Hookworm infection is endemic in many countries throughout the world; however, the information about the prevalence of each species, Necatoramericanus and Ancylostomaduodenale, is inaccurate in many South American countries. We aimed to determine the prevalence of human hookworm species by combining the results of both microscopy and PCR among endemic populations in Argentina, represented by natives and immigrants. A total of 140 serial fecal specimens were obtained from natives in the province of Misiones and an immigrant community living in the province of Buenos Aires. Samples were examined using the formalin-ethyl acetate concentration technique (FECT) and one flotation technique (screening tests) and specific PCRs for N. americanus and A. duodenale. We characterized samples containing N. americanus by sequencing a fragment of the cytochrome b gene. The observed hookworm prevalence as assessed by the screening tests and PCR were 24.3% and 32.8%, respectively. PCR positive samples were identified as N. americanus. PCR had 100% sensitivity compared with 73.9% of screening tests. A total of 12 samples from individuals with hookworm-infected household members were positive only by PCR. N. americanus sequences showed 90.5% identity, being more similar to each other than to any of the sequences obtained from GenBank. This is the first study that provides molecular data and characterization of N. americanus in Argentina. The complementary use of FECT and one flotation technique to screen hookworm infections, followed by PCR to differentiate the species contribute to produce better prevalence estimates.
La infección por Ancylostomideos es endémica en muchos países del mundo, pero la información sobre la prevalencia de las especies que la causan, Necator americanus y Ancylostoma duodenale, es inexacta en América del Sur. Nuestro objetivo fue determinar la prevalencia de especies de Ancylostomideos humanos en poblaciones de Argentina nativas o provenientes de áreas endémicas, combinando los resultados de microscopía y PCR. Un total de 140 muestras fecales seriadas fueron obtenidas de individuos nacidos en la provincia de Misiones con residencia en esta y de miembros de una comunidad oriunda del Paraguay establecida en la provincia de Buenos Aires. Las muestras fueron examinadas por la técnica de formol-acetato de etilo (FAE) y una técnica de flotación como pruebas de cribado, y se efectuaron PCR específicas para N. americanus y A. duodenale. Caracterizamos muestras que contienen N. americanus secuenciando un fragmento del gen del citocromo b. La prevalencia de Ancylostomideos según las pruebas de cribado y el método PCR fueron del 24,3 y 32,8%, respectivamente. Las muestras positivas por PCR se identificaron como N. americanus. La PCR tuvo una sensibilidad del 100,0% en comparación con el 73,9% de las pruebas de detección. Hubo 12 muestras de individuos con miembros de la familia infectados con anquilostomas que solo por PCR fueron positivas. Las secuencias de N. americanus mostraron un 90,5% de identidad y fueron más similares entre sí que a cualquiera de las secuencias obtenidas de GenBank. Este es el primer estudio que proporciona datos moleculares y la caracterización de N. americanus en Argentina. El uso complementario de FAE y una técnica de flotación para detectar infecciones por anquilostomas, seguido de PCR para diferenciar las especies, contribuye a producir mejores estimaciones de prevalencia.
Human hookworm infection ranks among the most important neglected tropical diseases in terms of causes of morbidity, with an estimated 500 million individuals infected in the tropics76. Globally, hookworm infections tend to be more prevalent in rural areas, in the tropics and subtropics, where favorable environmental conditions, such as temperature, soil moisture and soil type, converge with poverty and inadequate sanitary infrastructures30,43.
This infection is classically caused by Necator americanus and Ancylostoma duodenale; however, newer studies have shown that A. ceylanicum, a helminth of canids and felids, is also an important emerging parasite infecting humans in Southeast Asia38,48,49,68. N. americanus accounts for the majority of human hookworm cases worldwide, being A. duodenale more focally endemic in the Mediterranean region, in northern regions of India, China and North Africa.43
Hookworm species not only differ in their morphology, but also in their pathogenesis, life cycle, and clinical features. Although the treatment is similar for both hookworm species, the severity of anemia differs depending on the species and the worm load in the intestine. Actually, experimental studies have shown that A. duodenale caused 2–10 times more blood loss per worm than N. americanus35. Furthermore, A. duodenale has the ability to infect orally and remains arrested in human tissues75. Therefore, the specific identification of these species is an important public health measure for monitoring the severity of illnesses and the efficacy of mass and effective treatment63.
Several methods are available to determine the presence and intensity of hookworm infections, such as conventional techniques based on egg visualization, which are unable to differentiate between species (e.g. formalin-ether concentration and Kato–Katz), coproculture methods (e.g. Corticelli-Lai and Harada Mori) and PCR-based methods, which have already proven to be more effective for the detection of hookworm infection than conventional diagnosis12.
In a routine laboratory diagnosis or in large-scale prevalence screenings, the choice between any of these methods depends on many factors, such as the relative costs of the techniques, the methods for sample preservation and the simplicity to carry out diagnostic tests.
Although several studies have reported molecular-based methods to study the prevalence and species identity of human hookworm infections in endemic countries from Asia and Africa4,48,58,61, there are very few prevalence studies which apply these techniques in human populations from South American countries25,33,45,62.
