Biocontrol of the nematode Meloidogyne javanica was studied using the Argentinean strains Pseudomonas fluorescens MME3, TAE4, TAR5 and ZME4 and Bacillus sp. B7S, B9T and B19S. Pseudomonas protegens CHA0 was used as a positive control. Egg hatching and juvenile mortality were evaluated in vitro by exposure of nematodes to bacterial suspensions or their cell-free supernatants (CFS). The effect of bacteria on nematode infestation of lettuce was also studied. results showed that most of the tested strains and CFS reduced egg hatching and juvenile survival in vitro. The bacterial suspension of Bacillus sp. B9T produced the lowest hatching of eggs. Juvenile mortality was higher when M. javanica was exposed to Bacillus sp. than to Pseudomonas spp. suspensions. Except for CFS of B9T, all filtrates inhibited hatching at levels similar to or higher than the biocontrol strain P. protegens CHA0. The CFS of CHA0 showed the highest level of juvenile mortality followed by Bacillus sp. strains and P. fluorescens TAE4. None of the inoculated rhizobacteria reverted the negative effect of infestation on the aerial dry weight of lettuce plants. However, inoculation impacted on reproduction of M. javanica by reducing the development of galls and egg masses on roots and diminishing the number of individuals both on roots and in the substrate, as well as the reproduction factor. These results show that most of the analyzed native strains can control the nematode M. javanica. Among them, P. fluorescens TAE4 and Bacillus sp. B9T showed the most promising performances for the biocontrol of this pathogen and have a potential use in the formulation of commercial products.
Se estudiaron las cepas argentinas Pseudomonas fluorescens MME3, TAE4, TAR5 y ZME4 y Bacillus sp. B7S, B9T y B19S para el control del nematodo Meloidogyne javanica. Pseudomonas protegens CHA0 se utilizó como control positivo. La eclosión de huevos y la mortalidad de juveniles se evaluaron in vitro al exponerlos a suspensiones bacterianas y a sus sobrenadantes libres de células (SLC). Asimismo, se estudió la inoculación bacteriana sobre la infestación del nematodo en lechuga. Los resultados in vitro indicaron que la mayoría de las cepas, así como sus SLC redujeron la eclosión y la supervivencia de M. javanica. La suspensión de Bacillus sp. B9T produjo los menores niveles de eclosión. La mortalidad de juveniles fue mayor al exponerlos a suspensiones de Bacillus sp. respecto de Pseudomonas spp. Los SLC inhibieron la eclosión de huevos en niveles similares o superiores a P. protegens CHA0, excepto por el de B9T. La exposición a SLC de CHA0 registró la mayor mortalidad, seguido por las cepas de Bacillus sp. y P. fluorescens TAE4. La inoculación bacteriana no revertió el efecto de la infestación sobre el peso seco aéreo de las plantas, sin embargo, afectó la multiplicación de M. javanica lo que redujo el desarrollo de agallas y las masas de huevos, y disminuyó el número de individuos presentes tanto en la raíz como en el sustrato, así como el factor de reproducción. Los resultados indican que la mayoría de las cepas nativas evaluadas son capaces de controlar a M. javanica. Entre ellas, P. fluorescens TAE4 y Bacillus sp. B9T, se presentan como las más promisorias para el control de este patógeno, con potencialidad para ser utilizadas en la formulación de productos biológicos.
Root knot nematodes Meloidogyne spp. are cosmopolitan phytopathogens that cause significant crop losses worldwide. In Argentina, they are widely distributed through several regions, affecting both extensive and intensive crops6–8,27. The most widespread species in the country are Meloidogyne arenaria, Meloidogyne hapla, Meloidogyne incognita and Meloidogyne javanica, the latter two showing the greatest incidence on horticultural crops such as lettuce8.
Traditionally, the control of nematodes in horticulture has been done using chemical compounds that have known deleterious effects on the environment and human health. Therefore, it is desirable to find environmentally friendly options that promote integrated pest and disease management40. Biological control is an interesting alternative to chemical nematicides. Beneficial bacteria known as plant growth promoting rhizobacteria (PGPR) have been successfully used worldwide for the biocontrol of various plant pathogens. Among the most prominent biocontrollers are members of the Pseudomonas and Bacillus genera16,23,36.
Different mechanisms were proposed as responsible for the biocontrol of nematodes by PGPR. Direct control of bacteria on nematodes through the production of secondary metabolites (i.e., antibiotics and toxins) and hydrolytic enzymes, has been reported16,40,42. Furthermore, indirect mechanisms such as niche and nutrient competition, induction of plant systemic resistance and palliative effects by stimulating root growth, are also recognized36,37,40.
