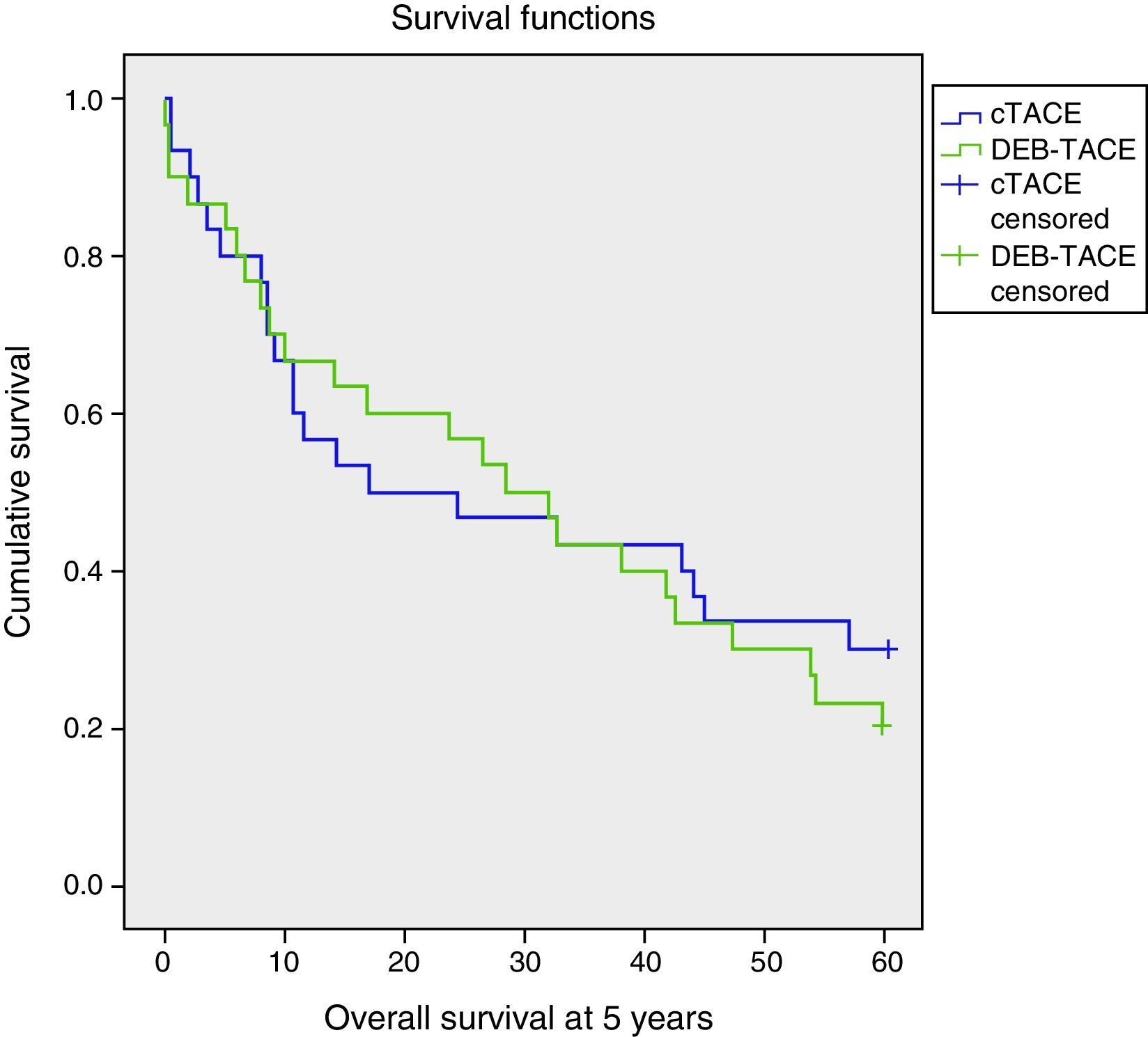
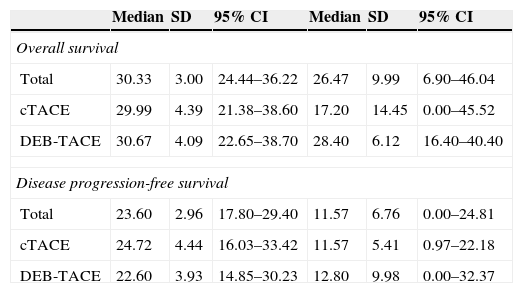
There were no significant differences of overall survival at 5 years between the two treatments (p=0.626) (Table 2; Fig. 1). With similar follow-up periods the analyses were carried out at 5 years–moment in which 25% of the patients were alive and the survival rates noted were 30% and 20% for cTACE and DEB-TACE, respectively (p=0.371).