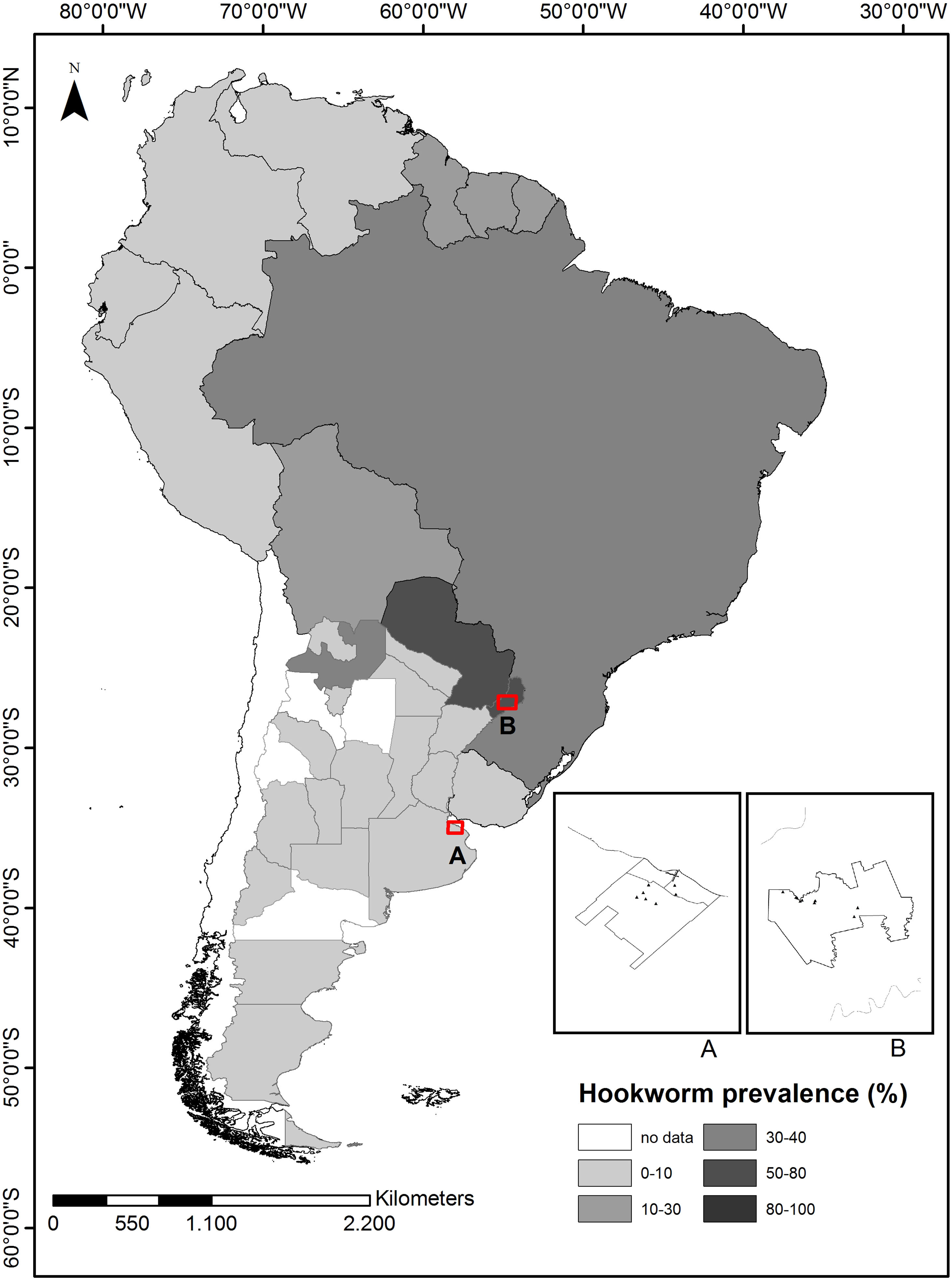
Argentina belongs to the southernmost region of America, and is characterized by high variability of its climatic and socioeconomic conditions. In this country, the frequency of intestinal parasites shows a declining trend from north to south and from east to west47. With regard to human hookworms, the infection was mainly recorded in the north of Argentina, being most prevalent in the provinces of Salta and Misiones (Fig. 1)9,12,78. In the province of Misiones, the physical environment favors the high prevalence of these parasites. Actually, Rivero et al.55 have shown that hookworms were the main pathogens detected in environmental samples studied in this province. Moreover, Cociancic et al.15 have found that most individuals infected by hookworms lived in areas with abundant arboreal-shrubby vegetation. On the other hand, in non-endemic areas of Argentina, such as Buenos Aires, there are no updated studies reporting hookworm infections and the previous ones described only sporadic cases10,23,70. The province of Buenos Aires, located in the center of Argentina, is characterized by temperate climate and the soil has a silty–loam texture with abundant organic matter. These climatic and environmental conditions are unfavorable for the hookworm life cycle. However, the high degree of immigrant people from endemic areas (mainly from Paraguay) living in the province of Buenos Aires makes it an important region to be studied42,44.