The study of native organisms as biological resources is important to contribute with biocontrol alternatives that are adjusted to the environmental conditions of the region. In the past years, different biological alternatives for the control of Meloidogyne spp., such as native arbuscular mycorrhizal fungi27, entomopathogenic nematodes7 and essential oils of Tagetesminuta26, were studied in Argentina. However, the antagonistic action of rhizobacteria native to Argentina against the root knot nematode M. javanica has not been explored yet.
In the present work, the antagonistic potential of several native strains of fluorescent Pseudomonas spp. and Bacillus sp. was evaluated against M. javanica under in vitro and in planta conditions. The objectives were: (i) to evaluate the effect of bacteria and their cell-free supernatants on egg hatching and juvenile mortality in vitro, and (ii) to determine if bacterial inoculation protects lettuce plants against the disease caused by nematode infestation.
Materials and methodsBacterial strains, isolates and molecular identificationThe strains of Pseudomonasfluorescens used in this study have been previously characterized as PGPR25 and are deposited in the collection of IMyZA, INTA-Castelar, Argentina. Strains TAE4 and TAR5 were both isolated from wheat, strain MME3 was recovered from tomato and strain ZME4 from maize25. The reference biocontrol strain Pseudomonas protegens CHA0 was used as a positive control39.
Bacillus sp. strains were isolated from wild potato (Solanum tuberosum) or surrounding typic paleargid soil in Abra pampa (Jujuy province, Argentina) and stored at −80°C in 20% glycerol slabs. Briefly, tuber and soil homogenates were pasteurized in a water bath at 80°C for 20min and serial dilutions were cultivated on nutrient agar (NA) with 50mM glucose. Isolates B7S, B19S and B9T were subjected to molecular identification by 16S rRNA gene sequencing. Genomic DNA extracts were obtained by suspending bacterial colonies in 50μl of ultrapure water and boiling for 10min. Universal primers 27F and 1492R were then used for 16S rRNA gene amplification from the extracted DNA by PCR10. The PCR products were visualized by TAE-agarose electrophoresis, excised and purified with QIAEX II gel extraction kit (Qiagen) for subsequent capillary sequencing at the Genomics Unit of the Biotechnology Institute of CNIA-INTA (http://www.inta.gov.ar/biotec). The retrieved 16S rRNA gene sequences of strains B7S, B19S and B9T were deposited in the GenBank database (http://www.ncbi.nlm.nih.gov/genbank/) under accession numbers MT160747, MT160746 and MT160745, respectively.
The derived sequences were compared to 16S rRNA gene sequences of type strains in the GenBank database using BLAST (http://blast.ncbi.nlm.nih.gov/Blast.cgi). Sequences were also analyzed with the EzTaxon tool available in the EzBioCloud portal (https://www.ezbiocloud.net/).
Selection of rifampicin-resistant mutantsNatural rifampicin-resistant mutants were isolated for all the studied strains. Bacterial cultures were plated on a rifampicin gradient (0–200μg/ml) and single colonies were isolated from the area with higher concentration of antibiotic. The selected clones were subcultured on antibiotic-free media for 20 generations and then tested for resistance stability on medium with and without rifampicin (50μg/ml)9. The Rifr mutants were stored in glycerol stocks at −80°C on LB supplemented with 50μg/ml rifampicin.
Culture conditions and preparation of cell-free supernatantsPseudomonas spp. were cultured on LB agar at 30°C while Bacillus spp. isolates B7S, B19S and B9T were grown at 37°C on potato-dextrose-agar (PDA). Pure colonies were transferred to LB broth (adding 50mM sucrose for Bacillus spp. isolates) and incubated with orbital agitation at 120rpm for 24h. Cells were harvested by centrifugation at 6000rpm for 15min and resuspended in sterile water. Bacterial concentration was estimated by measuring absorbance at 600nm and adjusted to 1 to obtain suspensions with 109CFU/ml for Pseudomonas spp. and 108CFU/ml for Bacillus spp. isolates.
Cell-free supernatants (CFS) were obtained by growing strains on 100ml of LB in 250ml flasks (adding 50mM sucrose for the Bacillus spp. isolates), with 120rpm agitation at 30°C during 48h. The bacterial cultures were centrifuged at 12000rpm at 4°C for 15min. The supernatants were collected and filtered with Minisart Syringe Filter 0.2μm.
Nematode inoculumThe M. javanica population was isolated from yacon Smallanthus sonchifolius (Tucumán) and maintained on susceptible tomato Lycopersicum esculentum cv. ‘Platense’ in the Nematology Lab, EEA-INTA Balcarce, Argentina. For inoculum preparation, egg masses were collected from the galls formed on the infected roots, pooled, and agitated in 0.5% NaOCl for 4min18. Egg suspensions were sifted through a 100-mesh sieve over 500-mesh coupled sieves, washed, and collected on water. Second stage juveniles (J2) were obtained from hatched eggs on sterile water at 28°C, J2 were collected and refrigerated until use.
