Edited by: Dr. José Luis del Cura Rodríguez - Servicio de Radiodiagnóstico, Hospital Universitario Donostia, Donostia-San Sebastián, España
More infoUltrasonography is a very good tool for guiding different interventional procedures in the chest. It is the ideal technique for managing conditions involving the pleural space, and it makes it possible to carry out procedures such as thoracocentesis, biopsies, or drainage. In the lungs, only lesions in contact with the costal pleura are accessible to ultrasound-guided interventions. In this type of lung lesions, ultrasound is as effective as computed tomography to guide interventional procedures, but the rate of complications and time required for the intervention are lower for ultrasound-guided procedures.
La ecografía es una muy buena herramienta para la guía de los diferentes procedimientos intervencionistas del tórax. Es la técnica ideal para el manejo de las patologías del espacio pleural y permite la realización de procedimientos como la toracocentesis, la biopsia o el drenaje. En el pulmón, tan solo aquellas lesiones que contacten con la pleura costal serán accesibles al intervencionismo con guía ecográfica. En este tipo de lesiones pulmonares, la ecografía es igual de efectiva que la tomografía computarizada como guía para estas intervenciones, pero con menor tasa de complicaciones y menor tiempo de ejecución.
Imaging-guided interventional techniques have brought about a revolution in the diagnosis and treatment of multiple diseases.1 There are multiple indications for imaging-guided interventional techniques in the chest. Haaga et al. reported the first two computed tomography (CT)-guided lung puncture procedures in 1974.2 This technique has undergone the most development as a guide for various interventional procedures in the chest.3 However, in the 1990s, several authors demonstrated the usefulness of ultrasound in guiding thoracic procedures, including in the lungs.4,5
At present, ultrasound is a first-line tool in multiple interventional pleural procedures.6–9 In the lungs, it may be equivalent to CT guidance in peripheral subpleural lesions. Contrast-enhanced ultrasound has proven useful before certain procedures, as it increases yield and precision.10–13
Below is a review of the advantages and disadvantages of ultrasound guidance in interventional procedures in the chest, with a focus on the lungs and pleurae.
Overview of interventional techniques in the chestUltrasound enables visualisation of thoracic lesions in the chest wall and costal pleura.14 The approach to lesions of this type is no different from the approach to lesions in other locations in the body. Simple visualisation of lesions with ultrasound enables an interventional approach to them. By contrast, lesions in the lung can only be approached when they are in contact with the costal pleura. Lesions in the mediastinum are more difficult to approach. The areas that are easiest to visualise on ultrasound correspond to the anterosuperior mediastinum and the internal thoracic lymph nodes. The mediastinal pleura is not accessible to ultrasound guidance.
As a general rule, interventional procedures in the chest are considered to entail a medium to low risk of bleeding and do not require major measures to be taken in advance.15
PleuraeUltrasound probably plays its most significant role in interventional techniques in the chest in pleural diseases. Ultrasound aids in directing procedures but also contributes a great deal of information about the type of pleural disease. Such information may prompt changes in decisions as to how to approach a particular procedure. For example, in patients with pleural effusion referred for diagnostic thoracentesis, detection on ultrasound of solid pleural implants should incite a change in procedure and the addition of a targeted biopsy to increase diagnostic yield. Next, the main interventional pleural procedures — thoracentesis, pleural drainage and pleural biopsy — shall be discussed.
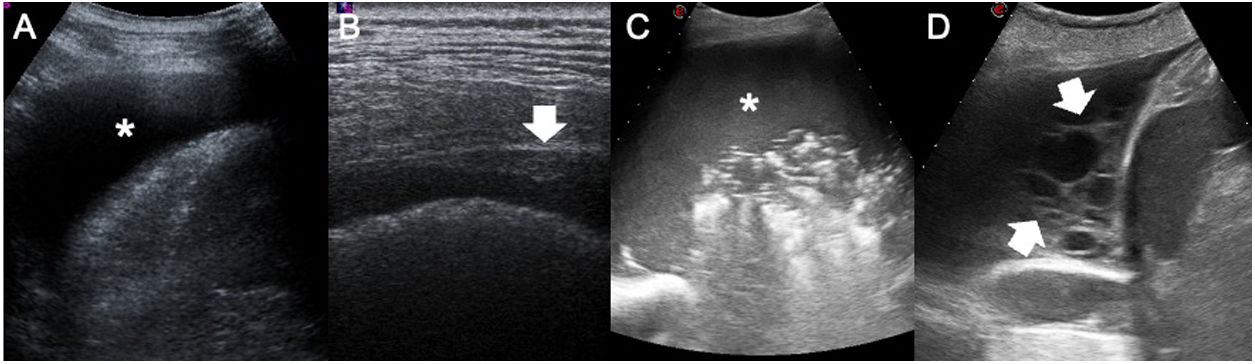
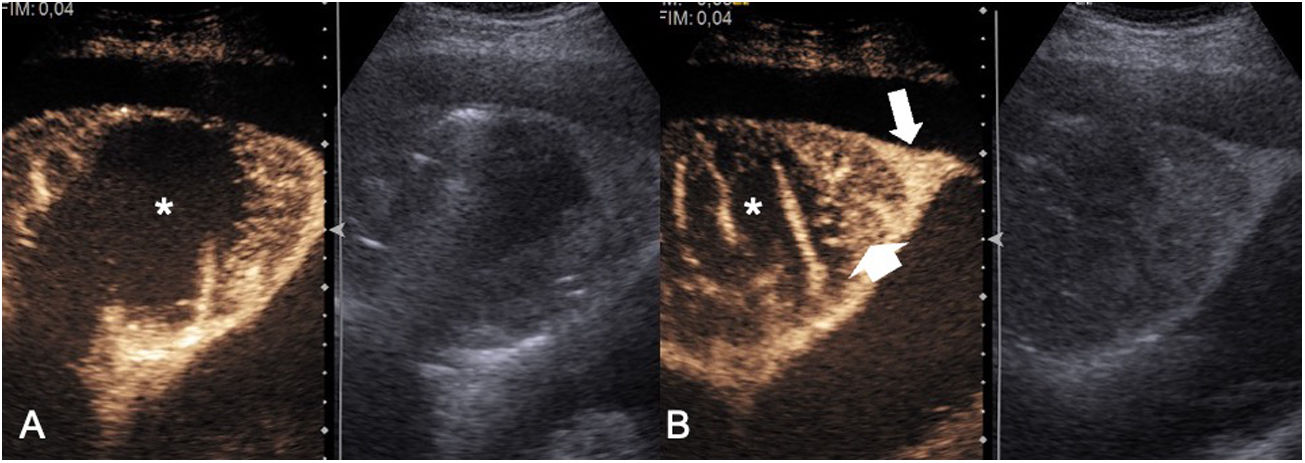
ThoracentesisEffusion is defined as a pathological accumulation of fluid in the pleural space. This space is virtual, with a separation between the two layers measuring 0.1-02 µm, and normally contains approximately 5 ml of fluid in an adult weighing 70 kg. Ultrasound is a highly sensitive technique for the detection of pleural effusion, as it is capable of detecting volumes of fluid corresponding to 5 ml or more.14,16 This means that any pathological accumulation of fluid in the pleural space could be detected on ultrasound. Pleural effusion is a very common disease, affecting 1.5 million people per year in the United States.16 Before diagnostic thoracentesis is performed, the pleural space should be thoroughly examined. It is mandatory to examine the diaphragmatic pleura because it is the most common site of pleural metastases.14 Practitioners should also determine not only whether pleural thickening is present but also whether the effusion contains echoes or septa. The presence of one of these three findings classifies the effusion as exudate.17 The absence of all three indicates likely transudate; however, it should be borne in mind that 20% of cases of exudate may manifest as anechogenic effusion (Fig. 1).
