MDR Klebsiella pneumoniae ST307 is a high-risk clone, whose genetic features contribute to its adaptation to hospital environments and the human host. This study describes the emergence and clonal dissemination of K. pneumoniae ST307, recovered during November 2018 to February 2019 in a hospital in Buenos Aires city, which concurrently harbored KPC-3 and NDM-1. These isolates were resistant to all β-lactams and to the ceftazidime/avibactam combination. Molecular studies showed that blaKPC-3 was located in Tn4401a platform, while blaNDM-1 was surrounded upstream by ISKpn14 followed by a partial sequence of ISAba125 and downstream by bleMBL-trpF, located in a 145.5kb conjugative plasmid belonging to the Inc A/C group. The dissemination of K. pneumoniae ST307 isolates co-producing KPC-3 and NDM-1 could lead to a worrisome scenario due to the remarkable features of this clone and its resistance profile.
Klebsiella pneumoniae ST307 es un clon de alto riesgo, cuyas características genéticas contribuyen a su adaptación al entorno hospitalario y al huésped humano. Este estudio describe la emergencia y diseminación clonal de aislamientos de K. pneumoniae ST307 productores de KPC-3 y NDM-1, recuperados en un hospital de Buenos Aires. Estos aislamientos fueron resistentes a todos los β-lactámicos y a la combinación ceftacidima/avibactam. Los estudios moleculares evidenciaron que el contexto genético de blaKPC-3 se correspondió con el Tn4401a, mientras que blaNDM-1 estuvo flanqueado corriente arriba por ISKpn14 y una secuencia parcial de ISAba125 y corriente abajo por bleMBL – trpF, localizado a su vez en un plásmido conjugativo de 145.5 kb perteneciente al grupo Inc A/C. La emergencia de aislamientos de K. pneumoniae ST307 coproductores de KPC-3 y NDM-1 pone de manifiesto una situación altamente preocupante debido a las características de este clon y a su perfil de multirresistencia.
Carbapenem-resistant Klebsiella pneumoniae (CR-Kp) infections are associated with high morbidity and mortality rates, mainly in patients admitted to intensive care units. Nowadays, the production of KPC-β-lactamases constitutes the main resistance mechanism among CR-Kp. KPC-producing K. pneumoniae (KPC-Kp) became endemic in 2010 in Argentinian hospitals, and has been attributed to the global expansion of the epidemic clone K. pneumoniae sequence type (ST) 258 and related strains of the clonal complex (CC) 2584. However, in recent years, the emergence of KPC-Kp belonging to non-CC258 has been reported3, including the high-risk clone K. pneumoniae ST307. Although ST307 was reported in several countries as early as 2013, it emerged in 1994, close to the estimated emergence of the international ST258 clone15. ST307 was initially associated with blaCTX-M-15; however, enough indexed literature supports that this lineage could acquire and disseminate carbapenemases (blaKPC, blaNDM, blaOXA-48, blaOXA-48, blaOXA-181, blaGES-5) and probably other clinically important antimicrobial resistance determinants14,15. K. pneumoniae ST307 is a highly successful MDR clone that has been proposed as a candidate for becoming the prevalent high-risk CR-Kp clone based on its greater aptitude, persistence and adaptation to hospital environments and to the human host14. K. pneumoniae ST307 was first noticed in Argentina during a surveillance study performed in 2015-2017. Two out of 76 KPC-Kp studied isolates, named Kp2 and Kp14, belonged to ST307. Kp2 corresponded to a KPC-2-producing K. pneumoniae isolate, which was recovered in January 2017, in a hospital located in Buenos Aires (HA), being the first KPC-Kp ST307 reported in Argentina. Kp14 was the first KPC-3-producing K. pneumoniae ST307 identified in this country, and corresponded to the first detection of this KPC allele3. Kp14 was isolated in December 2017 in another hospital in Buenos Aires (HB). The aim of this study was to investigate the presence of CR-Kp ST307 isolates and to characterize them based on their resistance mechanisms and clonal relationship.