Overall survival and disease progression-free survival at 5 years (expressed in months). Comparison between cTACE and DEB-TACE using the Kaplan–Meier method and the log-rank test.
| Median | SD | 95% CI | Median | SD | 95% CI | |
|---|---|---|---|---|---|---|
| Overall survival | ||||||
| Total | 30.33 | 3.00 | 24.44–36.22 | 26.47 | 9.99 | 6.90–46.04 |
| cTACE | 29.99 | 4.39 | 21.38–38.60 | 17.20 | 14.45 | 0.00–45.52 |
| DEB-TACE | 30.67 | 4.09 | 22.65–38.70 | 28.40 | 6.12 | 16.40–40.40 |
| Disease progression-free survival | ||||||
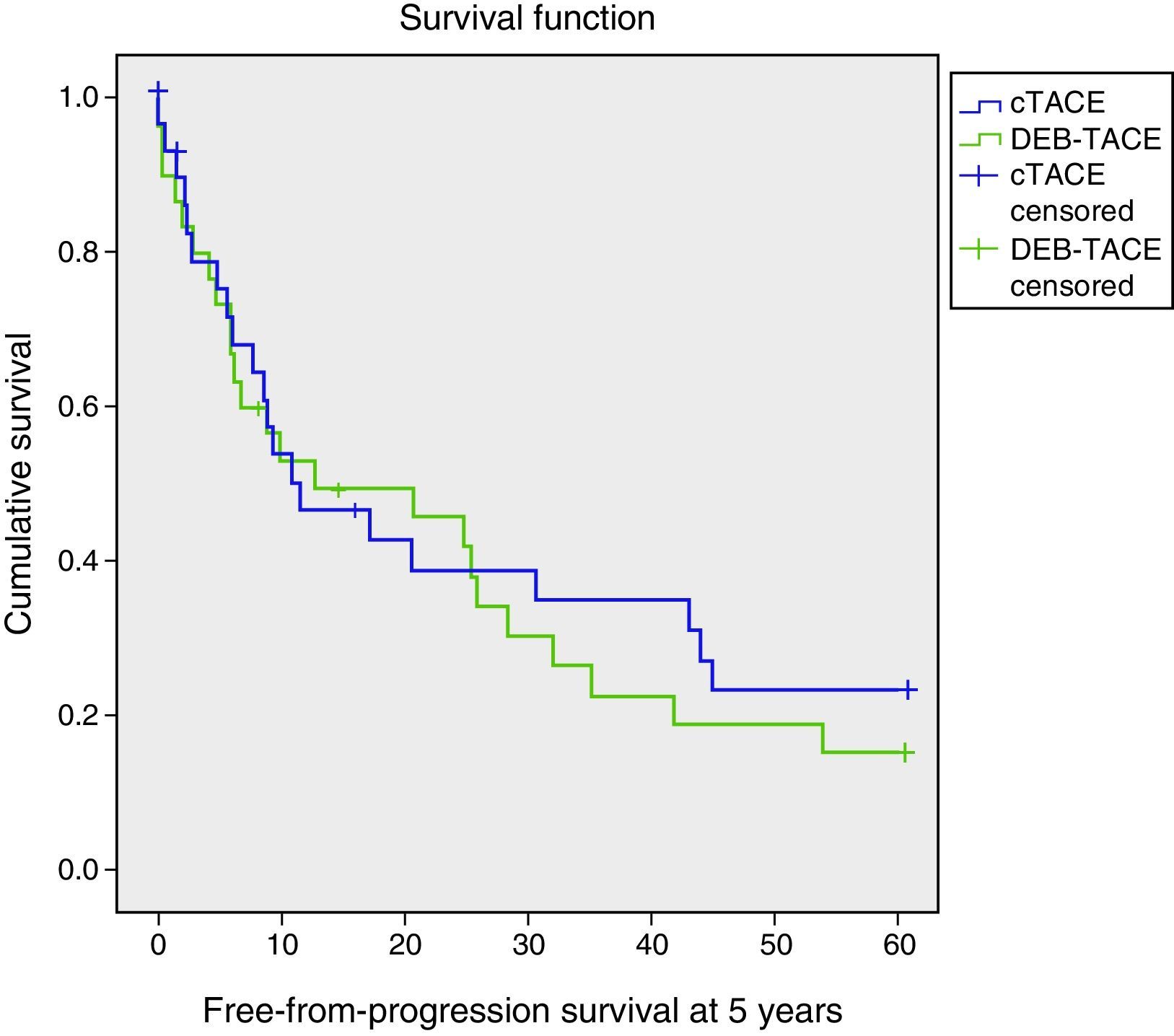
| Total | 23.60 | 2.96 | 17.80–29.40 | 11.57 | 6.76 | 0.00–24.81 |
| cTACE | 24.72 | 4.44 | 16.03–33.42 | 11.57 | 5.41 | 0.97–22.18 |
| DEB-TACE | 22.60 | 3.93 | 14.85–30.23 | 12.80 | 9.98 | 0.00–32.37 |
SD: standard deviation.
