The current study intended to isolate, characterize and identify biocontrol bacteria possessing broad-spectrum antifungal activity from the phyllosphere of different crops including maize, wheat and potato and to assess their growth-promoting activity. In this study 14/113 biocontrol bacteria showed antifungal activity. Bacterial isolates M11 and M33 from maize out of 113 were re-selected on the basis of their strong (more than 50%) broad spectrum antifungal activity after their assessment against four economically important phytopathogenic fungi including Alternaria alternata, Rhizoctonia solani, Fusarium oxysporum and Fusarium verticillioides. The isolates were further assessed for plant growth promoting traits, i.e., indole-3-acetic acid production, phosphate solubilization, production of cellulase, microbial volatile compounds, hydrogen cyanide and siderophores. All fourteen isolates showed positive results for the production of indole-3-acetic acid hormone and cellulase enzyme, 10 isolates were positive for hydrogen cyanide production; siderophores production was observed in 7 isolates while 5 isolates showed ability to solubilize inorganic phosphate. Microbial volatile compounds were only synthesized by M11 and M33, which were identified as Bacillus amyloliquefaciens and Bacillus subtilis respectively by 16S rRNA gene sequencing. The survival study revealed that biocontrol bacteria B. amyloliquefaciens and B. subtilis have the ability to survive in cost effective molasses containing carrier material up to a three-month period.
Este estudio tuvo como objetivo aislar, caracterizar e identificar bacterias de control biológico que poseen actividad antifúngica de amplio espectro de la filosfera de diferentes cultivos, incluidos el maíz, el trigo y la papa, así como evaluar su actividad en la promoción del crecimiento. En este estudio, 14/113 bacterias de control biológico mostraron actividad antifúngica. Los aislamientos bacterianos M11 y M33 (de maíz), del total de 113, fueron reseleccionados debido a su fuerte actividad antifúngica de amplio espectro (más del 50%) después de su evaluación contra cuatro hongos fitopatógenos que afectan cultivos de alta importancia económica, entre ellos, Alternaria alternata, Rhizoctonia solani, Fusarium oxysporum y Fusarium verticillioides. Los aislamientos se evaluaron, adicionalmente, para determinar los rasgos que promueven el crecimiento de las plantas, es decir, producción de ácido indolacético, solubilización de fosfato, producción de celulasa, compuestos orgánicos volátiles microbianos, cianuro de hidrógeno y sideróforos. Las 14 cepas aisladas mostraron resultados positivos para la producción de la hormona ácido indolacético y la enzima celulasa; 10 cepas fueron positivas para la producción de cianuro de hidrógeno. Además, se observó producción de sideróforos en el caso de 7 cepas, mientras que 5 pudieron solubilizar fosfato inorgánico. Los compuestos orgánicos volátiles microbianos solo fueron sintetizados por los aislamientos M11 y M33, que fueron identificados como Bacillus amyloliquefaciens y Bacillus subtilis, respectivamente, mediante secuenciación del gen ARNr 16S. El estudio de supervivencia mostró que las bacterias de control biológico, es decir, B. amyloliquefaciens y B. subtilis, tienen la capacidad de sobrevivir sobre un sustrato a base de melasa, por un período de tres meses.
Agriculture is undoubtedly the backbone of least developed countries, and plays a major role in ensuring food security and economic rise. Pakistan's economy is greatly reliant on agriculture23. Agricultural resources are critically threatened by plant diseases caused by microorganisms including bacteria, viruses and fungi. Among them, fungal pathogens are the most devastating infectious agents12. It is estimated that fungi are the major threat (approximately 65%) of pathogen-driven plant-host loss1. Some of the most common plant-pathogenic fungi belong to the genera Alternaria, Fusarium, Botrytis, Geotrichum, Penicillium and Sclerotinia. These fungi are responsible for causing considerable crop yield loss worldwide16. Efficient plant disease management is the prerequisite of sustainable agriculture. Plant diseases need to be contained to sustain the quantity and quality of food and fiber produced by agriculturists throughout the world. Various strategies might be utilized to avoid, alleviate or restrict plant diseases4. In the past few decades, synthetic fungicides played a fundamental function in suppressing plant diseases and enhancing crop yield to reduce food insecurity in today's world32. However, recent studies, reported that the prolonged use of chemicals could cause harmful effects, including environmental and health hazards, residual toxicity and resistance of pathogens, among others. Furthermore, agrochemicals are not very cost effective32. Consequently, scientists have focused their efforts on formulating alternate sources to synthetic agrochemicals for limiting pests and diseases. Among these alternatives, biological controls are much preferred. Biological control in plant pathology refers to the suppression of growth or reproduction of a pathogenic organism using another organism. It utilizes the natural enemies of pests or pathogens to eliminate or contain their population.