Sampling areas tested for hookworm infections. Buenos Aires and Misiones sampling areas are indicated (A and B, respectively). Distribution of human hookworm infections reported for Argentinian provinces and other South American countries was also indicated, showing the mean prevalence value for each geographic area. Adapted from 7, 11, 17, 29, 33, 37, 47. This map was created using QGIS 3.4.14 Madeira (version 3.4.14 Madeira), s. f. https://www.qgis.org/en/site/.
To date, most studies about hookworm infections in Argentina were conducted using microscopy-based diagnosis, while few reports have differentiated between species based on coproculture6,19,77 and only one was based on molecular analysis12. The single molecular study that differentiates hookworm species was performed in Salta and found that N. americanus (36.4%) was more prevalent than A. duodenale (19.1%)12.
The knowledge of the distribution of these parasites, both in endemic and non-endemic regions, is important to better understand the epidemiology and the clinical course of the infection of each parasite in each geographic area. This information would help to make a better diagnosis of patients and design control programs based on the characteristics of each region. To fulfill these goals, it is necessary to implement an improved diagnostic strategy in cross-sectional surveys. Furthermore, we aimed to determine the prevalence of human hookworm species by combining results of microscopy and PCR in human fecal samples from endemic populations in Argentina, represented by natives and immigrants. Moreover, we aimed to provide a molecular characterization of identified hookworm species in order to evaluate their genetic diversity and phylogenetic relationships, which will provide a basis to better understand the biology of these parasites.
Materials and methodsStudy area and subject samplingHuman fecal samples were collected from populations from two provinces in Argentina, representing two different ecoregions: the Province of Buenos Aires, located in the Pampas ecoregion and the Province of Misiones, located in the Paraná Atlantic Forest. All samples were obtained as part of a larger cross-sectional survey on intestinal parasitic infections, nutritional status, and socio-environmental conditions in human populations. In the Province of Buenos Aires, 34 fecal samples were collected from immigrants from Paraguay, aged 1–60 years old, who had resided in peripheral regions of the district of La Plata (34̊55′17.22″S 57̊57′16.31″W) for a period of residence ranging from 6 months to 22 years, being 8 years the average period. In the Province of Misiones, 106 samples were collected from individuals, aged 1–40 years old, from rural areas in neighboring villages in the locality of Aristóbulo Del Valle (27̊05′43″S 54̊53′49″W) (Fig. 1). Individuals who had decided to participate voluntarily and had given written and oral consent were included. Children or youths were included after their parents and legal guardians had given written and oral consent. However, those individuals who had received some antiparasitic treatment by the time of the research were excluded.
The area of study in Buenos Aires is a non-endemic region for hookworms, characterized by a temperate climate and poor structural and socio-environmental conditions. The area is inhabited by immigrants from Paraguay, a hookworm endemic country. Most of the individuals came from low-income families with parents having unskilled or informal jobs.
The rural areas studied in the Province of Misiones are characterized by favorable conditions for the persistence of human hookworm infection, such as humid climate and sandy soils with abundant arboreal-shrubby vegetation. Most of the individuals are tenant farmers who work on small-scale farming, and others belong to indigenous groups, who have their homes in open clearings in the jungle.
Sample collectionAfter consent was obtained, each individual was supplied a sterile plastic vial containing 70% ethanol. Each participant was instructed to collect a nut-sized stool sample each day, during 3–5 days, in the vials. When participants were children, samples were collected by their parents or legal guardians.
Fecal samples were submitted to Centro de Estudios Parasitológicos y de Vectores (La Plata, Buenos Aires, Argentina) from April 2018 to October 2019. During this period, the procedure included microscopic examination (screening tests) and molecular analyses (PCR) of human fecal samples to diagnose hookworm species.
Parasitological diagnosisScreening testsThe microscopic examinations performed after the formalin-ethyl acetate concentration technique (FECT) and the flotation technique were considered screening tests, which permitted the identification of hookworm eggs.
Fecal samples preserved in 70% ethanol were processed by the above techniques, followed by microscopic examination of hookworms and other intestinal parasites. First, each sample was filtered through a double layer of gauze to remove the ethanol solution and 5% formalin was added until it covered the entire sample. Then, samples were processed according to Cociancic et al.14 and WHO74. Briefly, for the FECT method, 10 ml of formalin fecal suspension was filtered and centrifuged at 1500rpm for 5min. Then, 7ml of formalin and 3ml of ethyl acetate were added to the sediment, mixed and centrifuged at 1500rpm for 5min. A saturated sodium chloride solution (S. G.=1.2g/ml) was used for the Willis flotation technique. Additionally, the anal swab method, performed according to Cociancic et al.14, was used for the specific detection of Enterobius vermicularis as part of the general parasitological analysis of the population studied.