In vitro antagonism assaysAssays were conducted in 24-well tissue culture plates (Costar®). To facilitate the counting of individuals, grid lines 2mm apart were scratched onto the underside of the plates. All assays were conducted using sterile materials in a laminar flow chamber.
To determine the effect on egg hatching, wells were supplied with 600μl of bacterial suspensions or CFS and 300μl of sterile water containing 100 M. javanica eggs. The percentage of hatched eggs was evaluated after 3, 6 and 9 days of incubation in a wet chamber at 28°C, under a stereomicroscope (Zeiss Stemi SV6, Carl Zeiss, Jena, Germany).
To study the effect on juvenile mortality, assays were set up similarly but replacing eggs with an equal number of second stage juveniles (J2). Nematodes were considered dead when they remained immobile after being touched with a fine needle. Number of dead juveniles was counted after 2 days of incubation and percentages of mortality were calculated.
The cell suspensions and CFS of Bacillus sp. B7S, B19S and B9T strains, P. fluorescens MME3, TAE4, TAR5 and ZME4 strains, and P. protegens CHA0 were tested against eggs and juveniles of M. javanica. Sterile water with nematode individuals without bacteria or CFS was used as control treatment (WC). Fresh LB was used as control (MC) for CFS assays. The treatments were tested in quadruplicate and each trial was repeated two times. All in vitro assays were carried out as completely randomized designs with rhizobacteria as factor. Data were checked for normality and homogeneity of variances and analyzed with one-way ANOVA and means compared by the LSD Tukey test (α=0.05). All statistical analyses were performed using the statistical software R version 2.15.233.
In planta biocontrol assaySeeds of Lactuca sativa L. cv. Elisa were disinfected in 1% NaOCl for 2min and washed four times with sterile distilled water. The seeds were then inoculated by immersion for 90min in a bacterial suspension containing 1×109CFU/seed for strains of Pseudomonas spp. (Rifr) and 1×108CFU/seed for strains of Bacillus spp. (Rifr). Uninoculated control seeds were imbibed in sterile water for 90min.
The assay consisted of seven treatments: strains B9T, B19S, TAE4, MME3, CHA0 (as positive control), control of infestation (MJC, with nematode but without rhizobacteria) and absolute control (PC, plant control without nematode or rhizobacteria).
After inoculation, lettuce seeds were individually sown in plug trays (cell size 288) with perlite: sterile sand (1:1) and cultivated in a growth chamber under 12:12h light–dark photoperiod and 25±2°C. Two weeks after sown, plants were transplanted to 2l plastic pots containing the perlite:sand mixture, and were re-inoculated at the stem base with 1ml of 1×109CFU/ml for Pseudomonas spp. or 1×108CFU/ml for Bacillus spp. strains. Control pots received the same amount of water. Plants were irrigated with distilled water by capillarity and fertilized with Hoagland solution (dilution 1:3) when necessary.
Bacterial colonization of roots was analyzed in a set of plants by CFU count using the drop plate method14 five days post-transplantation and one day after nematode inoculation. Plant roots were extracted from pots, agitated to release adhered substrate, gently washed, and weighed. Roots homogenates were obtained by grinding with sterile water (1:10) (w/v) using a mortar. Serial dilutions with sterile distilled water were seeded in triplicate as a drop (10μl) on LB or PDA adding rifampicin (50μg/ml) for Pseudomonas spp. and Bacillus spp., respectively. After 24h of incubation at 30°C, colonies were counted, and CFU/g root calculated for each treatment.
To infest lettuces, the inoculum of M. javanica was obtained as previously described. Suspensions consisting of a mix of eggs and juveniles were adjusted to infest at a dose of 1500 individual/plant. Four days after transplantation, the nematode inoculum was placed on two holes around the lettuce stem that were covered by substrate following the application. Sixty-five days after nematode infestation the number of galls and egg masses per root, egg and J2 per grams of root or substrate, fresh and dry weight of root and shoot per plant were determined.
Number of galls and egg masses per radical system were counted using a standing magnifier lamp 2×. Eggs were extracted from a portion of the middle root using Hussey and Barker's method18. Individuals of M. javanica were recovered from substrate using the sugar flotation-centrifugation technique20. Eggs and juveniles of M. javanica were counted on a 1ml counting slide under a stereomicroscope (Zeiss Stemi SV6, Carl Zeiss, Jena, Germany), and expressed as eggs or juveniles per gram of root or substrate. The reproduction factor was calculated as RF=final population/initial population, where the final population was the sum of the initial population (used for infestation) and the individuals present in root and substrate at the end of the assays.