Semiology of pleural effusion. A) anechogenic pleural effusion (asterisk). B) Focal pleural thickening (white arrow) in a patient with pleural metastases of pulmonary adenocarcinoma. This finding is the only specific sign of malignancy. C) Pleural empyema. Pleural effusion filled with internal echoes (asterisk) indicating increased cellularity inside the fluid. D) Metastatic pleural effusion. Multiple thick septa can be seen inside the pleural effusion (white arrows). The presence of thick septa should not be attributed to empyema, as this finding may also be detected in pleural effusion due to other causes.
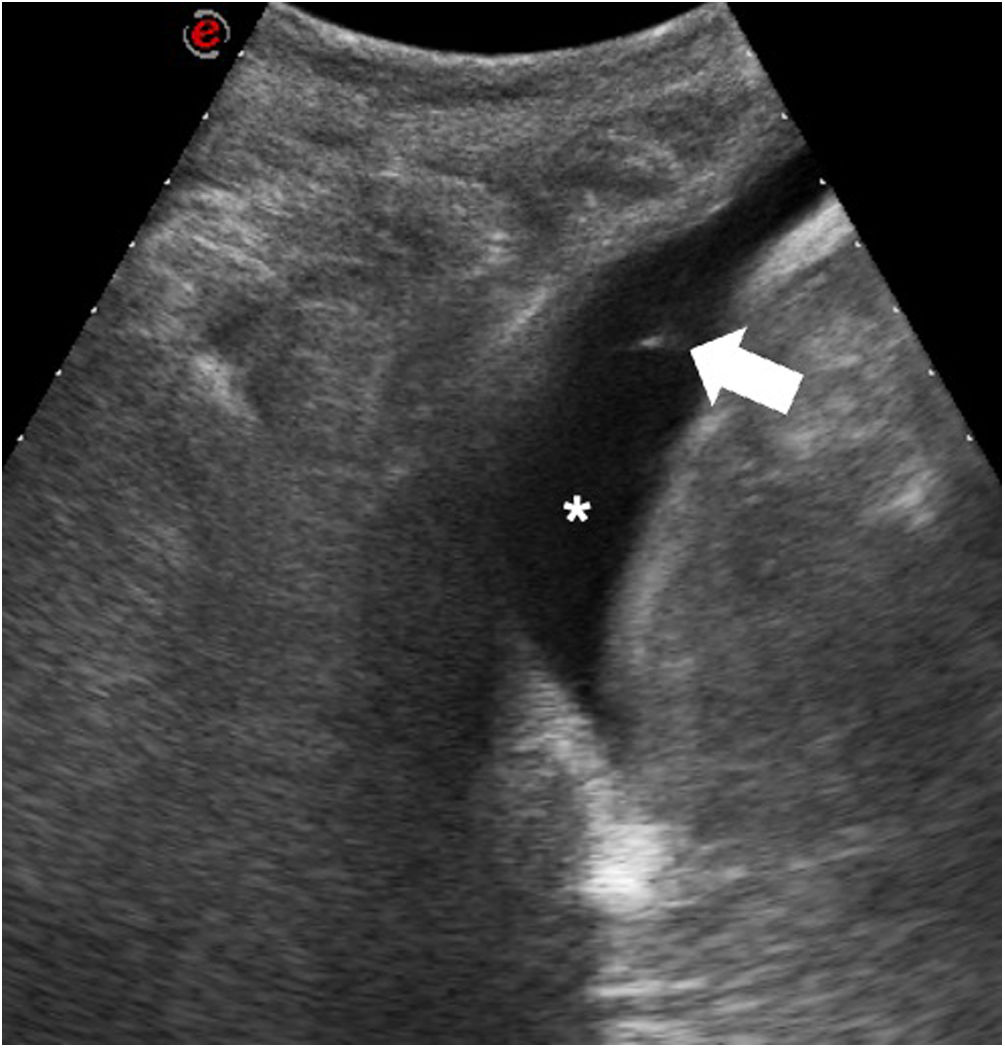
Thoracentesis is a diagnostic procedure that consists of taking samples of pleural fluid to be subsequently characterised. Thoracentesis should be performed whenever pleural effusion develops, unless there is rational cause for not doing so or when the patient has previously undergone puncture of the pleural space.18 The current clinical guidelines recommend the use of ultrasound both to confirm the presence of effusion and to guide thoracentesis.18,19 Thoracentesis is considered to entail a low risk of bleeding; therefore, it is not necessary to stop aspirin before the procedure; platelets should be administered in patients with platelet counts of less than 50,000 units/µl, and it is advisable to suspend clopidogrel 5 days beforehand and to suspend the dose of low–molecular-weight heparin prior to the procedure.15 Thoracentesis should be performed in a patient without mobility problems with the patient seated opposite the operator.20 The practitioner should look for an intercostal space where fluid is always present during respiratory manoeuvres and where the lung does not get in the way. Some guidelines recommend administering anaesthesia along the trajectory of the needle, but this is not necessary because the injection is as uncomfortable as the thoracentesis itself and not doing so avoids delaying the procedure. The practitioner should look for the upper border of the rib, although it should be borne in mind that, in posterior regions, especially in elderly people, the intercostal arteries run a tortuous course.21 If the skin is marked for another specialist to perform thoracentesis with a delay, it should be marked in the same position in which the procedure is to be performed.20 However, we recommend performing the procedure in the same room in which thoracentesis is performed, without a delay, because such a delay is associated with the same complications as unguided puncture. The procedure must sometimes be performed in patients with mobility problems. In these cases, thoracentesis can be performed (Fig. 2) in different ways (Table 1).22 When ultrasound guidance is used, the rate of success of thoracentesis increases, reaching 92% at the start of the technique in some published series,23 although in our experience this figure is clearly higher. The main complications are pneumothorax, vasovagal reaction, haemothorax, haematoma at the puncture site and post-expansion pulmonary oedema in evacuation thoracentesis.23–25 Pneumothorax may occur in 0%-3% of ultrasound-guided thoracenteses; this range may be as high as 11%-12% in blind thoracenteses.23–25 Therefore, ultrasound-guided thoracentesis significantly decreases the odds of developing pneumothorax during or after the procedure.26 Taking a chest X-ray after thoracentesis is not justified if ultrasound-guided thoracentesis has been performed.27 The only factor correlated with the onset of pneumothorax after ultrasound-guided thoracentesis is aspiration of air.28,29 While there is debate in the scientific literature as to whether there is a limit on the amount that can be aspirated in evacuation thoracentesis, with the onset of symptoms such as cough, pain and difficulty breathing, the procedure should be stopped.30,31 In addition, fluid aspiration should be stopped in cases in which it becomes difficult, as this indicates changes in pressure within the pleural cavity that may lead to the development of post-expansion pulmonary oedema.30