Nowadays more attention is paid to developing useful approaches for environmentally friendly and sustainable agricultural practices, mainly depending on the application and use of beneficial microorganisms, particularly plant growth promoting bacteria (PGPB) as biofertilizers or bio-pesticides9. PGPB are capable to stimulate plant yield and restrict phytopathogens and could be used as an alternative source to limiting the usage of chemical fungicides. PGPBs are defined as a wide array of bacteria that colonize the plant phyllosphere/rhizosphere and are usually involved in several beneficial activities such as the improvement of plant growth by increasing seed development, plant weight, and crop yield. They can influence plant health either directly or indirectly. The direct mechanisms include production of hormones (indole acetic acid, cytokinins, gibberellins, among others), phosphate solubilization, nitrogen fixation, biofilm formation, siderophores production, mineral solubilization and plant resistance to abiotic stresses. The indirect mechanisms include restricting plant pathogens by producing enzymes (i.e. cellulase, chitinase, glucanase), volatile compounds (VC), secondary metabolites and by competing for space and nutrients9. The most reported biocontrol bacteria belong to the Bacillus and Pseudomonas genera that can possibly promote plant growth and inhibit fungal pathogens8,17. Furthermore, a wide range of bacteria including Agrobacterium spp., Burkholderia spp., Azotobacter spp., Frankia spp., Azospirillum spp., Bradyrhizobium spp., Rhizobium spp., Serratia spp. and Thiobacillus spp. are also considered PGPBs2.
Unfortunately, laboratory results for the application of bacterial inoculants such as biofungicides usually do not correlate with in vivo results21; therefore, an extensive research and market survey is required to bring a successful bacterial inoculum from the laboratory to the farms. Microbial incorporation for plant disease management and growth promotion in agricultural frameworks and eradication of the use of agrochemical is reliant on the effective selection, screening and safety analysis of potential PGPR strains28. A number of bacterial biocontrol and plant growth-promoting products are currently in the developmental stages. Most products are generally based on gram positive bacteria such as Bacillus spp. because of their easy formulation and long-term survival due to spore-producing quality5.
This study focuses on the assessment of bacterial antagonism against fungal pathogens and plant growth-promoting traits. Furthermore, the study also aims to determine the survival of biocontrol bacterial strains in cost-effective liquid carrier materials for mass production, which is one of the initial steps toward the commercialization of formulated biofungicides.
Materials and methodsIsolation of bacteria in pure cultureSamples of the phyllosphere from different crops including maize (Zea mays), potato (Solanum tuberosum) and wheat (Triticum aestivum) were collected from different fields of Quetta, Pakistan. The collected plant samples were packed in sterilized polythene bags and referred to “Applied Biotechnology Laboratory, BUITEMS”, Quetta, for the isolation of biocontrol bacteria.
Bacteria were isolated from the phyllosphere of different crops according to Ramzan et al.22. Briefly, 1g of the collected samples was thoroughly rinsed under running tap water and subsequently crushed in saline solution (0.85% NaCl). The resulting suspension was serially diluted to 10−3 and 1ml of each dilution was spread on LB agar plates. The plates were incubated at 37°C for 24h pure cultures were obtained by sub-culturing on additional Luria-Bertani (LB) plates.
Preliminary screening of bacterial isolates for antifungal activityThe bacterial isolates were screened for antifungal activity against phytopathogenic fungi. The phytopathogenic fungi used in this study Alternaria alternata, Fusarium oxysporum, Fusarium verticillioides and Rhizoctonia solani were kindly provided by ARS USDA. The fungi were cultured on potato dextrose agar (PDA) and incubated at 28°C for 7 days for further experimentation. The bacteria with inhibitory activity were selected for further characterization.
Qualitative and quantitative assessment for antagonistic activity in vitroThe qualitative assessment was performed according to Awais et al.28. The bacterial strains were cultured against each of the fungus on separate PDA plates using the agar plug method. About 4mm agar discs of previously cultivated phytopathogenic fungi were inoculated at the center of PDA plates. Bacterial isolates were inoculated with a sterilized wire loop on two sides of Petri dishes at a distance of 3cm from the fungal plug. The dishes were incubated at 28°C for 5 days.
The bacterial isolates with antifungal properties were subjected to quantitative evaluation. The dual culture method was performed according to Haidar et al.7. Briefly, fungal strains were grown on PDA plates for about 7 days, a 4mm agar disc was excised with a disinfected scalpel and placed at the center of a separate plate. The bacterial isolates to be assessed were co-inoculated on the same agar plate at a distance of 2.5cm from the fungal disc in a straight line. Control plates were cultured only with the fungal pathogen. Dishes were incubated for 5 days at 28°C. The experiment was performed in triplicate. The inhibition percentage was calculated by using a given formula;
where R2 is the growth of fungus toward the control side of the Petri dish and R1 is the outward growth of the fungus in the direction of the antagonistic bacteria.Molecular identification and phylogenetic analysisThe selected bacterial strains were primarily identified by Gram staining and endospore staining followed by molecular identification.
DNA extractionThe total genomic DNA from freshly cultured bacterial isolates was extracted by using Thermo Scientific™ #K0721 as per the manufacturer's protocol. Proteinase K and RNase A were used for protein digestion and removal of RNA respectively.