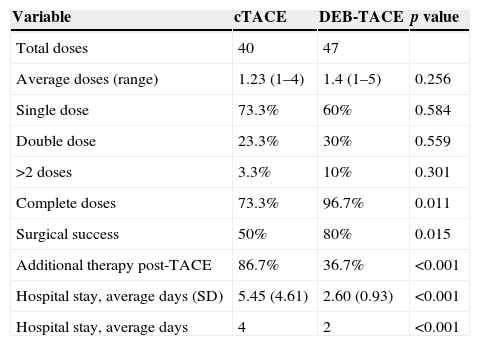
The number of doses received by each patient was recorded, as well as the percentage of symptoms related with the procedure and the mean hospital stay with each and everyone of the techniques (Table 3). With DEB-TACE the number of doses administered was greater as well as the success percentage of the procedure (p=0.015) and the number of complete doses administered (p=0.011) while the postoperative symptoms were fewer (p<0.001) and hospital stays shorter (p<0.001).
Clinical state associated with the TACE intervention. Comparison between cTACE and DEB-TACE using the χ2 test.
| Variable | cTACE | DEB-TACE | p value |
|---|---|---|---|
| Total doses | 40 | 47 | |
| Average doses (range) | 1.23 (1–4) | 1.4 (1–5) | 0.256 |
| Single dose | 73.3% | 60% | 0.584 |
| Double dose | 23.3% | 30% | 0.559 |
| >2 doses | 3.3% | 10% | 0.301 |
| Complete doses | 73.3% | 96.7% | 0.011 |
| Surgical success | 50% | 80% | 0.015 |
| Additional therapy post-TACE | 86.7% | 36.7% | <0.001 |
| Hospital stay, average days (SD) | 5.45 (4.61) | 2.60 (0.93) | <0.001 |
| Hospital stay, average days | 4 | 2 | <0.001 |
SD: standard deviation.
Grade 2–4 toxicity was greater in the cTACE group (p<0.001)–especially nauseas (p=0.001) and fever (p=0.005) (supplementary material, Table 2) that required treatment.
Free from progression-survivalThere were no significant differences (p=0.618) (Table 2, Fig. 2). The deaths at five years of follow-up were similar in both groups (overall 75%: 70% for cTACE and 80% for DEB-TACE). The relapse rate was 46.7% for cTACE and 60% for DEB-TACE (p=0.301). The main causes for relapse were intrahepatic spread (73.3%), vascular invasion (10%), clinical intolerance to TACE (3.3%), liver failure (9.9%) and others (3.5%). The distributions for the type of TACE are summarized in Table 3 of the supplementary material, where intrahepatic dissemination stands out as the main cause for relapse in the DEB-TACE group (p=0.018). Secondary causes were more frequent, but not significant, with cTACE (20% and 10%, p=0.278).