Thoracentesis in a bedridden patient. This was a male patient admitted to the intensive care unit (ICU) with fever and persistent pleural effusion. The procedure was performed with the patient in right lateral decubitus with a lateral access. The image shows the tip of the needle (white arrow) inside the pleural effusion (white asterisk).
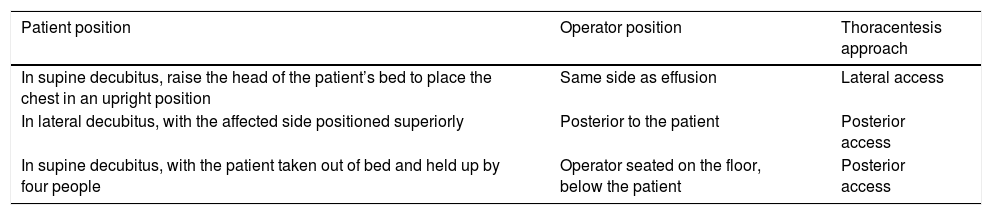
Positioning of patient and operator in thoracentesis in cases of confinement to bed.
| Patient position | Operator position | Thoracentesis approach |
|---|---|---|
| In supine decubitus, raise the head of the patient’s bed to place the chest in an upright position | Same side as effusion | Lateral access |
| In lateral decubitus, with the affected side positioned superiorly | Posterior to the patient | Posterior access |
| In supine decubitus, with the patient taken out of bed and held up by four people | Operator seated on the floor, below the patient | Posterior access |
This table summarises the different options that have been reported for performing thoracentesis in patients with mobility problems.
Although adding ultrasound guidance to thoracentesis would seem to lead to an increase in the cost of the test, it has been shown to not only decrease complications and increase yield but also decrease overall healthcare expenditure.32 Other advantages of using ultrasound to guide thoracentesis are the ability to determine the volume of effusion and the presence of complications within the fluid (such as septa, echoes and nodules), the possibility of precisely measuring the distance from the skin to the effusion and the possibility of detecting complications such as pneumothorax and bleeding.27
Pleural drainageA pleural drain may be placed in cases of pneumothorax or effusion. Pleural drainage, like thoracentesis, is considered to entail a moderate risk of bleeding, and the same preventive measures should be taken.
Although there are various X-ray–based classifications on when pneumothorax must be drained, this article focuses on ultrasound assessment. When pneumothorax is suspected, the ultrasound examination should target the superior areas of the affected hemithorax and the practitioner should look for signs suggestive of this disease: absence of lung sliding, absence of B-lines, presence of posterior linear reverberations and detection of the lung point sign.33–37 The latter is the most significant for determining the need to place a pleural drain in a case of pneumothorax. If the patient is in supine decubitus and the lung point sign is found to be anterior, this indicates that the pneumothorax is mild, with just 8% of patients requiring a drain. If instead this sign is found to be lateral, 90% of patients require a drain.1 Drain placement is simple and can be performed under ultrasound guidance. The practitioner only has to consider that once the tip of the drain enters the pleural cavity it cannot be visualised due to the major change in acoustic impedance between the chest wall and the pneumothorax.
The indications for pleural drainage in patients with pneumonia or suspected pleural infection are the following: voluminous pleural effusions occupying more than half the hemithorax, loculated effusions, effusions with thickening of the parietal pleura, when culture or Gram staining is positive, when pH is less than 7.20 or when pus has been obtained in thoracentesis.38 Catheters with a French (F) size of 8F-14F can be placed using the Seldinger technique or directly. To do this, as in thoracentesis, the practitioner should look for an intercostal space where pleural fluid is always present and where the lung does not get in the way during respiration. While there are no differences in outcomes between the Seldinger technique and the direct trocar technique, the latter is quicker. In cases in which the pleural collection is small or difficult to access, it is advisable to use the Seldinger technique.