16S rRNA gene amplificationThe amplification of the 16S rRNA gene was performed according to the method described by Ramzan et al.22. The primers used to amplify a 1.5kb gene fragment were 27F and 1492R.31 The polymerase chain reaction was performed in a thermal cycler (T100 Thermal Cycler) in 20μl reaction mixture containing 0.1μmol/l of each primer, 2.5μmol/l MgCl2, 1μmol/l of each deoxynucleoside triphosphate (dNTP), 50ng/μl of template DNA and 2U/μl Taq DNA polymerase (Thermoscientific, USA). The PCR amplification consisted of DNA denaturation at 95°C for 5min followed by 35 cycles of denaturation at 95°C for 1min, annealing at 57°C for 1min, extension at 72°C for 1.5min and final extension at 72°C for 10min. The PCR products were analyzed by electrophoresis in a 1% agarose gel in 1× TBE (Tris–borate–EDTA) buffer with ethidium bromide staining. The purified PCR products were sequenced by using the facility of Macrogen (Korea).
Similarity analysis and dendrogram constructionThe 16S rRNA gene sequences were compared with the NCBI database using BlastN. Further, identification of phylogenetic neighbors and calculation of pairwise similarity of these sequences were done using ClustalW and MEGA 6.414 software as described by Ramazan et al.22. The phylogenetic tree was constructed by using a neighbor-joining method. The bootstrap replications (1000 replicates) were used for statistical support.
Evaluation of biocontrol bacteria for plant growth promoting traitsThe biocontrol bacteria were evaluated for their plant growth-promoting traits. The parameters selected for evaluating PGP activity were: phosphate solubilizing activity, indole acetic acid (IAA), hydrogen cyanide (HCN), cellulase, siderophore and VOC production.
Phosphate solubilization assayNational Botanical Research Institute Phosphate growth medium (NBRIP) agar containing insoluble tri-calcium phosphate was prepared as previously described18. The pH of NBRIP agar was adjusted to 7.0. The bacterial strains were spot inoculated at the center of NBRIP agar plates. NBRIP agar without bacterial inoculation was taken as control. The dishes were kept at 37°C for 72h and were observed for formation of a clear halo zone. The quantification of phosphate solubilization was performed according to Chandra et al.3 by measuring the yellow color on a spectrophotometer at 880nm.
Indole-3-acetic acid assayThe bacterial isolates were screened for indole 3-acetic acid (IAA) production according to Sivasankari27. Briefly, isolated bacteria were cultured in autoclaved LB broth in a 100ml Erlenmeyer flask with and without the addition of 0.1g of l-tryptophan (Sigma Aldrich). The 100ml Erlenmeyer flask containing uninoculated sterile LB broth was taken as negative control. All flasks were incubated in a shaking incubator at 37°C and 100rpm for 48h. Salkowski's reagent (1ml of 0.5M FeCl3·6H2O in 50ml of 35% perchloric acid) was prepared for IAA detection. In properly labeled test tubes, 1ml of culture supernatants was mixed with the addition of 2ml Salkowski's reagent. The color change was observed. The experiment was repeated in triplicate. The quantitative screening of the isolates was performed according to Chandra et al.3. Briefly, after the development of a red color from the qualitative screening, the isolates were placed in the dark for about 2h and analyzed on a spectrophotometer at 530nm against different standards ranging from 20 to 200μg/ml.
Detection of cellulolytic activityThe bacterial strains were inoculated in carboxymethylcellulose media (KH2PO4, Na2HPO4·7H2O, (NH4)2SO4, MgSO4·7H2O, CaCl2, FeSO4·7H2O, MnSO4, CMC and agar, pH 7.0) as described by Kumar et al.13 with slight modifications, followed by incubation for 24–48h at 37°C. The 2ml of Gram iodine was added for 10min followed by the addition of a 2M NaCl solution. The plates were examined for clear halo zones.
Hydrogen cyanide productionHydrogen cyanide formation was detected using the method described by Passari et al.19. Briefly, each isolate was streaked on a nutrient agar medium added with glycine (4.4g/L). The freshly streaked agar was covered with filter paper (Whattman no. 1) previously flooded with solution (0.5% picric acid and 2% sodium carbonate). The parafilm wrapped plates were incubated at 37°C for 7 days. The change of color from yellow to orange indicates positive results for hydrogen cyanide production.
Siderophore productionThe bacterial strains were assessed for siderophore production by using Chrome Azurol-S agar medium, as described by Sheeba et al.25. The isolates were point inoculated at the center of a CAS agar medium plate and incubated at 30°C for 48–72h; the formation of a yellow to orange halo zone around the colony shows siderophore production.
Molecular volatile organic compound production assayThe divide plate method was used to evaluate the production of VOC as described by Yuan et al.33 with slight modifications. Briefly, the two compartments of divide plates were filled with LB agar and PDA respectively. The LB agar containing sides were inoculated with bacterial isolates except the control plate. The PDA side was used for fungal strains. The bacterial isolates that showed positive results were further evaluated by the dry fungal biomass assay as mentioned by Khan et al.11. Briefly, one portion of a centrally partitioned plate was filled with 10ml of LB agar for the growth of biocontrol bacteria while the other side with 10ml PDA for the fungal counterpart. For control, the LB agar side of the divide plate was left uninoculated. The plates were sealed with parafilm and kept at incubation for 7 days at 37°C. The plates were observed for the antifungal effect of volatile organic compounds released by bacterial isolates in terms of the reduction of the fungal dry mass in the treated plate as compared to control. For the observation of the fungal dry mass, the PDA portion was placed on pre-weighed filter paper and desiccated at 65°C. Readings were taken for assessing the reduction in the fungal dry biomass. The experiment was performed in triplicate.