From November 2018 to February 2019, a total of 35 CR-Kp isolates were recovered from inpatients with invasive infections in HB. These isolates were anonymously delivered to the Instituto de Investigaciones en Bacteriología y Virología Molecular (IBaViM) – FFyB, Universidad de Buenos Aires for further characterization. Those previously mentioned K. pneumoniae ST307 isolates, Kp2 and Kp14, were included in this study. Except for Kp2, the isolates were recovered in HB. Susceptibility to β-lactams, quinolones, aminoglycosides and trimethoprim-sulfamethoxazole (TMS) was assessed by automated systems (BD Phoenix). Aztreonam and ceftazidime/abivactam (CZA) susceptibilities were determined by the disk diffusion method. Antimicrobial susceptibilities were interpreted according to CLSI M100-ED29 2019 (https://clsi.org/all-free-resources/). Colistin minimum inhibitory concentration was determined by the broth microdilution method according to EUCAST 2019 (http://www.eucast.org/). Double disk synergy tests were performed in order to detect KPC and/or metallo-β-lactamases (MBL) using phenyl boronic acid (300μg) and EDTA (1μmol) disks, respectively9,11.
The presence of the most prevalent carbapenemase encoding genes (blaVIM, blaIMP, blaSPM, blaKPC, blaNDM and blaOXA-48-like) was investigated by multiplex-PCR, using specific primers and total DNA as template13. Moreover, blaKPC and blaNDM were confirmed as previously described using plasmid extracted DNA as template1,12. PCR products were sequenced at external facilities (Macrogen, Korea) and analyzed using the BLAST tool at the National Center for Biotechnology Information (http://www.ncbi.nlm.nih.gov/BLAST). The genetic context of blaKPC was studied by PCR mapping and sequencing in order to infer the Tn4401 structures or blaKPC-bearing non-Tn4401 elements.3 To investigate blaNDM-1 surrounding regions, specific primers were designed based on previously reported structures (Table 1). In those isolates that displayed colistin resistance, screening for plasmid located colistin resistance genes (mcr-1,-2,-3,-4,-5) was conducted by multiplex PCR as previously described.7 Plasmid conjugation assays were performed in Luria Bertani broth (LB) using E. coli J53AzR as recipient strain and K. pneumoniae isolates as the donor strains. Isolates were grown overnight in LB (5ml) at 37°C. After incubation, the bacterial cultures were mixed 1:10 and 5:10 donor-recipient, and incubated at 37°C for 18h without shaking. Transconjugants were selected on Mueller Hinton agar plates containing sodium azide (200μg/ml) and imipenem (0.5μg/ml). Plasmids from transconjugants were extracted and further characterized in terms of their replicons2,13. Plasmid size was estimated by treatment with S1 nuclease followed by pulse-field gel electrophoresis (PFGE).
Primers designed in this study.
| Primer | Sequence (5′→3′) | Amplicon length (bp) | Reference for primer design |
|---|---|---|---|
| ISAba125-FW | GAACTCATTAAAAGACATCCTAG | 1099 | JN872329 |
| NDM-UP-RV | CCCGCTCAGCATCAATGCA | ||
| ΔOXA-10-FW | TTTAGCCACCAATGATGCCCTC | 993 | KP770033 |
| NDM-UP-RV | CCCGCTCAGCATCAATGCA | ||
| ISKpn14-FW | GTGATGCTACCAACTTGCTGATT | 1024 | KP770024 |
| NDM-UP-RV | CCCGCTCAGCATCAATGCA | ||
| NDM-END-FW | GATGCCGACACTGAGCACTACG | 1090 | JN872329KP770033KP770024 |
| TrpF-RV | GACGCCGCTGGAGGTATCGA |
Multilocus sequence typing was performed amplifying and sequencing the following seven house-keeping genes: gapA, infB, mdh, pgi, phoE, rpoB, tonB, in accordance with www.pubmlst.org. Clonal relationship was analyzed by PFGE after digestion of genomic DNA with XbaI. Among the 35 CR-Kp isolates, 4 were assigned to ST307 according to www.pubmlst.org. These CR-Kp ST307 isolates, KpM24, KpM25, KpM30, KpM34 and those previously recovered CR-Kp, Kp2 and Kp14, were further characterized. All of them displayed resistance to ampicillin, ampicillin-sulbactam, piperacillin-tazobactam, ceftazidime, ceftriaxone, aztreonam, imipenem, meropenem, ciprofloxacin, levofloxacin and gentamicin. KpM24, KpM25 and KpM34 isolates were resistant to CZA, while Kp2, Kp14 and KpM30 remained susceptible. Except for Kp2, they also displayed resistance to colistin (Table 2). Phenotypic screening of KPC enzymes rendered positive results in Kp2, Kp14 and KpM30, while the production of carbapenemases could not be phenotypically detected in the remaining isolates.