The rate of success of drains in parapneumonic effusions has been found to be between 75% and 88%.20 The main complications of ultrasound-guided pleural drainage with a thin catheter are obstruction, pneumothorax and pachypleuritis.20 By contrast, the main complication of thick surgical drainage is improper positioning, which may occur in up to 25% of cases and which may not be identified on a posteroanterior or anteroposterior X-ray in 60%-95% of cases.20 The indications for thick and thin catheters are the same, except in acute haemothorax, in which the viscosity of the blood and the speed at which the effusion occurs mandate the placement of a thicker tube.39 Thin tubes are better tolerated by patients and complications of improper positioning do not occur when ultrasound guidance is used.40,41 However, in cases in which the substance retrieved in thoracentesis is thick pus or in which pleurodesis must be performed through a percutaneous drainage tube, the use of sizes greater than 8F is indicated. In addition, ultrasound enables the practitioner to determine the course and outcome of a complex pleural effusion. Cases of empyema or parapneumonic effusion with internal septa have a worse response to percutaneous drainage and a worse prognosis associated with a higher rate of intensive care unit (ICU) admission and a higher mortality rate.42 Outcomes of percutaneous drainage depend on ultrasound characteristics, with a better yield in anechoic effusions (92.3%) than in complex non-septated ones (81.54%) or complex septated ones (62.5%).43 Therefore, a pleural drain should be placed early, before ultrasound characteristics worsen. In cases of complex septated effusions, the mechanical septolysis technique can be used; this consists of breaking the septa with the drainage trocar. The objective of this technique is to increase the efficiency of thrombolysis, but for it to be effective and safe, it should always be performed under ultrasound guidance so as not to injure extrapleural structures.22 The use of fibrinolytics in patients with septated effusions or no response decreases the need for surgical intervention, as well as treatment failure, although it has not been shown to reduce mortality.44
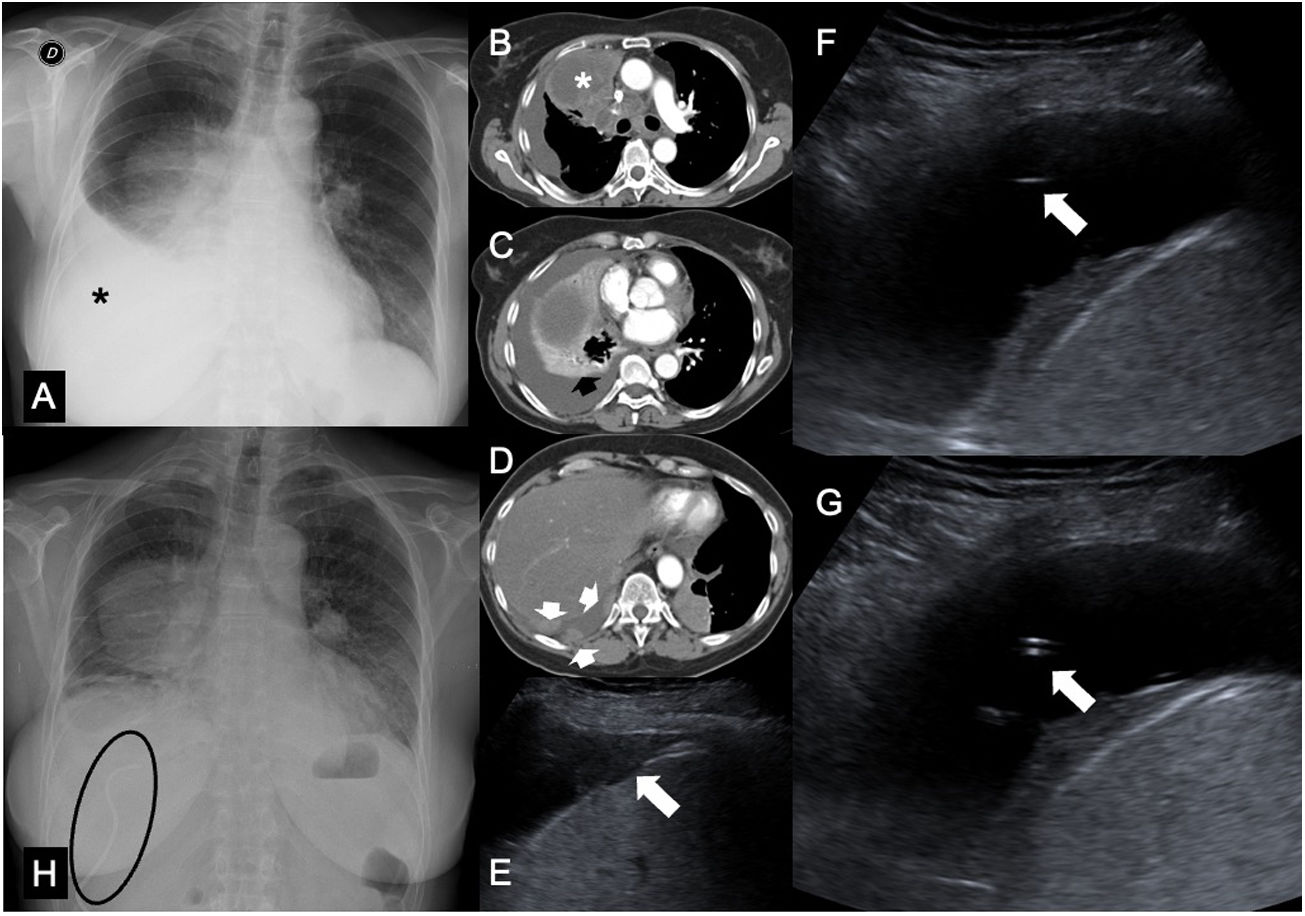
The indications for malignant pleural effusion drainage tend to be clinical and driven by the need to mitigate the patient's symptoms or to assess the capacity for re-expansion of the lung prior to pleurodesis. The technique is similar to that described for parapneumonic pleural effusion and empyema, with a few exceptions. In cases of malignant pleural effusions, the crossing of pleural implants should be avoided to decrease any potential for spread along the trajectory of the needle,1 and it is advisable to use an intercostal access relatively far from the diaphragm in the presence of inversion thereof, as the catheter could graze the diaphragm while the fluid is being drained and cause the patient to cough or feel pain (Fig. 3). Pneumothorax ex vacuo may be seen, especially in drains in oncology patients. It is not uncommon, especially in obstructive pulmonary disease associated with pleural effusion, and consists of the onset of pneumothorax that is moderate or significant after evacuation thoracentesis or pleural drainage, or mild but persisting for more than three days.45 The placement of a new pleural drain in asymptomatic patients is unnecessary and unlikely to have clinical benefits.45
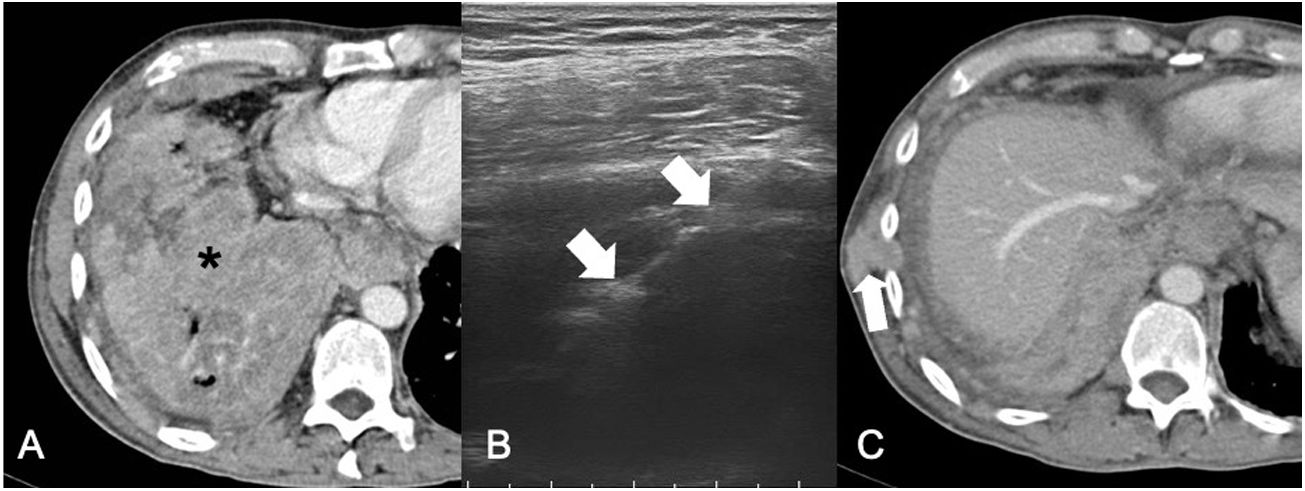