The trial was designed as a randomized complete block with seven treatments. The experiment was repeated twice, each consisting of 11 replicates (3 of which were used for bacterial recount) with a total of 77 pots. Data were compared by one-way analysis of variance (ANOVA) followed by the Tukey-LSD test (α=0.05).
ResultsMolecular identification of Bacillus sp. isolatesIsolates B7S, B19S and B9T were selected from a collection of potato-associated bacilli due to their strong in vitro antifungal activity against Botrytis cinerea and Fusarium sp. (data not shown). Analysis of 16S rRNA gene sequences showed that the three isolates exhibit high identity to members of the genus Bacillus. Although the sequences obtained from B9T and B19S were identical, they exhibited differences in colony morphology (data not shown). Based on the results of the EzBioCloud database, B9T and B19S isolates belong to the Bacillus siamensis species, sharing 99.93% identity with B. siamensis KCTC 13613T. Moreover, the BLAST alignments of these sequences in the NCBI GenBank database showed 99.72% identity with both Bacillus subtilis subsp. subtilis and Bacillus amyloliquefaciens 168T. Analysis of the 16S rRNA gene sequence of strain B7S indicated that it belongs to the atrophaeus species, showing 99.93% identity to Bacillus atrophaeus JCM 9070T in both the EzBioCloud and NCBI GenBank databases.
In vitro antagonism assaysAll rhizobacterial suspensions showed a significant inhibition of egg hatching when compared to the control (WC) (Table 1). The lowest rates of M. javanica egg hatching were produced by B9T treatment during the whole period of incubation. Levels of hatching of B9T were statistically similar to B19S and TAR5 on day 3, to MME3 and TAE4 on day 6, and to B19S, MME3 and TAR5 on day 9.
In vitro effect of PGPR suspensions on egg hatching and juvenile J2 mortality of M. javanica.
| Treatments | Egg hatching (%) | Mortality of J2 (%) | ||
|---|---|---|---|---|
| 3rd day | 6th day | 9th day | ||
| WC | 55.5±3.5a | 70.8±5.7a | 74.5±3.2a | 33.8±2.1g |
| B19S | 21.3±2.4cd | 42.4±2.1bc | 44.9±2.9de | 85.4±2.5b |
| B7S | 27.0±3.7bc | 51.8±3.7b | 56.4±2.3b | 98.6±0.8a |
| B9T | 14.7±1.0d | 32.7±4.3d | 42.8±1.3e | 72.1±4.7c |
| CHA0 | 27.7±1.0bc | 49.7±3.1b | 52.2±2.0bc | 58.5±2.5de |
| MME3 | 23.8±2.2bc | 38.7±3.5cd | 45.2±2.1de | 64.4±3.8cd |
| TAE4 | 27.9±1.2bc | 36.8±1.5cd | 51.6±2.1bcd | 61.6±3.7de |
| TAR5 | 21.8±2.3cd | 43.0±4.2bc | 49.4±2.3cde | 55.8±2.7e |
| ZME4 | 29.2±2.9b | 44.9±1.8bc | 51.3±1.7bcd | 47.2±1.4f |
WC: water control (without bacteria, with nematode). Results are presented as mean±standard error. Within each column, different letters indicate significant differences in means according to the LSD-Tukey's test (p<0.05).
The effectiveness of bacterial suspensions in controlling egg hatching decreased with incubation time. Three days after incubation with PGPR suspensions, egg hatching was 47–73% less than control, while hatching inhibition dropped to 26–53% and 24–42% on days 6 and 9, respectively.
The mortality of M. javanica juveniles was also significantly affected by all tested PGPR suspensions (Table 1). Mortality was higher when juveniles were exposed to Bacillus sp. than to Pseudomonas sp. suspensions.
All CFS from bacteria tested exhibited inhibitory activity of egg hatching considering both controls (MC and WC), except for CFS from B9T on days 6 and 9 (Table 2). Overall, treatment with Pseudomonas filtrates showed the lowest hatching values, matching or outperforming the positive control CHA0 (Table 2).