Hookworm L3 larvaeIn order to determine PCR sensitivity, filariform larvae (L3) were isolated from fresh fecal samples. These samples were obtained from three volunteers, who were not part of the sampling performed as object of this study, with a positive parasitological test for hookworm eggs. For this purpose, 4g of fresh fecal samples with hookworm eggs were cultured at 25̊C–28̊C using the Harada Mori technique. Cultures were prepared in a thick smear to individual 1-cm-wide strips of filter paper and placed into open 15ml conical tubes. Sterile water was added to the bottom of each tube so that the meniscus was about 0.5cm below the fecal mass. The volume of liquid in each tube was monitored daily and adjusted as needed, and larvae were collected from the bottom of the tubes on day 763. The morphological differentiation of the L3s of N. americanus from those of A. duodenale was based on the following criteria: measuring the total length of the sheath; measuring the total length of the larva; examining the buccal cavity for the presence of a visible spear; examining the shape of the larval tail and measuring the larval tail from the anus to the tip of the tail and establishing whether the cuticular striation was faint or strong2. All larvae were identified as N. americanus.
Molecular analysisDNA isolation from fecal samplesPrior to extraction, 500mg of fecal/ethanol suspension was centrifuged at 8000×g, and the pellet was washed three times with 1ml of sterilized saline buffer. After centrifugation, the pellet was subjected to mechanical disruption by 3 freeze–thaw cycles. DNA was isolated using the ZR Fecal DNA MiniPrep™ Kit (Zymo Research, California, USA) following the manufacturer's instructions.
DNA from larvae (obtained by the coproculture technique) was extracted using the Wizard® Genomic DNA Purification Kit (Promega, Madison, WI, USA) according to the manufacturer's instructions.
DNA isolation from larvaeDNA from larvae (obtained by the coproculture technique) was extracted using the Wizard® Genomic DNA Purification Kit (Promega, Madison, WI, USA) according to the manufacturer's instructions.
Hookworm-specific PCRHookworm-specific primers were designed using Primer BLAST software of the National Center for Biotechnology Information (http://www.ncbi.nlm.nih.gov). The N. americanus-specific primers were designed based on reference sequences of the cytochrome b (cob) gene available in GenBank (Acc. Nos. NC_003416, AJ556134, NW_013561619); and A. duodenale-specific primers were designed based on reference sequences of the ITS1 rRNA region available in GenBank (Acc. Nos. EU344797, AJ001594, MK271367, MG271919, KC632570). Sequences were aligned by using the CLUSTALW program with the default option32. The primer sequences to identify N. americanus were NecF 5′-GGTGTGGGCTCAAATGAGAT-3′ and NecR 5′-CAACAAACCTCAAGGCAACA-3′. The primer sequences to identify A. duodenale were AncyF 5′-GAATGACAGCAAACTCGTTGTTG-3′ and AncyR 5′-ATACTAGCCACTGCCGAAACGT-3′.
PCR conditions were optimized in a final volume of 20μl as follows: 1X GoTaq® buffer (Promega, Madison, USA), 0.2μM dNTPs, 1U/μl GoTaq® Hot Start polymerase (Promega, Madison, USA), 0.5μM of each primer, 0.1μg/μl BSA, 3mM MgCl2 and 4μl of DNA as template. Cycling conditions were: 3min at 95°C, 35 cycles of 45s at 95°C, 1 min at 60°C (for N. americanus primers) or 55̊C (for A. duodenale primers), and 90s at 72°C, with a final extension at 72°C for 5min.
Some PCR products were further purified and sequenced (Macrogen, Seoul, Korea) to validate positive PCR results and perform phylogenetic analyses. Forward and reverse sequences obtained were assembled using the PREGAP and Gap4 programs of the Staden package66. The consensus sequences were compared to the previously published sequences of hookworm by using the Basic Local Alignment Search Tool (BLAST) (www.ncbi.nlm.nih.gov/blast).
Internal PCR controlIn each fecal sample, axenic culture epimastigotes (1×104) from the protozoa Trypanosoma cruzi, was added before DNA isolation to act as an internal control. T. cruzi-specific PCR was performed on all the samples in parallel to identify false-negative PCR results. DNA was amplified with primers: TCZ1: (5′-CGAGCTCTTGCCCACACGGGTGCT-3′) and TCZ2: (5′-CCTCCAAGCAGCGGATAGTTCAGG-3′ under the following conditions: 3min at 95̊C, 35 cycles of 45 s each at 95̊C, 1min at 55̊C and 45s at 72̊C with a final extension step of 5min at 72̊C54.
Positive control samplesPositive DNA samples were obtained from hookworm-positive human fecal samples provided by the Division of Parasitic Diseases and Malaria (Centers for Disease Control and Prevention).
Negative control samples and other parasite samplesHookworm-specific PCR was also performed on samples from individuals infected with related nematodes or other pathogens: Entamoeba coli, Entamoeba histolytica, Entamoeba dispar, Giardia lamblia, Blastocystis sp., Strongyloides stercoralis, and Ascaris lumbricoides. Individuals without parasite species were included as negative controls.