Pleural drainage in a patient with colorectal cancer metastasis. A) Posteroanterior chest X-ray showing a right parahilar mass and a moderate right pleural effusion occupying a third of the right hemithorax (asterisk). B-D) Images from computed tomography (CT) of the chest in a mediastinal window showing a pulmonary mass occluding the right upper lobe bronchus (asterisk), with moderate pleural effusion with metastatic solid implants (white arrows) and passive collapse of the right (black arrow) and middle lower lobes. E) Ultrasound examination revealing metastatic pleural implants (white arrow) in the lateral costophrenic recess. F) Placement of a pleural drain using the Seldinger technique. Cephalic access to the implants seen in E was performed. G) Pleural drainage catheter deployed within the pleural effusion. H) Posteroanterior chest X-ray showing resolution of pleural effusion 24 hours after drain placement (visible inside the ellipse).
The use of ultrasound guidance is widely recommended given that not using this technique has been found to be a cause of morbidity and mortality in patients who undergo pleural drainage.46 The recommendation to place pleural drains requires an intermediate level according to the British criteria for competency in pleural procedures.47
Pleural biopsyPleural biopsy is indicated in suspected malignancy, in persistent pleural effusions that do not show improvement and in cases of suspected but unconfirmed tuberculosis.18 The complications of this procedure when performed under ultrasound guidance occur at a low rate (less than 1%) and comprise pneumothorax, haemothorax, haematoma at the puncture site and seeding (spread along the trajectory of the needle). The latter is very common in percutaneous pleural biopsy of mesothelioma (Fig. 4). The first series published found dissemination in 40% of patients,48 though more recent series have arrived at lower if still significant rates of up to 13.2%.49 However, while many guidelines once indicated prophylactic radiotherapy along the puncture trajectory in cases of mesothelioma, more recent studies have shown that it is not necessary and that it should only be done in patients with relapse with clinical repercussions.50 The clinical guidelines recommend that it be performed by expert physicians under ultrasound guidance. The number of samples to be obtained will depend on the clinical suspicion, ranging from a single core in cases of metastasis or tuberculosis to three or four cores in mesothelioma. Ultrasound is the ideal technique for performing the procedure because it detects small areas of pleural thickening and clearly distinguishes them from the pleural fluid that usually accompanies solid pleural disease. Pleural biopsy increases the yield of thoracentesis in cases of suspected neoplastic or tuberculous disease, as does the use of ultrasound guidance to take the sample,51,52 managing to achieve the same yield as thoracoscopic biopsy. Hence, imaging-guided pleural biopsy should be considered a first-line option in the diagnosis of pleural effusion suspected of malignancy or tuberculosis, over traditional closed pleural biopsy and thoracoscopic biopsy. One factor involved in a better diagnostic outcome is the thickness of the pleural involvement, with a yield of nearly 100% achieved in patients with a thickness in excess of 20 millimetres.7 Using a 16G needle rather than an 18G needle also increases diagnostic yield.7 While the procedure is being performed, a smaller angle of incidence in relation to the pleura increases the length of the fragment obtained. This is particularly important in pleural thickening of just a few millimetres (Fig. 5).
Malignant pleural mesothelioma. A) CT of the chest in a mediastinal window revealing a large heterogeneous pleural mass (asterisk). B) Ultrasound-guided pleural biopsy. The white arrows indicate the biopsy needle. In these cases, it is important to note that this type of lesion should be punctured from the extrapleural fat so that the pathologist may locate the fragment obtained. C) CT of the chest performed six months after biopsy showing a soft-tissue mass in the chest wall (white arrow) corresponding to spread along the trajectory of the biopsy.