Survival study of bacterial isolates in different liquid carrier materialThe selected isolates were analyzed on a cost-effective liquid broth that may have the potential of being used as carrier material on a commercial scale. Three recipes were prepared, with different C, N and P ratios, which can be used as carrier materials (i.e. all of them contain molasses as carbon source, KH2PO4 as phosphorus source and NH4SO4 as nitrogen source), for evaluating the survival of selected bacterial strains. LB broth (enriched media) was used as control. The biocontrol bacteria were inoculated separately in all treatments. Three replicates were prepared for each treatment. The bacterial population was tested at different time intervals from day 0 up to 3 months using the serial dilution method on LB agar medium. The medium after inoculation was kept at 37°C for 72h. Then, the number of colony forming units (CFUs) was counted.
ResultsThe aim of this study was to isolate and characterize potential broad spectrum antifungal bacterial isolates that can be used on a commercial scale as bacterial control agents. Along with their antifungal properties, the isolates were characterized for plant growth-promoting activities. The selected isolates were kept on a cost-effective liquid culture to analyze the survival of the isolates.
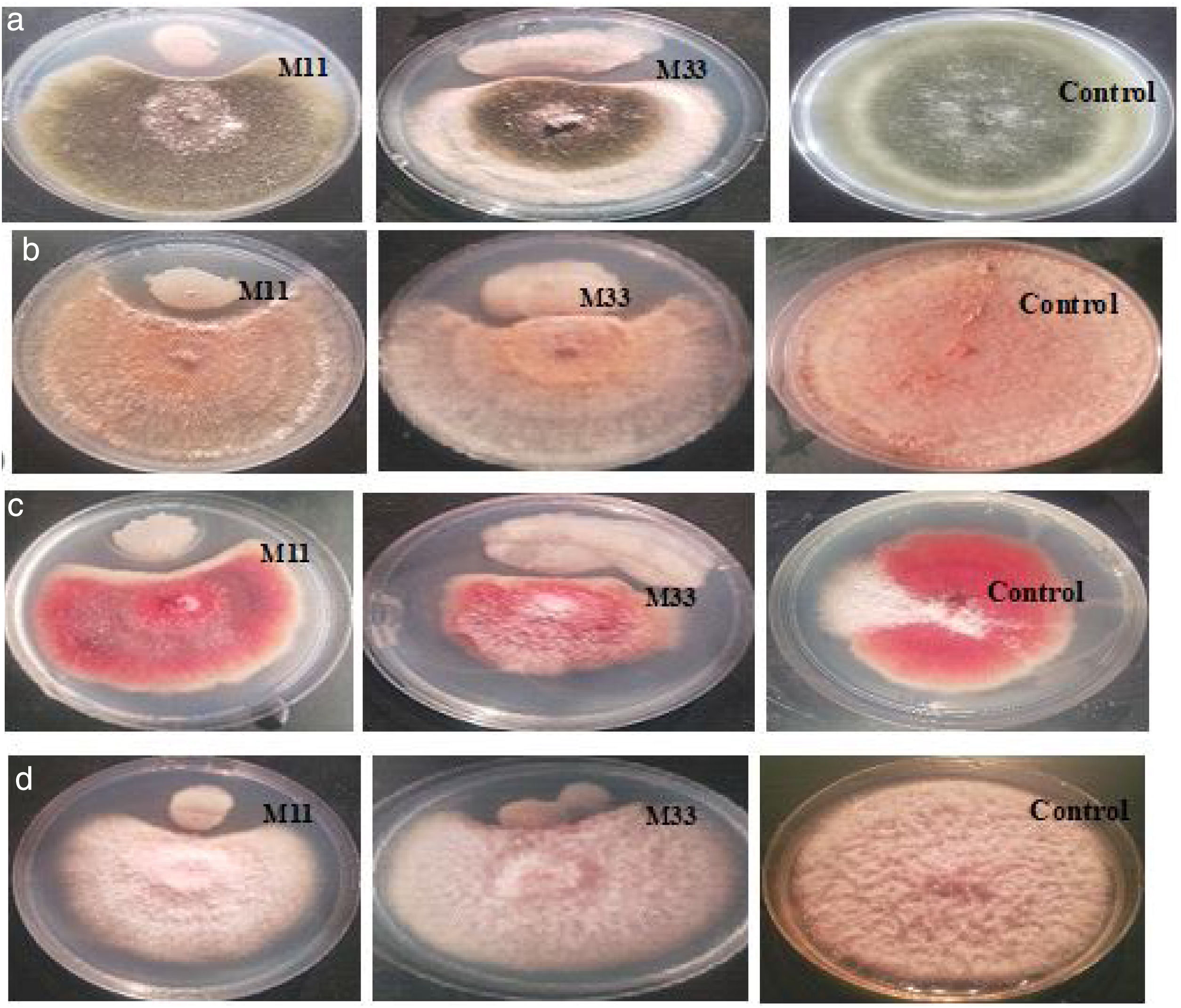
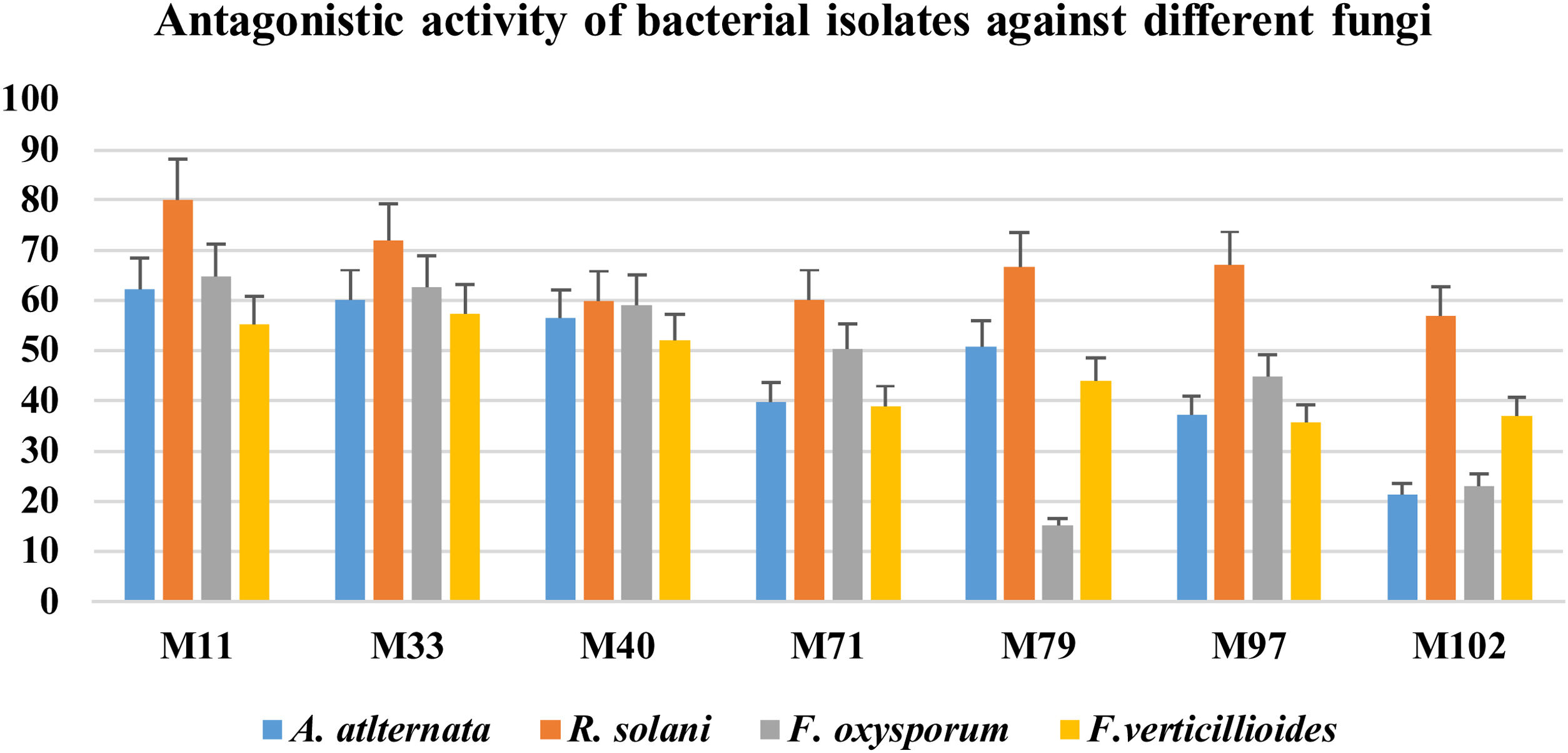
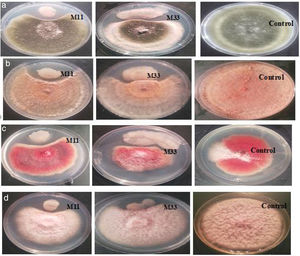
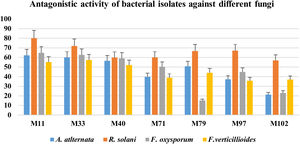