Phylogenetic analysisPhylogenetic analysis was carried out using newly obtained hookworm sequences and matching sequences of other representatives of hookworm species available in GenBank. Furthermore, the available GenBank sequence of the strongylid nematode Oesophagostomum dentatum was used as an outgroup (supplementary table S1). DNA sequence data was aligned using the CLUSTALW program32, with default options for introducing gaps into the alignment. When needed, inconsistencies were checked and manually edited. Phylogenetic relationships were inferred by Maximum Likelihood (ML) using MEGA X software41 and the Bayesian inference (BI) as implemented in MrBayes Ver. 3.2.6 software57. The best DNA substitution model (HKY+G+I) was estimated using the jModelTest program50. Nodal support for the ML analysis was estimated by performing 1000 bootstrap replications. The BI analysis was performed using Markov chain Monte Carlo (MCMC) chains for 1000000 generations with sample frequency set at 100. The first 25% of the trees sampled were discarded as ‘burn-in’. This number of generations was considered sufficient because the SD dropped below 0.01.
A pairwise distance matrix among the hookworm sequences was calculated using the nucleotide p-distance algorithm implemented in MegaX41.
Statistical analysesPrevalence was estimated with 95% confidence intervals. A sample was considered positive if it was positive by any technique, and was considered negative if all diagnostic techniques were negative.
In order to compare the performance of the diagnostic tests, a minimum sample size of 125 individuals was calculated assuming a prevalence of about 80% based on previous surveys conducted in populations from the same regions here studied42,47,65. The combined results of the techniques employed served as the gold standard27. Sensitivity and negative predictive value (NPV) of the techniques were calculated using R software version 3.6.3 (R Core Team 2020) through library bdpv60. These values were given with their 95% confidence intervals. Complementary evaluation was conducted using Cohen's kappa test for two categorical variables under the interpretation of values from Watson and Petrie71 and according to the criteria of Fleiss et al.22 through library irr24. This test was performed to determine the statistical concordance between the techniques performed.
Sequences obtained were deposited at GenBank under accession numbers MT757751–MT757759 and MW711817–MW711819.
ResultsPrevalence and parasitological screeningA total of 140 fecal samples from individuals from the provinces of Buenos Aires and Misiones were subjected to the initial screening tests for hookworm infection consisting of microscopic examination. Screening tests detected 34 out of 140 (24.3%) samples positive for hookworm eggs (30 samples were detected positive by FECT and 22 samples by flotation techniques). The highest proportion of hookworm infection corresponded to individuals from the Province of Misiones (31/34; 91.2%), and only three samples (3/34; 8.8%) were positive in Buenos Aires (Table 1).
Description of the performance of the diagnostic methods conducted on 140 samples from Buenos Aires and Misiones.
| Technique | Buenos Aires | Proportion of positives (%)a | Misiones | Proportion of positives (%)b | Total | Proportion of positives (%)c |
|---|---|---|---|---|---|---|
| FECT | 3 | 60.0 | 27 | 65.8 | 30 | 65.2 |
| Flotation | 2 | 40.0 | 20 | 48.8 | 22 | 47.8 |
| Screening testsd | 3 | 60.0 | 31 | 75.6 | 34 | 73.9 |
| PCR | 5 | 100.0 | 41 | 100.0 | 46 | 100.0 |
The prevalence of hookworm based on FECT was 21.42% CI [15.13%–29.14%] in the total population analyzed, 25.47% CI [17.64%–34.77%] in Misiones and 8.82% CI [2.45%–22.87%] in Buenos Aires. On the other hand, the prevalence based on flotation technique was 15.71% CI [10.33%–22.69] in the total population analyzed, 18.86% CI [11.96%–27.41%] in Misiones and 5.88% CI [1.05%–18.83%] in Buenos Aires.
Globally, the prevalence of hookworm calculated based on screening test results was 24.28% CI [17.65%–32.04%] in the total population analyzed, 29.24% CI [20.97%–38.59%] in Misiones and 8.82% CI [2.45%–22.87%] in Buenos Aires.
The overall prevalence of parasites was 76.5% in Buenos Aires and 77.3% in Misiones, being Blastocystis sp. and E. vermicularis the most prevalent parasites in both provinces (supplementary table S2).
Validation of PCR approachThe PCR for the amplification of hookworm DNA from feces was established using positive control DNA samples.
When primers Ancy/AncyF were tested, a fragment of approximately 71bp could be amplified from DNA samples known to contain A. duodenale. Similarly, when primers NecF/NecR were tested, amplicons of 172 bp were obtained (supplementary Fig. S3).
The analytical specificity of N. americanus and A. duodenale PCR was evaluated with DNA samples from patients with related nematodes or other pathogens, and no cross-reactions were observed (supplementary Fig. S3). N. americanus larvae obtained by coproculture were used to test this specific-PCR.
Analytical sensitivityAnalytical sensitivity of PCR was tested with different counts of N. americanus larvae (1 L3, 30 L3, 50 L3 and 100 L3). All these samples showed amplification, except the one with a single filariform larva L3 (supplementary Fig. S3).
Differential diagnosis by PCRBased on the results from the validation, all the fecal samples (n=140) were subjected to PCR for the differential diagnosis of A. duodenale and N. americanus, using the approach described herein.
Thirty-four out of 34 (100%) hookworm egg-positive samples were successfully amplified. In addition, 12 samples that were found to be negative by microscopy were found to be positive only by PCR. All these samples corresponded to individuals with at least one hookworm-positive family member. These results were validated by sequencing, obtaining the corresponding 12 sequences successfully.