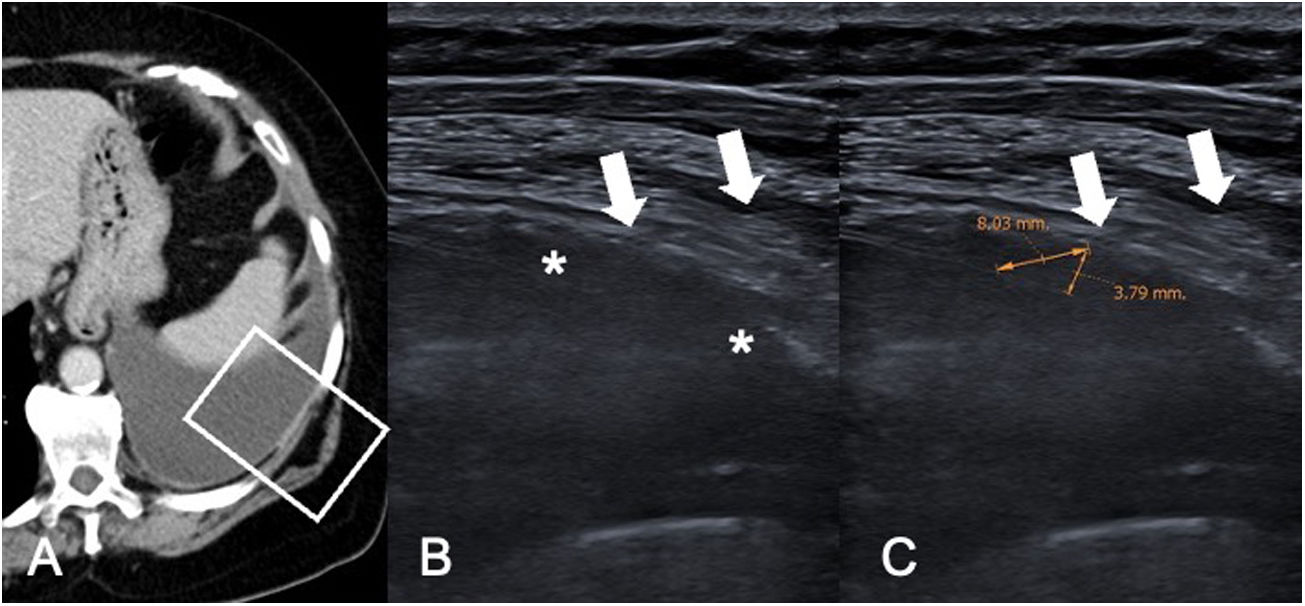
Pleural metastases of breast cancer. A) CT of the chest in a mediastinal window revealing moderate left pleural effusion with regular thickening of the parietal pleura. The white rectangle indicates the location of the ultrasound images shown in B and C. B) Ultrasound examination to perform a pleural biopsy revealing hypoechogenic pleural thickening (white asterisks). The white arrows indicate the trajectory of the biopsy needle. C) Same image as B with measurement of the thickening perpendicular to the pleura (3.79 mm) and along the trajectory of the biopsy (8.03 mm). The performance of a biopsy needle approach with a smaller angle of incidence on the pleural thickening enables acquisition of longer histology cores.
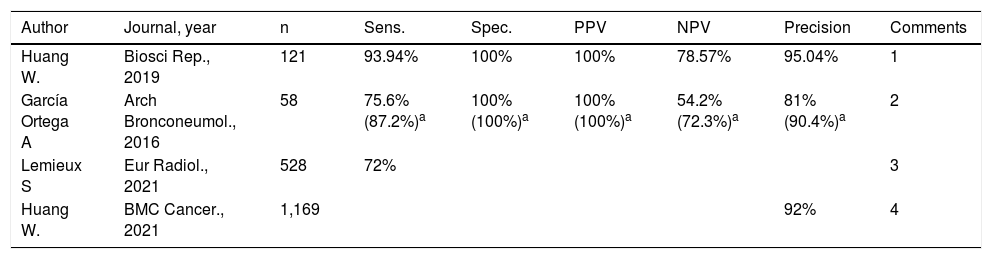
Traditionally, lung punctures have been CT-guided since Haaga et al.2 published the first two cases. The first publications that assessed the use of ultrasound as a guide for lung puncture appeared in the 1990s.4,5,53 Only lesions on the lung periphery and in contact with the costal pleura will be accessible using ultrasound guidance. Lesions located in the vicinity of the mediastinal pleura are not accessible using ultrasound guidance, nor are lesions that are peripheral but not in contact with the pleura. It has been estimated that just 5% of pulmonary nodules are in contact with the pleura; therefore, the proportion of lesions that can be approached using ultrasound will always be lower than the proportion of lesions that can be approached using CT guidance (Fig. 6). However, when it is possible to perform the procedure using either technique, ultrasound has proven to be quicker and have a lower incidence of complications, with an equivalent yield.54,55 A study by Lee et al. concluded that the use of ultrasound guidance should be recommended for peripheral pulmonary lesions measuring more than 10 mm because it is safer, quicker and possibly more precise then CT guidance.55 The overall yield of ultrasound-guided lung puncture has exceeded 90% in the most recently published studies (Table 2).56–59 The yield of CT-guided lung punctures has been seen to be influenced by lesion size. Different studies have found CT guidance to have a better yield in lesions with diameters greater than 5 cm or less than 1-2 cm than in lesions or masses in between those sizes.60 By contrast, a study by Huang56 found that size did not influence the yield of ultrasound-guided lung puncture. When ultrasound monitoring is used to perform the procedure, the area of pleural contact has a greater influence than lesion diameter.61 A series by Lemieux et al.58 confirmed that the best outcomes are achieved in the puncture of lesions with more than 30 mm of pleural contact and that the puncture of nodules of a size less than 10 mm should be avoided. Moreover, Guo et al.62 confirmed that lesion size also modifies outcomes, with a decrease in yield under 20 mm and over 50 mm. They confirmed that the rate of necrosis gradually increases as the size of a lesion increases, which would account for the drop in precision at sizes exceeding 50 mm. By contrast, the lower yield in smaller lesions could be attributed to technical factors arising from the difficulty of puncturing such a small lesion.