In vitro effect of cell-free supernatants of bacterial on egg hatching and juvenile J2 mortality of M. javanica.
| Treatments | Egg hatching (%) | Mortality of J2 (%) | ||
|---|---|---|---|---|
| 3rd day | 6th day | 9th day | ||
| MC | 28.5±2.9a | 41.7±3.0b | 43.7±4.1b | 50.0±1.9f |
| WC | 32.5±3.6a | 51.9±3.3a | 52.8±3.2a | 45.0±1.0f |
| B19S | 8.2±1.8cd | 17.6±4.0c | 19.8±2.7cde | 84.7±1.6b |
| B7S | 11.3±1.6c | 12.3±2.5cd | 21.4±2.6cd | 79.3±3.7bc |
| B9T | 19.5±3.0b | 36.7±2.6b | 43.8±1.7b | 74.6±2.3c |
| CHA0 | 13.7±1.8bc | 17.9±0.6c | 23.2±2.2c | 95.1±0.4a |
| MME3 | 7.9±0.4cd | 10.1±1.7d | 17.2±2.1cde | 59.1±3.5e |
| TAE4 | 4.3±2.2d | 5.9±0.7d | 18.9±3.8cde | 76.1±2.8c |
| TAR5 | 4.8±1.4d | 5.5±1.4d | 12.0±2.4e | 66.9±3.0d |
| ZME4 | 8.2±1.5cd | 8.2±1.5d | 14.5±2.1de | 61.1±3.2de |
MC: medium control (LB medium without bacteria, with nematode). WC: water control (without bacteria, with nematode). Results are presented as mean±standard error. Within each column, different letters indicate significant differences in means according to the LSD-Tukey's test (p<0.05).
Bacterial metabolites increased juvenile mortality by more than 31.3% regarding water control (WC) and by 18.2% when compared to medium control (MC) (Table 2). Metabolites of the positive control CHA0 showed the highest level of mortality. Metabolites from Bacillus spp. isolates caused greater deaths of juveniles than Pseudomonas spp. filtrates (Table 2), except for TAE4, which matched the mortality levels caused by B7S and B9T.
In planta biocontrol assayConsidering the best in vitro antagonist behavior (hatching and mortality) against M. javanica, one strain per bacterial genus and per bioassay was selected to continue with in planta analyses. Strains B9T and MME3 were selected from the suspension bioassay and strains B19S and TAE4 from the CFS bioassay. Strain CHA0 was again included as a positive control.
One day after nematode infestation, the levels of bacterial count were in the order of 105CFU/g of roots for Bacillus spp. and 107–108CFU/g for Pseudomonas spp. (Table 3).
Colonization and effects of rhizobacteria on the growth of M. javanica infested-lettuce.
| Treatments | Root colonization (CFU/g) | Aerial dry weight (g/plant) | Root dry weight (g/plant) |
|---|---|---|---|
| PC | – | 2.19±0.15a | 0.83±0.07c |
| MJC | – | 1.74±0.12b | 1.12±0.09a |
| B19S | 1.4±0.4×105 | 1.73±0.06b | 0.99±0.07abc |
| B9T | 6.6±1.0×105 | 1.70±0.06b | 1.04±0.08ab |
| CHA0 | 7.0±3.9×107 | 1.64±0.08b | 0.97±0.04abc |
| MME3 | 2.2±0.8×108 | 1.73±0.08b | 0.93±0.06bc |
| TAE4 | 1.2±0.4×107 | 1.71±0.08b | 1.02±0.11ab |
PC: plant control (without bacteria, without nematode). MJC: nematode infestation control (without bacteria, with nematode). Results are presented as mean±standard error. Within each column, different letters indicate significant differences in means according to the LSD-Tukey's test (p<0.05).
Lettuce aerial dry weight was significantly affected by infestation with M. javanica (p<0.01). The inoculation of lettuce with rhizobacteria did not compensate for losses in aerial dry weight caused by nematode infestation (Table 3).
A significant increase in root dry weight was produced by nematode infestation as can be observed by comparison to the control (PC vs MJC) (Table 3, p<0.05). Inoculation with strains B19S, CHA0 and MME3 restored root dry weight to the PC control value; however, only MME3 ameliorated the effect of nematode infestation on this parameter (Table 3).