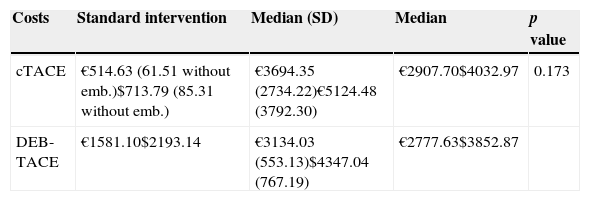
Cost analysisWe calculated a cost of €514.63 (US$713.79) for cTACE (which was €61.51 [US$85.31] when embolization was not performed due to complications during the procedure), and €1581.10 (US$2193.14) for DEB-TACE according to the purchase price of its components. The mean hospitalization period fixed by EPIMED35 including the hospitalization GRD due to low severity-pancreatic and hepatobiliary neoplasm was about 7.5 days, and its average cost was 4487 € (US$6223) according to the official rates of the National Healthcare Service36 with a daily cost of €598.27/day (US$829.80/day). Knowing the administration costs of each procedure and the length of hospitalization, it was possible to estimate the mean cost per intervention based on the technique used. As summarized in Table 4 the costs were €3694.35 (US$5124.48) for cTACE and €3.134 (US$4347.04) for DEB-TACE (p=0.173).
Cost of the interventions (in Euros and US dollars). Comparison between cTACE and DEB-TACE using the Student t-test.
| Costs | Standard intervention | Median (SD) | Median | p value |
|---|---|---|---|---|
| cTACE | €514.63 (61.51 without emb.)$713.79 (85.31 without emb.) | €3694.35 (2734.22)€5124.48 (3792.30) | €2907.70$4032.97 | 0.173 |
| DEB-TACE | €1581.10$2193.14 | €3134.03 (553.13)$4347.04 (767.19) | €2777.63$3852.87 |
SD: standard deviation; emb: embolization.
Conversion rate (€1=US$1.39).
The results of our study suggest that both chemoembolization techniques are equally effective in unresectable HCC, without any significant differences in its overall cost, but with less toxicity and better tolerance to DEB-TACE.
So far the studies conducted with TACE have demonstrated a limited improvement of survival compared to the standard therapy. Out of the five random Phase II clinical trials that have compared survival with TACE and with conservative treatment,3,4,7–9 two have shown advantages with TACE3,4 and three meta-analyses have corroborated this improvement.5–7 Similarly there was no clear evidence that DEB-TACE was more effective than cTACE.16,23–26 The new technique allows us to minimize the free-drug dose and the possible systemic adverse effects.6,11 The results of the published initial studies have been good despite the short follow-up periods in most of them.12–22
Few studies have compared the two procedures directly. One randomized clinical trial with 212 patients with unresectable HCC in Child–Pugh Stage B showed a significant increase of objective response with DEB-TACE in the subgroups of patients with worse prognosis (Eastern cooperative Oncology Group 1 [ECOG 1], in patients with recurrent disease and bilobular tumor) compared to cTACE. Fewer hepatobiliary toxicity and systemic adverse effects resulting from the doxorubicin used both types of TACE.16 A subsequent observational study compared the two groups of 22 patients with unresectable HCC (Child–Pugh A) treated with DEB-TACE with epirubicin and cTACE with cisplatin. The overall survival was statistically greater with DEB-TACE (21.7±2.5 and 13.8±1.4 months), without any significant differences in toxicity.23 In both studies there was a trend (though not a significant one) to a greater complete response, objective response and disease control with DEB-TACE. Other studies have shown similar results in effectiveness but more toxicity with cTACE.24,25 In the most recent comparative studies (which were also observational), the response to treatment was significantly greater with DEB-TACE than with cTACE, as was the time until disease progression (11.7 and 7.6 months, respectively). There were no significant differences in this study with respect to the toxicity seen between the two therapies.26 In our case, sample size and the result variables used make it difficult to compare it with other studies. In any case, we obtained very similar values in overall and progression-free survival at 5 years of follow-up with both techniques, but DEB-TACE was the procedure with the least incidents, a greater number of complete doses and a shorter postoperative period without complications. It also allowed us to administer a larger number of doses. Relapse was more frequent in patients treated with DEB-TACE though the difference was not statistically significant. The causes for relapse varied depending on the procedure. cTACE had several primary relapse causes, among which intrahepatic dissemination stands out, and to a lesser extent, vascular invasion, while with DEB-TACE the main cause was intrahepatic dissemination. Secondary causes for relapse were detected more frequently with cTACE and they were due to different causes, but with DEB-TACE vascular invasion was the only secondary cause. This distribution can reflect that relapse with DEB-TACE is more related with tumor progression, greater tolerance to the procedure, liver failure and vascular invasion than with cTACE, maybe related with less control of chemoembolization. All these results can justify the change to DEB-TACE, whose main objective is to reduce to the minimum the amount of free drug in circulation and the concomitant systemic adverse effects of chemotherapy.