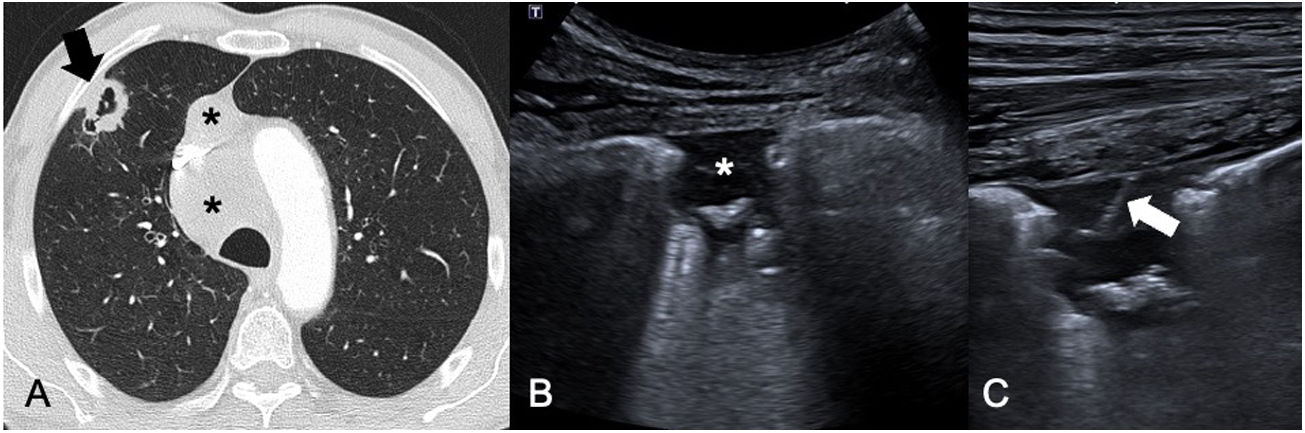
Primary pulmonary adenocarcinoma. A) CT of the chest in a lung window showing a cavitated subpleural lung nodule in the right upper lobe (black arrow) with mediastinal lymphadenopathy (black asterisks). B) Chest ultrasound showing the cavitated lesion identified in A, with extensive pleural contact, enabling core-needle biopsy to be performed. C) Post-biopsy ultrasound image showing a white line corresponding to the trajectory of the needle. The puncture confirmed the diagnosis of adenocarcinoma and enabled the performance of techniques to evaluate its mutational status.
Summary of recently published results on ultrasound-guided lung puncture.
| Author | Journal, year | n | Sens. | Spec. | PPV | NPV | Precision | Comments |
|---|---|---|---|---|---|---|---|---|
| Huang W. | Biosci Rep., 2019 | 121 | 93.94% | 100% | 100% | 78.57% | 95.04% | 1 |
| García Ortega A | Arch Bronconeumol., 2016 | 58 | 75.6% (87.2%)a | 100% (100%)a | 100% (100%)a | 54.2% (72.3%)a | 81% (90.4%)a | 2 |
| Lemieux S | Eur Radiol., 2021 | 528 | 72% | 3 | ||||
| Huang W. | BMC Cancer., 2021 | 1,169 | 92% | 4 |
NPV: negative predictive value; PPV: positive predictive value; sens.: sensitivity; Spec.: specificity.
Another indication for ultrasound-guided lung puncture is the determination of the causal agent of pneumonia. It is usually performed in bedridden ICU patients in whom antibiotic treatment does not lead to radiological or clinical improvement.63 Ultrasound guidance helps identify abscesses and necrosis in pneumonia.
Regarding complications, a wide range of outcomes from 0%56,57 to 44% has been reported.64 It should be borne in mind that the latter study was conducted in paediatric patients and that all types of complications were taken into account. Regarding pneumothorax, the various series published have reported percentages ranging from 0%56,57 to 15%,58 although many studies have shown incidences under 3%.59,65,66 A study by Cozzolino66 compared the complications that occurred in 80 patients who underwent CT-guided or ultrasound-guided punctures and found that the ultrasound-guided procedures had a lower rate of pneumothorax (2.5% with ultrasound versus 22.5% with CT). In addition, ultrasound may be useful in detecting the onset of pneumothorax after a lung puncture with a yield comparable to that of X-ray65 (Fig. 7).
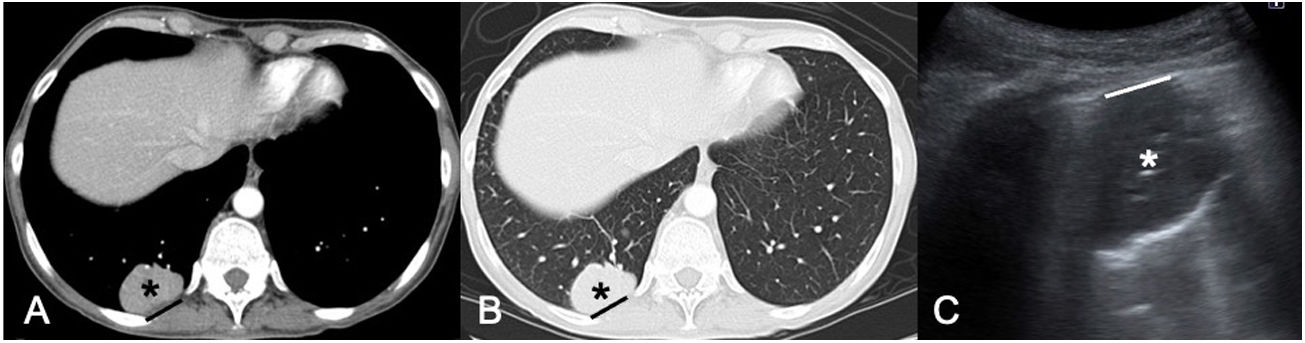
Lung metastasis of kidney cancer. A and B) CT of the chest in a mediastinal window (A) and a lung window (B) showing a subpleural lung mass in the right lower lobe (black asterisks) with extensive contact with the costal pleura (black line). C) Ultrasound image prior to core-needle biopsy enabling evaluation of the lung lesion (white asterisk). Note that the pleural contact distance (white line) is smaller in the ultrasound examination, probably due to the effects of gravity in placing the patient in a prone position.