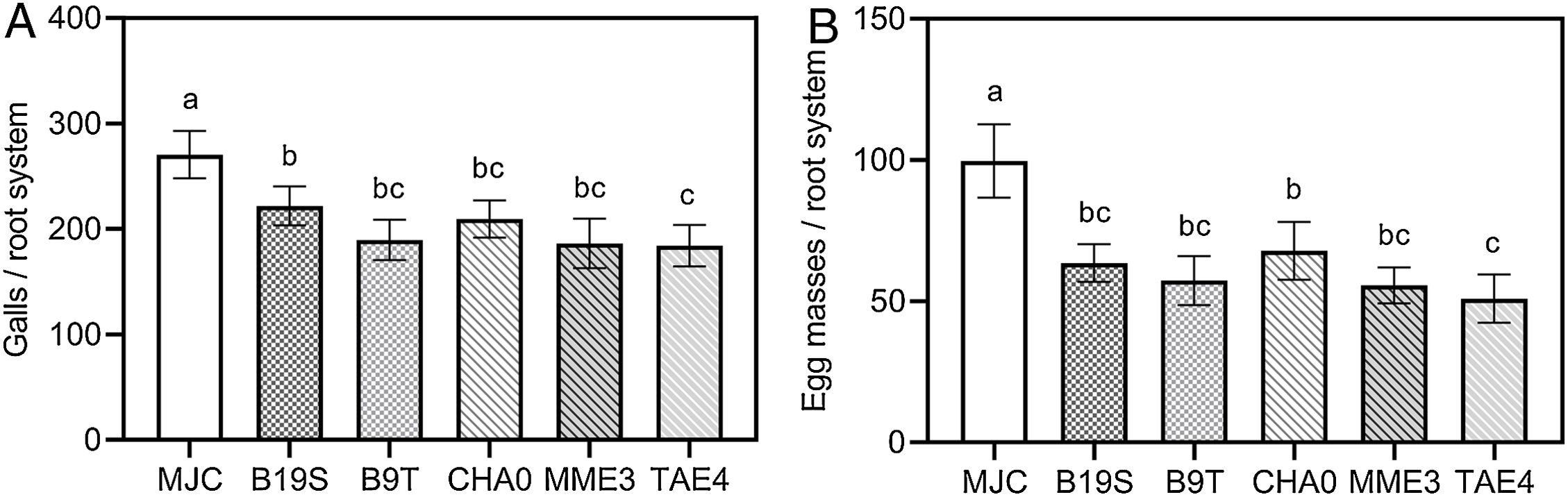
Inoculation with PGPR strains statistically reduced the number of galls and egg masses caused by M. javanica compared to the infestation control (Fig. 1). The level of galling on lettuces inoculated with strains of Bacillus spp. and Pseudomonas spp. was similar to that recorded for positive control treatment CHA0 (Fig. 1A). A similar behavior was found when analyzing the number of egg masses (Fig. 1B), except for TAE4.
Effect of rhizobacteria inoculation on the number of galls (A) and egg masses (B) on the root system of lettuce infested with M. javanica. MJC: nematode infestation control (with nematode, without bacteria). B19S: Bacillus sp. strain B19S. B9T: Bacillus sp. strain B9T. CHA0: P. protegens strain CHA0. MME3: P.fluorescens strain MME3. TAE4: P.fluorescens strain TAE4. Error bars correspond to standard error means. Different letters indicate significant differences in means according to the LSD-Tukey's test (p<0.05).
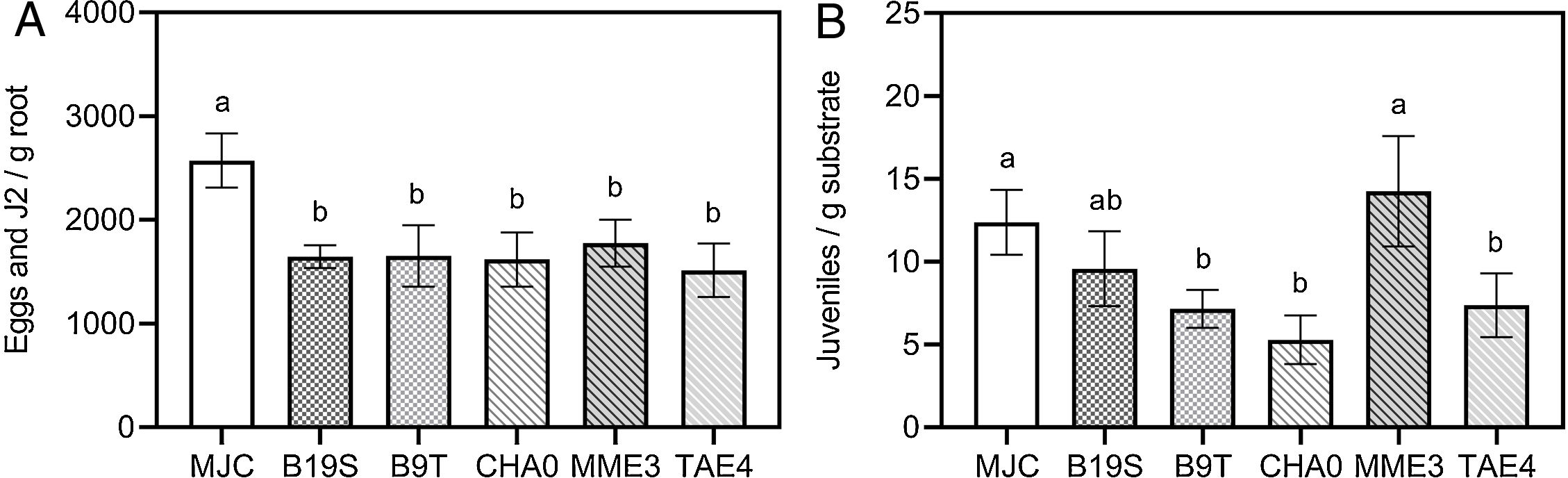
Bacterial treatments reduced the number of eggs and juveniles present at the root in the range of 31–41% regarding the infestation control (MJC) (Fig. 2A). The presence of individuals in the substrate was also significantly lowered by B9T, CHA0 and TAE4 (Fig. 2B). Consequently, a reduction in RF values can be observed (Table 4).