Isolation and screening of bacteria with antifungal activityInitially, 113 bacteria were isolated from the phyllosphere of different crops (i.e. Zea mays, Solanum tuberosum, and Triticum aestivum), 14 isolates (M11, M33, M35, M40, M42, W71, W75, W79, W88, P92, P97, P102, P107 and P110) were selected on the basis of antifungal activity. Looking for bacteria with a wide array of fungicidal activity, further two isolates (M11 and M33) from maize were selected after evaluating them against a panel of phytopathogenic fungi (A. alternata, R. solani, F. oxysporum and F. verticillioides). The two selected bacteria showed strong inhibitory activity against four fungi under study (Fig. 1). The inhibitory percentages are shown in Figure 2.
Graphical representation of the% suppression of growth of phytopathogenic fungi under study using biocontrol bacteria. The y-axis shows the inhibition percentages, while the x-axis represents the bacterial isolates. The bars show the standard error mean computed by using Microsoft Excel.
Morphologically, isolates M11 and M33 were rod-shaped, endospore-producing Gram positive bacteria. Molecular identification showed that both isolates belonged to the Bacillus genera. Bacterial isolate M11 showed 100% sequence similarity with B. amyloliquefaciens while M33 shared 100% sequence homology with B. subtilis. The phylogenetic tree constructed by using the MEGA X 64 program is shown in Figure 3. The bootstrap support was >75%. The sequences of M11 and M33 were deposited in Genbank under accession numbers MW531180 and MW531181 respectively.