All PCR-positive samples (n=46) corresponded to N. americanus.
The prevalence of hookworm, calculated based on PCR assays, was 32.85% [25.18%–41.02] in the total population analyzed, 38.67% CI [29.53%–48.58%] in Misiones and 14.70% CI [5.97%–30.39%] in Buenos Aires.
Comparison among techniques and combined resultsA total of 46 out of 140 selected samples were found to be positive for hookworms by at least one technique; 73.9% (34/46) of the samples were found to be positive by the screening tests, and 100% (46/46) was positive by PCR (Table 1).
PCR had the highest sensitivity for N. americanus detection (100.00% CI [92.29%–100.00%]), followed by FECT (65.21% CI [49.75%–78.74%]) and flotation (46.82% CI [32.88%–63.05%]). When FECT and flotation were considered together as screening tests, sensitivity was 73.91%. In addition, the higher value of NPV was for PCR (100% CI [(99.46%–100.00%]) (Table 2).
Sensitivity and negative predictive values (NPV) of the formalin-ethyl acetate concentration technique (FECT), flotation and PCR for N. americanus detection.
| Technique | Sensitivity% (95% CI) | NPV% (95% CI) |
|---|---|---|
| FECT | 65.21 (49.75–78.74) | 84.97 (79.34–88.98) |
| Flotation | 46.82 (32.88–63.05) | 79.46 (74.65–83.43) |
| Screening testsa | 73.91 (58.86–85.73) | 88.02 (82.10–91.81) |
| PCR | 100.00 (92.29–100.00) | 100 (99.46–100.00) |
Cohen's kappa index showed good agreement between the employed techniques, being moderate between PCR and flotation (0.54 CI [0.39–0.68]), and substantial between PCR and FECT (0.70 CI [0.54–0.86]). When FECT and flotation were considered together, substantial agreement was found with PCR (0.78 CI [0.61–0.94]) (Table 3). Fleiss’ Kappa index was calculated among PCR, flotation and FECT techniques, and was 0.617.
Results of Cohen's Kappa index between the screening tests (FECT and Flotation) and PCR.
| Techniques | PCR | |||||
|---|---|---|---|---|---|---|
| Positive | Negative | Total | Cohen's Kappa index (95% CI) | S.E. | Level of agreement | |
| FECT | ||||||
| Positive | 30 | 0 | 30 | 0.70 (0.54–0.86) | (0.08) | Substantial |
| Negative | 17 | 93 | 110 | |||
| Flotation | ||||||
| Positive | 22 | 0 | 22 | 0.54 (0.39–0.68) | (0.07) | Moderate |
| Negative | 25 | 93 | 118 | |||
| Screening testsa | ||||||
| Positive | 34 | 0 | 34 | 0.78 (0.61–0.94) | (0.08) | Substantial |
| Negative | 13 | 93 | 106 | |||
Based on combined microscopy plus the PCR detection approach, a prevalence of 32.85% [25.18%–41.02] was detected for N. americanus in the total population analyzed, 38.67% CI [29.53%–48.58%] in Misiones and 14.70% CI [5.97%–30.39%] in Buenos Aires. Most of the infected individuals in Buenos Aires were 20–45-year-old adults (4/5; 75%), while most of the infected ones in Misiones were 1–15-year-old children (40/41; 97.6%).
Molecular and phylogenetic analysisA total of 12 cob sequences of 169 bp were successfully obtained from N. americanus. The BLAST similarity analysis confirmed that the correct regions had been amplified. All the sequences obtained aligned closest to N. americanus from China (GenBank accession number AJ417719) (96–99% identity). Sequences were 90.5% (153/169) identical, showing 16 polymorphic sites, ten of which corresponded to singleton variable sites with two variants, and six to parsimony informative sites with two variants.