Effect of bacterial inoculation on the number of M. javanica individuals present in the root of lettuce (A) or in the substrate (B). MJC: nematode infestation control (with nematode, without bacteria). B19S: Bacillus sp. strain B19S. B9T: Bacillus sp. strain B9T. CHA0: P. protegens strain CHA0. MME3: P.fluorescens strain MME3. TAE4: P.fluorescens strain TAE4. Error bars correspond to standard error means. Different letters indicate significant differences in means according to the LSD-Tukey's test (p<0.05).
Effect of bacterial inoculation on the reproduction factor of M. javanica in lettuce plants.
| Treatments | Reproduction factor |
|---|---|
| MJC | 17.00±2.07a |
| B19S | 11.57±0.57bc |
| B9T | 11.13±2.14bc |
| CHA0 | 10.80±1.66bc |
| MME3 | 14.25±2.11ab |
| TAE4 | 7.50±1.02c |
MJC: nematode infestation control (without bacteria, with nematode). Nematode reproduction factor (RF) was calculated as final population/initial population. Results are presented as mean±standard error. Different letters indicate significant differences in means according to the LSD-Tukey's test (p<0.05).
In the quest for indigenous microbes as a natural resource of root-knot nematode biocontrol agents adapted to the edaphic conditions of Argentina, we challenged Meloidogyne javanica with locally isolated strains of Pseudomonas spp. and Bacillus spp. This study demonstrated that several of these strains are capable of successfully limiting the growth and survival of the nematodes in vitro and also of reducing parasitism in lettuce plants.
Both bacterial suspensions and cell-free supernatants inhibited egg hatching and increased juvenile mortality. In general, hatching inhibition was greater when cell-free supernatants were used, particularly those produced by Pseudomonas spp. strains. It has been reported that antibiotic 2,4-diacetylphloroglucinol (2,4-DAPG), hydrogen cyanide (HCN) and protease AprA produced by P. protegens CHA0 contribute to the biocontrol activity against eggs and juveniles of Meloidogyne spp., which would explain their performance in this study34–36. However, the other P. fluorescens strains used in this work are not expected to generate 2,4-DAPG2 while only strain MME3 produces HCN25. Our results show that some P. fluorescens strains inhibited the hatching of M. javanica eggs at a higher level than P. protegens CHA0, suggesting the action of other metabolites. Several compounds, including diketopiperazines13,19, antibiotics21, cyclic lipopeptides16,22 and hydrolytic enzymes such as proteases, lipases, and chitinases16,34,40 could determine the ability and efficacy of CFS to control M. javanica by both P. fluorescens and Bacillus spp. strains. An interesting case is the behavior of Bacillus sp. B9T on egg hatching, because CFS had no effect on this parameter while the bacterial suspension exhibited the highest inhibition. The fact that Bacillus sp. B9T may require physical contact with M. javanica to exert its biocontrol mechanism cannot be ruled out. Although, diverse mechanisms that require cell to cell contact have been described in gram-positive bacteria12, to the best of our knowledge, no data is available about the role of this type of mechanisms on nematode antagonism by Bacillus sp.
Except for P. protegens CHA0, all cell suspensions caused higher juvenile mortality rates than their respective CFS, especially with Bacillus spp. strains. This behavior could be explained by in situ generation of compounds with volatile characteristic that may have been lost during the filtration of CFS. Volatile organic compounds (VOC) such as hydrogen cyanide, hydrogen sulfide, dimethyldisulfide, decanal, 2-undecanone and 2-nonanone have been reported to have nematicidal activity against Meloidogyne spp.1,5,17,35,43 Any of these metabolites could be produced by the Bacillus spp. and Pseudomonas spp. strains studied here. Alternatively, the production of hydrolytic enzymes may be induced in bacterial cell suspensions under a starving state. Pseudomonas spp. and Bacillus spp. enzymes such as chitinases1,24,29, proteases34, collagenases and lipases1 were already described for the control of Meloidogyne spp. eggs and juveniles. In addition to the diffusible secondary metabolites described above, intermediates of purine biosynthesis41, sphingosine11, uracil, dihydrouracil and 9H-purine30 produced by Bacillus spp. have nematicidal properties on juveniles of Meloidogyne spp. and may contribute to the control exerted by our strains. The potential presence of these secondary metabolites together with VOC and hydrolytic enzymes in cell suspensions could be the reason why Bacillus spp. strains affect juvenile survival to a greater extent than Pseudomonas spp. strains. The specific case of P. fluorescens MME3, whose aggressiveness against juveniles was notably higher in the cell suspension than CFS with respect to other native Pseudomonas spp. strains, may be explained by in situ production of HCN25. Although P. protegens CHA0 also produces HCN, its CFS showed higher juvenile mortality than the bacterial suspension, an exception to general behavior observed for other strains. This may be due to the additional production of 2,4-DAPG, pyrrolnitrin and pyoluteorin in CFS, metabolites known not only to affect hatching but also to induce mortality in nematode juveniles28,36,37.