Screening for PGPR traitsIAA production and phosphate solubilizationThe 14 isolates showing inhibitory activity were assessed for IAA production. All of them showed positive results in the presence of l-tryptophan by development of the color (Table 1). The phosphate solubilization assay showed that 5 out of 14 isolates were able to solubilize tricalcium phosphate, while the bacterial isolates that showed strong antifungal activity (M11 and M33) were unable to solubilize phosphate (Table 1). The quantification data of IAA and phosphate solubilization is shown in Suppl. Table 1.
Summary of plant growth-promoting traits.
| Bacterial isolates | IAA | PS | Cel | HCN | Sid |
|---|---|---|---|---|---|
| M11 | + | − | + | + | + |
| M33 | + | − | + | − | + |
| M35 | + | + | + | + | − |
| M40 | + | + | + | − | + |
| M42 | + | − | + | − | + |
| W71 | + | + | + | + | + |
| W75 | + | + | + | + | − |
| W79 | + | + | + | + | − |
| W88 | + | − | + | + | − |
| P92 | + | − | + | + | + |
| P97 | + | − | + | − | + |
| P102 | + | − | + | + | − |
| P107 | + | − | + | + | − |
| P110 | + | − | + | + | − |
IAA: indole acetic acid; PS: phosphate solubilization; Cel: cellulase production; HCN: hydrogen cyanide; Sid: siderophores. The (+/−) indicates positive and negative results respectively.
The hydrolyzing zones were clearly observed on CMC agar dishes after the addition of Gram iodine, in the case of all 14 biocontrol bacterial strains including M11 and M33 (Table 1).
Production of HCN and siderophoresOur data suggested that 10 isolates, including M11, produced HCN as the change of color from yellow to orange, which was clearly observed, while M33 showed a negative result for HCN production (Table 1). Siderophores were produced by 7 isolates, including M11 and M33 (Table 1).
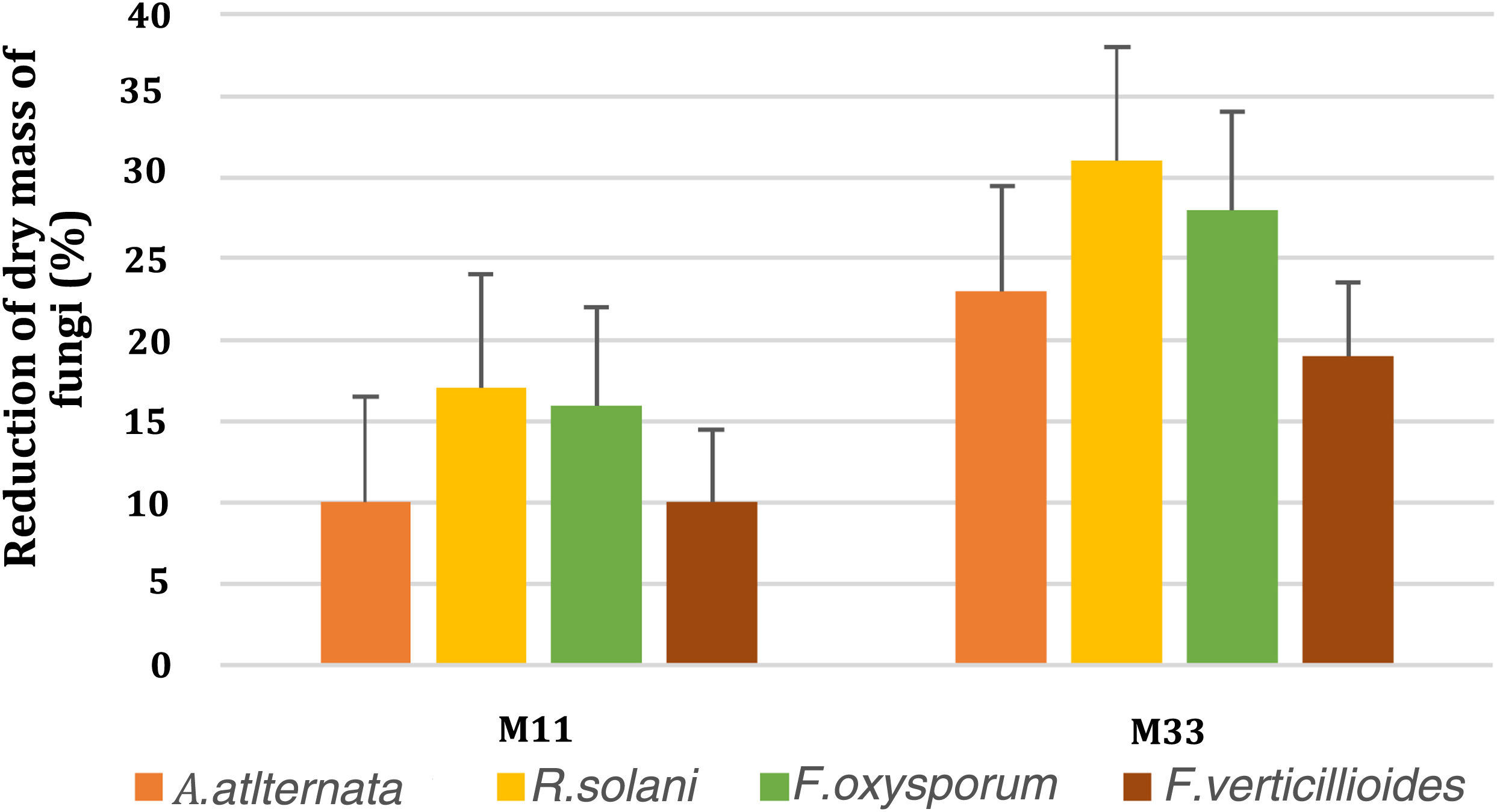
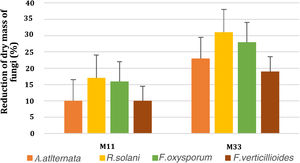
Volatile organic compound productionVolatile organic compound production was assessed by using the divide plate method. Only two bacterial isolates (M11 and M33) showed positive results for VOCs. The significant reduction of fungal dry mass was observed in the treated plates as compared to control. The reduction in the fungal dry mass of A. alternata, R. solani, F. oxysporum and F. verticillioides, due to VOCs, produced by M33 and M11 respectively, is shown in Figure 4.