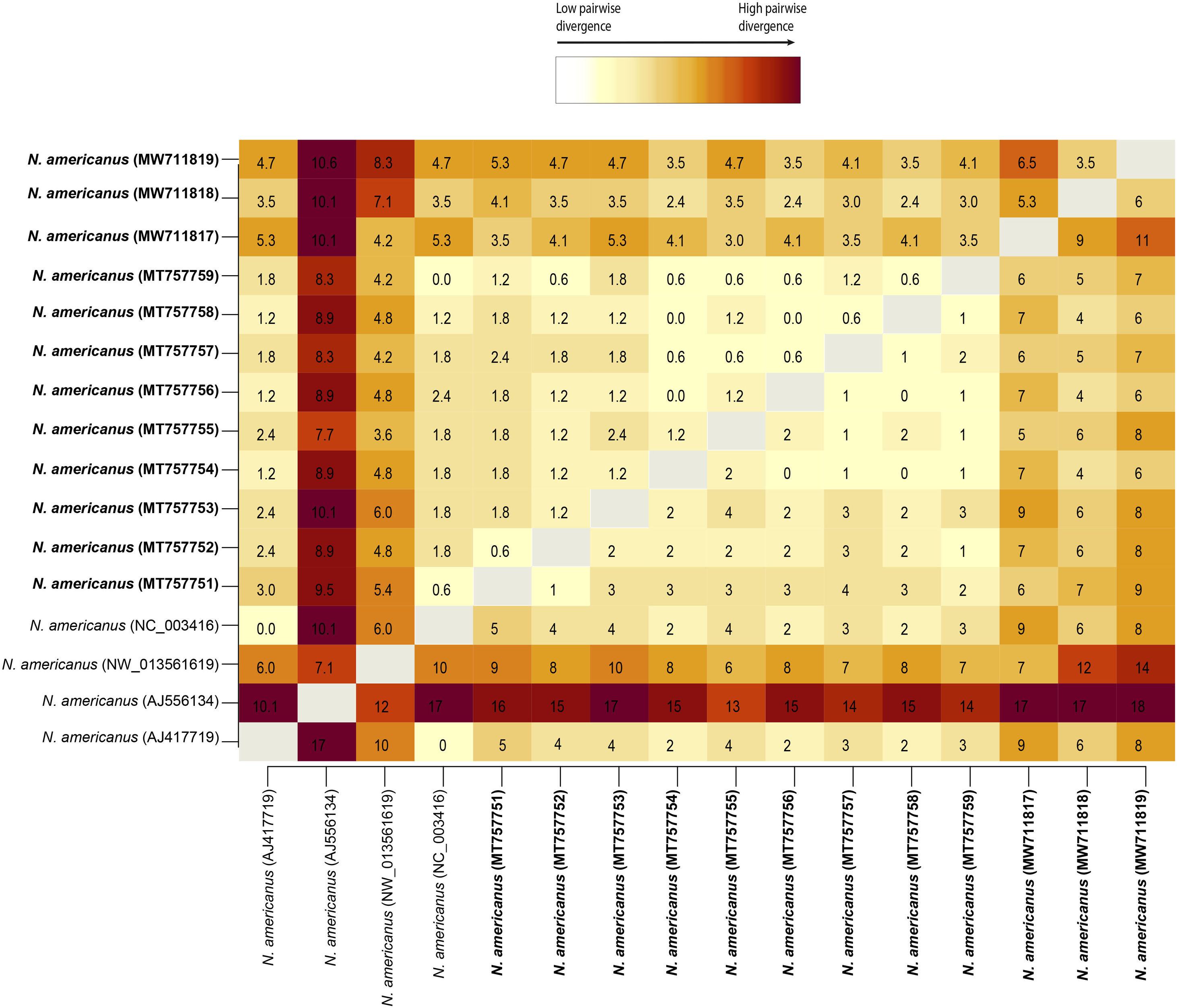
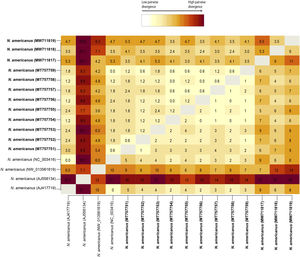
Pairwise DNA analyses revealed that the divergence among newly obtained sequences of N. americanus ranged from 0 to 6.5% (0–11 nucleotides). Three isolates from the Province of Misiones (Acc. Nos. MT757754, MT757756 and MT757758) were completely identical. This analysis also showed that the genetic distance of the cob gene among N. americanus sequences included in this study ranged from 0 to 10.6% (0–18 nucleotides). The sequence of N. americanus from Africa (AJ556134) showed higher values of genetic distance, ranging from 7.1% to 10.6%, when it was compared with each one of the remaining sequences analyzed (Fig. 2).
Heat-map distance matrix showing percentage (above diagonal) and number (below diagonal) of base differences per site based on pairwise comparison of the partial fragment of the cob gene among the sequences obtained in this study, and 4 sequences of available at the GenBank database. There was a total of 169 positions in the final dataset. Sequences obtained in the present study are in bold.