The successful colonization of the plant rhizosphere is a prerequisite for pathogen suppression by rhizobacteria. Lettuce roots were effectively colonized by the bacterial strains studied in this work. Pseudomonas sp. showed better survival than Bacillus sp. one day after infestation with M. javanica. Once located in the roots, bacteria can interfere with nematode vital cycle through the production of secondary metabolites and hydrolytic enzymes, by competing for niche and nutrients, or by inducing the plant systemic resistance36,37,40,42. Additionally, rhizobacteria can also have a palliative effect by promoting plant growth. In the present study, the losses on lettuce aerial biomass caused by M. javanica could not be recovered through inoculation with any of the bacterial strains. Similar results were reported with field-cultivated lettuce infested by Meloidogyne spp. and treated with B. subtilis and Pseudomonasaeruginosa32. Nevertheless, other authors reported the effect of growth promotion by Bacillus spp. and Pseudomonas spp. as attenuating the infestation by Meloidogyne spp. in lettuce and tomato3,4,15,31,38. An unexpected behavior was observed for root dry weight as infestation with M. javanica incremented this parameter compared to healthy plants, while bacterial inoculation did not show clear trends. It is possible that the formation of galls on the radical system with a consequent decrease in the ability to absorb water could have incremented root dry weight. In addition, a growth-promoting effect of bacteria on root might have been slanted by the increase in dry weight due to M. javanica infestation. Similarly, Hoffmann-Hergarten et al.15, described an increase in fresh root weight of cherry tomato produced by M. incognita infection.
Species of Bacillus spp. and Pseudomonas spp. were reported as suppressors of root knot infestation in various crops, with magnitudes that depend on the mode and dose of bacterial application3,31,36. Our results show that all bacterial strains studied here controlled the infestation and reproduction of M. javanica on lettuce. Bacterial inoculation decreased nematode infestation by reducing the number of galls developed on roots by 18–32%. This effect may be due to direct mechanisms on free nematodes in soil, as suggested by our in vitro results. Nevertheless, we cannot rule out the possibility of an indirect action on plants, such as the induction of systemic resistance36,37,40. The reproduction of M. javanica in infested lettuce was also affected by bacterial inoculation, as revealed by the lower number of egg masses, individuals in the roots, juveniles in the substrate and reproduction factor. In particular, the presence of Bacillus sp. B9T, P. protegens CHA0 and P. fluorescens TAE4 would seem to affect to a greater extent the multiplication of M. javanica, as shown by the number of individuals in the substrate and the reproduction factor.
More research is necessary to validate the potential of these rhizobacteria for the biocontrol of M. javanica under natural conditions. In addition, combinations with other PGPR in order to find environmentally friendly alternatives that point to a double purpose, promotion of plant growth and biocontrol of nematodes, could be an outstanding goal in next studies.
ConclusionArgentinean strains of Pseudomonas spp. and Bacillus spp. could control the root knot nematode Meloidogyne javanica, either under in vitro or in planta conditions. Bacterial suspensions and their cell-free supernatants exerted a direct antagonism on eggs and juveniles of M. javanica. These PGPR strains were also effective for reducing Meloidogyne javanica reproduction on inoculated lettuce plants; however, they did not show positive effects on plant growth. Bacillus sp. B9T and P. fluorescens TAE4 are the most promising native strains for the control of this pathogen.
Financial supportThis work and the data herein contained were supported by Universidad Nacional de Mar del Plata (Grant 473/15 SECyT-UNMDP and PICT4650/16 to CMC) and by INTA (Grant E4 2019-PD-E4-I069-001 to EAM), in Argentina. MBP and MF are CONICET fellowship, GM is CONICET researcher, EAM is INTA researcher and CMC is University professor.
Conflict of interestThe authors declare that they have no conflicts of interest.
The present work is part of the thesis of María Paula Borrajo in partial fulfillment for the requirements for the Doctor's degree at Facultad de Ciencias Agrarias, Universidad Nacional de Mar del Plata, Argentina.