Graphical representation of fungal dry mass reduction (%) by VOCs produced by bacterial isolates (M11 and M33). The y-axis represents reduction percentages, while the x-axis shows two selected bacterial isolates (M11 and M33). The bars show the standard error mean computed using Microsoft Excel.
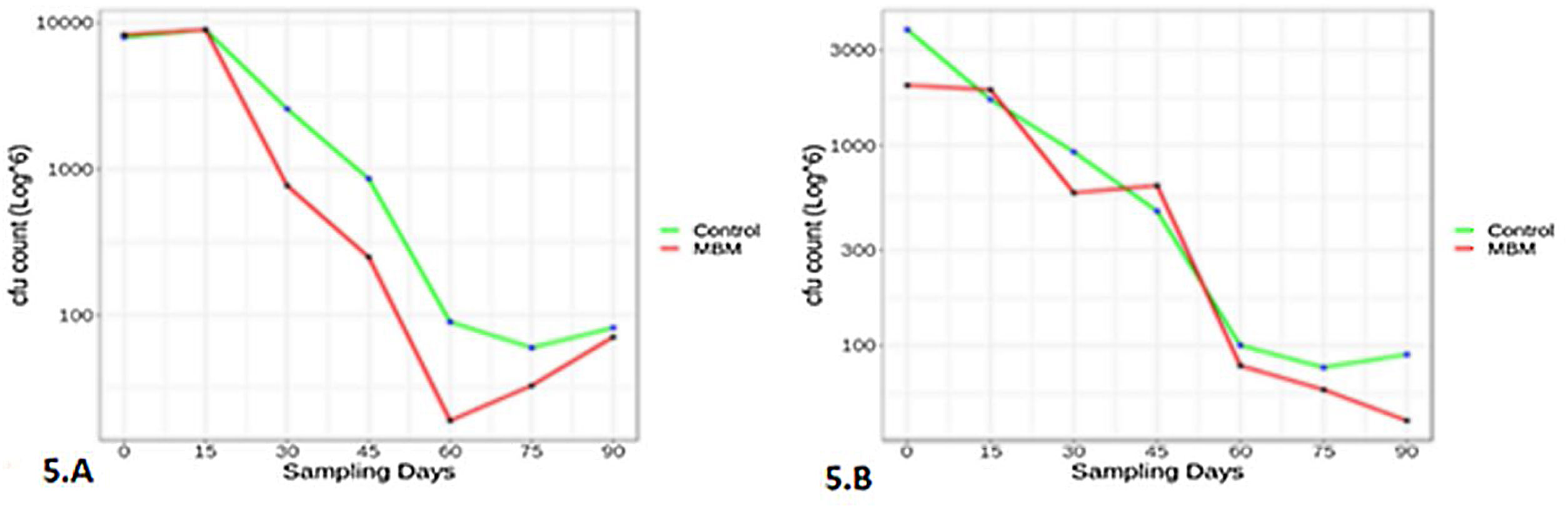
The survival study was conducted only for M11 and M33 as they showed a strong broad spectrum biofungicidal activity. The results showed that B. amyloliquefaciens (M11) and B. subtilis (M33) survived in all three treatments containing molasses with negligible difference in population rate, up to a test duration of 3 months. Therefore, the composition of only one treatment is documented in this paper. The survival rate of isolates in LB media was high as expected; however, with no significant difference between enriched and molasses-based medium (MBM). The survival rate after 90 days of B. amyloliquefaciens (M11) in LB and molasses media was 8.2×107 and 7.1×107 (CFU/ml) respectively, while the survival rate of B. subtilis (M33) was 9×107 and 4.2×107 (CFU/ml) respectively. The graph was plotted using R software (Fig. 5).
Survival of biocontrol bacteria in molasses-based medium (MBM) compared to control, where the x-axis represents the sampling days (0–90 days) and the y-axis shows the CFU count. (A) B. amyloliquefaciens (M11) survival in MBM compared to LB control medium; (B) B. subtilis (M33) in two different media, LB medium (control) and molasses-based medium (MBM).