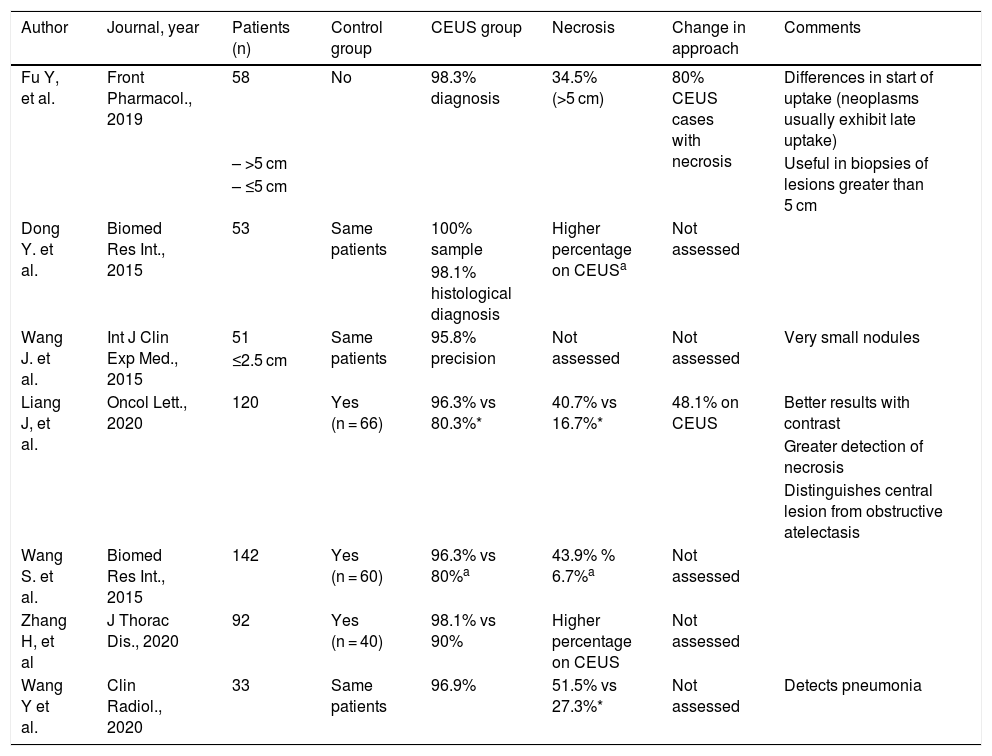
Contrast-enhanced ultrasound has brought about a revolution in multiple areas of the body.67 As with ultrasound itself, the potential indications for this technique in the chest have taken longer to be published.68 In recent years, several articles evaluating the usefulness of contrast-enhanced ultrasound in guiding puncture of pulmonary lesions have been published.10,11,13,69–72Table 3 summarises the main findings reported in these articles. The use of ultrasound contrast prior to the procedure enables more precise detection of necrosis within lung lesions and increases yield (Fig. 8). Studies that have compared to a control group (conventional ultrasound-guided puncture) have demonstrated superior yield with the use of contrast prior to the procedure.71–73 In 48.1%-80% of cases, contrast has resulted in a change to the planned interventional approach.69,71
Summary of the publications on the use of contrast-enhanced ultrasound (CEUS) in lung punctures.
| Author | Journal, year | Patients (n) | Control group | CEUS group | Necrosis | Change in approach | Comments |
|---|---|---|---|---|---|---|---|
| Fu Y, et al. | Front Pharmacol., 2019 | 58 | No | 98.3% diagnosis | 34.5% (>5 cm) | 80% CEUS cases with necrosis | Differences in start of uptake (neoplasms usually exhibit late uptake) |
| – >5 cm | Useful in biopsies of lesions greater than 5 cm | ||||||
| – ≤5 cm | |||||||
| Dong Y. et al. | Biomed Res Int., 2015 | 53 | Same patients | 100% sample | Higher percentage on CEUSa | Not assessed | |
| 98.1% histological diagnosis | |||||||
| Wang J. et al. | Int J Clin Exp Med., 2015 | 51 | Same patients | 95.8% precision | Not assessed | Not assessed | Very small nodules |
| ≤2.5 cm | |||||||
| Liang J, et al. | Oncol Lett., 2020 | 120 | Yes (n = 66) | 96.3% vs 80.3%* | 40.7% vs 16.7%* | 48.1% on CEUS | Better results with contrast |
| Greater detection of necrosis | |||||||
| Distinguishes central lesion from obstructive atelectasis | |||||||
| Wang S. et al. | Biomed Res Int., 2015 | 142 | Yes (n = 60) | 96.3% vs 80%a | 43.9% % 6.7%a | Not assessed | |
| Zhang H, et al | J Thorac Dis., 2020 | 92 | Yes (n = 40) | 98.1% vs 90% | Higher percentage on CEUS | Not assessed | |
| Wang Y et al. | Clin Radiol., 2020 | 33 | Same patients | 96.9% | 51.5% vs 27.3%* | Not assessed | Detects pneumonia |
Primary pulmonary lymphoma. A) Images combining the contrast-enhanced examination (left) and baseline imaging (right). Notice how contrast-enhanced ultrasound reveals that the tumour necrosis (white asterisk) is more extensive than expected on conventional imaging. B) Images combining the contrast-enhanced examination (left) and baseline imaging (right). The use of contrast makes it possible to detect the central necrosis (white asterisk) and the vessels that cross the necrotic areas and to distinguish between collapsed lung parenchyma (long arrow) and tumour parenchyma (short arrow).
Drainage of lung abscesses is performed very uncommonly, but may replace surgical intervention. A lung abscess should be drained when there is a poor response to antibiotic treatment after 10 days, if the patient is not expected not to comply with antibiotic treatment or when the abscess is larger than 4 cm.10,14 Catheters with a size of 6F-12F can be placed, although 8F catheters are most commonly used. Ultrasound guidance is particularly useful for draining abscesses in contact with the peripheral pleura or in pneumonia. The main complication of this procedure is pneumothorax.
ConclusionsUltrasound guidance is very helpful in non-vascular interventional techniques in the chest. While it plays a limited role in the lung, it is of choice in lesions in contact with the costal pleura, with outcomes comparable to those of CT, but with a shorter procedure time and fewer complications. In pleural disease, it is the ideal technique for performing thoracentesis, drainage and biopsy procedures. Radiologists must know the indications for this technique, as well as its potential complications and limitations. The use of ultrasound contrast prior to the procedure may be a tremendously useful tool with encouraging outcomes published to date and a promising future to explore.
Authorship- 1
Responsible for study integrity: GI, IV
- 2
Study concept: GI, IV
- 3
Study design: GI, IV
- 4
Data collection: GI, IV
- 5
Data analysis and interpretation: GI, IV
- 6
Statistical processing: GI, IV
- 7
Literature search: GI, IV
- 8
Drafting of the article: GI, IV
- 9
Critical review of the manuscript with intellectually significant contributions: GI, IV
- 10
Approval of the final version: GI, IV
This study has not received any type of funding.
Conflicts of interestThe authors declare that they have no conflicts of interest.
Please cite this article as: Isus G, Vollmer I. Intervencionismo torácico con guía ecográfica. Radiología. 2021;63:536–546.