Characteristics of carbapenem-resistant Klebsiella pneumoniae ST307 isolates included in this study.
| Isolate | Month/Year of isolation | Sample | Susceptibility profile | Synergy test for KPC/MBL | Carbapenemase encoding gene | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BLa | CZA | CIP | LEV | GEN | AKN | COL | TMS | ||||||
| HA | Kp2 | January/2017 | Blood | R | S | R | R | R | S | S | R | +/− | blaKPC-2 |
| HB | Kp14 | December/2017 | Blood | R | S | R | R | R | I | R | S | +/− | blaKPC-3 |
| HB | KpM24 | November/2018 | Urine | R | R | R | R | R | R | R | S | −/− | blaKPC-3+blaNDM-1 |
| KpM25 | December/2018 | Blood | R | R | R | R | R | R | R | S | −/− | blaKPC-3+blaNDM-1 | |
| KpM30 | January/2019 | Blood | R | S | R | R | R | S | R | S | +/− | blaKPC-3 | |
| KpM34 | February/2019 | CSF | R | R | R | R | R | R | R | S | −/− | blaKPC-3+blaNDM-1 | |
H: hospital; Kp: Klebsiella pneumoniae; CSF: cerebrospinal fluid; MBL: metallo-β-lactamases.
BL: β-lactams. Includes: ampicillin, ampicillin-sulbactam, piperacillin-tazobactam, ceftazidime, ceftriaxone, aztreonam, imipenem and meropenem. CZA: ceftazidime/avibactam, CIP: ciprofloxacin, LEV: levofloxacin, GEN: gentamicin, AKN: amikacin, COL: colistin, TMS: trimethoprim-sulfamethoxazole. R: resistant, I: intermediate, S: susceptible
The presence of blaKPC was detected in all CR-Kp ST307 isolates, corresponding to either blaKPC-2 or blaKPC-3. KpM24, KpM25 and KpM34 harbored both blaKPC-3 and blaNDM-1 (Table 2). Tn4401a was recognized as the genetic platform for blaKPC-2 and blaKPC-3. Moreover, the presence of ISKpn14 was detected in the upstream region of blaNDM-1, followed by a partial sequence of ISAba125, while bleMBL and trpF were detected downstream. blaNDM-1 harboring plasmids were successfully transferred by conjugation to an Escherichia coli J53 recipient strain, and all blaNDM-1 plasmids from transconjugants corresponded to the A/C incompatibility group and were approximately 145.5kb in size. blaKPC harboring plasmids could not be transferred under the assayed conditions. Screening for plasmid located colistin resistance genes rendered negative results.