The practice of using biological control in fungal disease suppression is an effective alternative to the use of chemical fungicides, which often has deleterious effects on living things and the environment. Nowadays, the researchers are trying to develop eco-friendly methodologies based on biological sources. Although various microorganisms have been used as biological control agents, biofungicidal bacteria are widely utilized for the suppression or inhibition of fungal phytopathogens20. The present study demonstrates that biocontrol bacteria isolated from the corn phyllosphere are potential candidates to be used as antagonists to phytopathogenic fungi. The screening strategy carried out in this paper consists of the isolation of bacterial strains capable of inhibiting phytopathogenic fungi.
Fourteen bacterial strains (M11, M33, M35, M40, M42, W71, W75, W79, W88, P92, P97, P102, P107 and P110) with antifungal activity were isolated; the two isolates from the maize plant (M11 and M33) showed strong inhibition against all four phytopathogenic fungi under study. The bacterial isolates with strong antifungal activity were Gram positive and endospore-producing. They were identified as B. amyloliquefaciens and B. subtilis respectively by 16S rRNA gene sequencing. Among the bacterial antagonists, the Bacillus species are widely reported biocontrol agents24.
VOC production, cell-wall degrading activities and production of secondary metabolites, i.e. HCN and siderophores, seemed to be the possible mechanisms responsible for antagonism in the case of B. amyloliquefaciens and B. subtilis, since cellulolytic activity, VOCs, HCN and siderophore production were clearly detected. It has been previously reported that enzymatic activities, VOCs, HCN and siderophore production correlate with antifungal activity15. The antagonistic bacterial isolates showed positive results for VOCs and siderophore production whereas HCN production was detected only in the case of B. subtilis. The role of VOCs and HCN (produced by the Bacillus species) in plant-bacterial interactions (i.e. triggering plant growth and defense) has been well-documented26,30. Bacillus species are known to produce several VOCs, such as, 2-propanone, phenol, 1-decanol and so on11. The production of HCN is an important plant growth-promoting activity and has significant toxicity against the number of phytopathogens26. Bacterial HCN is effective in the control of fungal infections, restraining the cytochrome-C terminal oxidase in the fungal respiratory chains and cause extreme damage to the fungal metabolism. Furthermore, bacterial siderophores indirectly enhance plant growth via inhibiting phytopathogens and creating competition for ferric iron resources in soil29. Thus, siderophores play a role as iron solubilizing agents by solubilizing iron from minerals or organic compounds under low iron conditions10.
M11 and M33 isolates were found to produce IAA in the presence of L-tryptophan but were unable to solubilize inorganic phosphate. Approximately 80% of the isolated bacteria from the rhizosphere/phyllosphere of different crops can produce and release auxins as secondary metabolites10. Bacterial IAA promotes plant growth, especially stimulates the overproduction of root hairs to increase the surface area so that the plant can easily uptake water and soil nutrients6. The studies proved that significant increase is observed in the growth rate of plants inoculated with bacterial IAA when compared to non-inoculated plants28.
Previous studies suggest that using bacterial inoculants such as Bacillus spp., Pseudomonas spp., Azospirillum spp. and others, is beneficial for plant growth promotion and the control of fungal diseases. Based on these qualities (i.e. growth promotion and biocontrol activity) along with their eco-friendly behavior, microbial inoculants are potential candidates for a vast market share around the globe with 15% growth rate per year28. However, a number of biocontrol agents perform quite well in the laboratory and greenhouse conditions but failed to perform in the field9. The major limitation for commercialization is the development of an effective bacterial formulation with high survival and rhizosphere colonization rate. Generally, PGPRs are inoculated directly to plant material without a suitable carrier or in inappropriate quantities that are insignificant under field conditions for rhizosphere colonization. In addition, the fumigation of soils with broad spectrum biocidal chemicals affect soil–microbial interaction, causing a lower rate of rhizosphere colonization. The issue can be resolved by a comprehension of the ecological parameters that influence biocontrol agents. To develop the final bioproduct, the selection criteria of the optimal strategy will depend on the balance between stability, efficacy, economic feasibility, and ease of use. Additionally, the transition of PGPR from laboratory to farmers requires an extensive research about survival and mass production of these strains in cost-effective carrier materials. Our data suggested that B. amyloliquefaciens and B. subtilis can easily survive in a cost effective self-prepared media (containing molasses as carbon source) up to 3 months with a significant population rate.
ConclusionThe purpose of this study was to characterize the indigenous population of biocontrol bacteria that have broad spectrum fungicidal activity to be used as biofungicides for commercial purposes. It is concluded that the bacterial isolates mentioned in the study not only had broad spectrum fungicidal activity but also additional properties of plant growth-promoting activity. The mentioned isolates had good survival rate in the molasses-based medium, which is a cost-effective nutrient medium compared to the commercial media available in the market. Therefore, the mentioned isolates could be used for commercial purposes to control phytopathogens on a cost-effective nutrient medium.
FundingThe authors wish to thank the Higher Education Commission, Pakistan (HEC) for the financial support for this study in the form of research grant HEC-TDF02-13.
Conflict of interestThe authors declare that they have no conflicts of interest.