The analyses showed that despite the fact that the cost of the procedure with DEB-TACE is almost three times more expensive than that of cTACE, the overall costs were €560.32 (US$777.16) more expensive with cTACE due to its worse tolerance. We have not calculated additional costs derived from the treatment of adverse reactions, which are significantly more expensive with cTACE, since it was a historical, non-prospective series, and there can be some information bias in some patients though the studies used to calculate these costs35,36 included the medication administered during hospitalization. The costs of these therapies have been evaluated on very few occasions and never in Spain. In previous cost analysis studies, direct costs per patient and per treatment of up to €4265 (€2674 with the official rates of the GRD)27 were obtained with values for DEB-TACE similar to those obtained in our study. A French study observed significant differences when comparing both treatments, with lower costs associated to DEB-TACE (€3577 and €4332 in hospitalization costs and €2852 and €4507 in official rates in France).28 However, in another subsequent French study no significant differences were found between the two techniques after a cost minimization analysis (€3960.10 with DEB-TACE and €2869.05 with cTACE), possibly due to the fact that hospital stays were similar for both procedures.29 A recent study also conducted in France showed that the introduction of the DEB-TACE procedure did not modify the effectiveness but otherwise reduced hospital stays and costs.30 The main limitation of this study was that it compared cTACE with DEB-TACE, cTACE or both.
The study that we have carried out has some limitations including, as we have already pointed out, the small size of the sample, which can limit the statistic value of the results, especially when it comes to evaluating effectiveness. The retrospective design can also introduce some sort of information bias, such as the security registration of the procedure in all patients. In addition, the chemotherapy used was different in the two techniques which can make it difficult to establish comparisons between the techniques. Another limitation is the length of the follow-up, since five years can be a period too short to be able to compare the two techniques appropriately, especially when there is a separation for different periods of the time in which they had been performed. And finally, the cost analysis included the cost of acquiring the drugs and hospital stays yet indirect costs were not included.
In sum the effectiveness of the two techniques is similar, but DEB-TACE has greater costs yet the overall cost is similar. The advantage of DEB-TACE lies in reducing hospital stays because of the better tolerance of the doxorubicin preloaded particles. Studies with larger samples and longer follow-up times are necessary to confirm these findings.
Ethical responsibilitiesProtection of people and animalsThe authors declare that the proceedings used abide by the ethical rules set forth by the human experimentation committee and the World Health Organization (WHO) and the Helsinki Declaration.
Data confidentialityThe authors declare that their center protocols have been followed on the publication of data from patients records.
Right to privacy and informed consentThe authors declare that they have obtained prior written consent from patients and/or other subjects referred to in this article. This document belongs to the corresponding author.
Authors- 1.
Manager of the integrity of the study: JEMV, RGM, ELB, JJMR, JLPA.
- 2.
Study Idea: JEMV, RGM, ELB, JJMR, JLPA.
- 3.
Study Design: JEMV, RGM, ELB.
- 4.
Data Mining: JEMV, RGM, FGM, JRR.
- 5.
Data Analysis and Interpretation: JEMV, RGM, ELB, FGM, JRR.
- 6.
Statistical Analysis: JEMV, ELB.
- 7.
Reference Search: JEMV, RGM, ELB, FGM, JRR.
- 8.
Writing: JEMV, RGM, ELB, FGM, JRR, JJMR, JLPA.
- 9.
Critical review of the manuscript with intellectually relevant remarks: JEMV, RGM, ELB, FGM, JRR, JJMR, JLPA.
- 10.
Approval of final version: JEMV, RGM, ELB, FGM, JRR, JJMR, JLPA.
The authors declare no conflict of interests.
We wish to thank Ms. Carmela Borrell, DPharm, Jesús Esteban, Ph.D., Maximiliano Lloret, M.D. and Daniel Pérez-Enguix, M.D. for their help and assistance taking care and managing patients.
Please cite this article as: Megías Vericat JE, García Marcos R, López Briz E, Gómez Muñoz F, Ramos Ruiz J, Martínez Rodrigo JJ, et al. Quimioembolización transarterial con partículas liberadoras de doxorrubicina frente a quimioembolización transarterial convencional en carcinomas hepatocelulares irresecables: un estudio de eficacia, seguridad y gastos. Radiología. 2015;57:496–504.