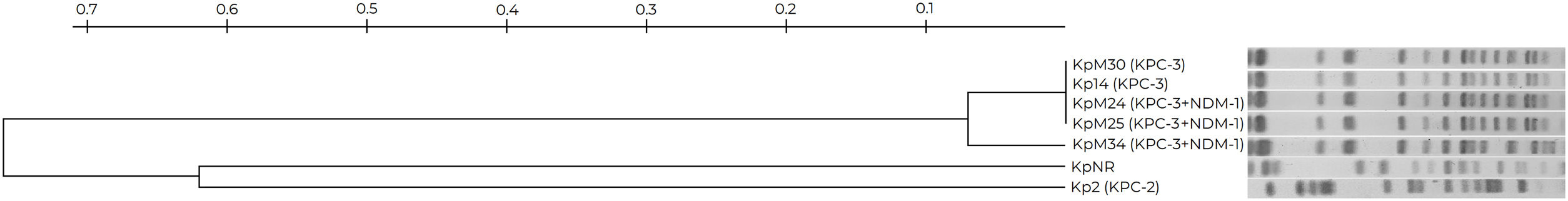
KpM24, KpM25, KpM30 and Kp14 displayed a unique pulsetype in the XbaI-PFGE, closely related to KpM34 (Fig. 1). As previously mentioned, all these isolates were recovered in HB. Kp2, which was recovered in HA, exhibited a different pulsetype. An increase of K. pneumoniae ST307 was observed in HB compared with previous periods3, despite the fact that a longer time should be monitored for a conclusive epidemiological definition. ST307 has become a globally disseminated high-risk clone, and was responsible for several worldwide nosocomial outbreaks3,5,14,15. Although this clone emerged during the mid-1990s, it remained largely unnoticed for almost 20 years, and was not detected in Argentina until 20173. It is worth noting that three K. pneumoniae ST307 isolates were positive for blaKPC-3 and blaNDM-1, being its first description in indexed literature in Argentina. The synergy tests performed in this study were not able to detect any carbapenemases in KPC+NDM-producing isolates; alternative methodologies such as a chromatographic test should be useful. The presence of metallo-carbapenemase encoding genes in blaKPC positive K. pneumoniae isolates is infrequent; however, the presence of blaNDM-1+blaOXA-48 is most commonly described, including several outbreaks caused by K. pneumoniae belonging to ST3075. blaNDM-1 had not been previously recognized in K. pneumoniae in this hospital (HB); nevertheless, a rise in the detection of this marker is being observed in Enterobacterales in Argentina.
blaNDM-1 harboring plasmids corresponded to IncA/C, low-copy-number, conjugative and self-transferable plasmids6. The genetic environment of blaNDM-1 is similar to that described in 2015 in Pn2 plasmid from Enterobacter cloacae (Accession Number KP770024), and in a type 1-IncC plasmid from Klebsiellaquasipneumoniae subsp. quasipneumoniae isolated in Argentina8. Compared to IncC blaNDM-1 harboring plasmids recently reported in Uruguay, the plasmids analyzed in the present study displayed different size and upstream genetic context for blaNDM-110.
The former KPC-3-producing isolate, Kp14, was recovered in the same hospital (HB) and displayed the same PFGE pulsetype as those isolates that produce both KPC-3 and NDM-1, recovered in the present period. Probably this strain, which was circulating in HB, acquired the blaNDM-1 harboring IncA/C epidemic plasmid. In those isolates recovered from HB, inactivation of mgrB may be involved in the colistin resistance phenotype, as it was previously observed in the clonally related Kp14 isolate3.
In conclusion, the present study provides information about the dissemination of this high-risk clone in HB, although it is still infrequently reported in Argentina. Here we alert about the emergence of K. pneumoniae ST307 isolates co-producing KPC-3 and NDM-1, which could lead to a worrisome scenario in this country, regarding the remarkable features of K. pneumoniae ST307 and its resistance profile to CZA. Association of blaNDM-1 to epidemic plasmids, such as the IncA/C group, along with the introduction of avibactam in HB in 2018, may have favored the selection and dissemination of NDM-1-producing isolates. The potential for dissemination of carbapenemase-producing K. pneumoniae ST307 warrants larger and more continuous epidemiological surveillance studies aimed at the early detection of this high-risk clone.
FundingThis work was supported by UBACyT to M. Radice and G. Gutkind (20020150100174BA and 20020170100473BA, respectively); PICT to G. Gutkind (2015-1925); PIP to M. Radice (PIP11220200102425CO).
Ethical approvalThe ethics committee of FFyB-UBA approved this study (Res CD 894-2019). All isolates were delivered anonymized from HB to IBaViM-FFyB-UBA, in order to preserve patient's identity. Isolates have been designated using numbers, and hospitals were mentioned using capital letters.
Conflict of interestThe authors declare that they have no conflicts of interest.