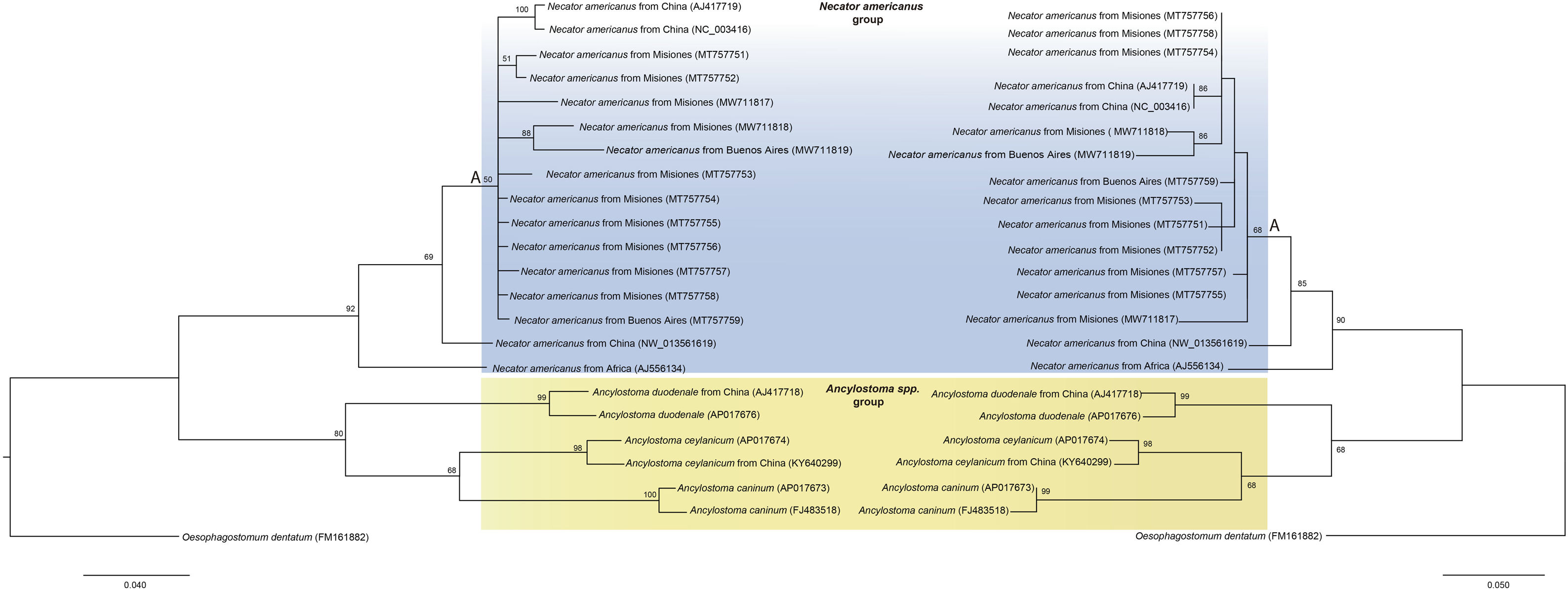
When the ML and BI analyses were performed, almost identical tree topologies were obtained. However, there were differences in the resolution of clades which included the newly obtained sequences (Fig. 3).
Phylogenetic trees constructed by BI (on the left) and ML (on the right) methods based on 20 sequences of a partial region of the cob gene under the substitution model HKY+G+I Sequences are identified by taxon name and locality of origin (when this data was available) and GenBank Acc. Nos. Sequences obtained in the present study are in bold. Nodal support (>50%) is indicated above internodes.
All N. americanus sequences clustered together in a monophyletic clade, named N. americanus group, highly supported by both the ML and BI methods (90% and 92%, respectively). In this group, the newly obtained sequences clustered altogether (clade A), separately from the isolates from China and Africa (Fig. 3).
The Ancylostoma sequences also formed a monophyletic clade, named Ancylostoma spp. group, and represented a sister clade of N. americanus group. Within Ancylostoma spp. group, sequences of A. caninum and A. ceylanicum, both isolated from dogs, clustered together and separately from A. duodenale sequences isolated from humans (Fig. 3).
Taken together, N. americanus and Ancylostoma spp. sequences clustered as a major clade (Ancylotomatidae group).
DiscussionThe etiologic agent of hookworm infection has often been unknown because the differentiation between species is not usually performed in the diagnostic laboratory, particularly in resource-limited countries such as Argentina12,30. Although differential diagnosis contributes to a further comprehension of the dynamics of infection, hookworm species have been considered to be identical for treatment and control strategies13. Actually, both species can cause anemia, but clinical studies have indicated a stronger association of A. duodenale infection with anemia than N. americanus35,64. Moreover, unlike N. americanus, A. duodenale can be orally ingested, and potentially result in Wakana syndrome (nausea, vomiting, pharyngeal irritation, cough and dyspnea)72.
More sensitive diagnostic tools are essential for an accurate assessment of parasitic infections in low-endemicity areas, their reliable mapping and surveillance3,40. Moreover, designing optimal strategies for the diagnosis, which takes into account ease of use, test performance, and costs, is particularly important in developing countries. Numerous PCR-based assays have been developed for the identification of human hookworm species4,13,18,26,48,75. However, the current and definitive diagnosis is mainly based on the microscopic visualization of eggs in feces and the Kato–Katz method is the recommended technique by the WHO for monitoring helminth control programs because of its simplicity and relatively low cost74. Despite this latter recommendation, the Kato–Katz method has low sensitivity; particularly if only a single stool sample is examined in areas characterized by low-intensity infections, and can only be performed on fresh fecal samples16,40.
In Argentina, very few studies have reported the differential diagnosis of hookworm species; one study has performed molecular techniques12 while most have carried out coproculture as a means of species identification6,12,19,77.
In this work, we proposed to determine the prevalence of hookworm species by morphological and molecular techniques, as a strategic way to study endemic populations for these parasites represented by natives and immigrants in Argentina.
Morphological techniques (sedimentation and flotation methods) were selected as screening tests for hookworm infection because they are standard methods for gastrointestinal parasites; they are technically simple and low-cost techniques58. These methods allowed us to detect 73.9% (34/46) of the total hookworm-positive samples in the populations studied. In parallel, the PCR assay detected 100% (46/46) of the positive cases. This method provided 12 additional positive results in addition to those detected using the screening tests. These cases corresponded to individuals with at least one household member positive for hookworms. With regard to the last point, many authors indicated that the predisposition to human hookworm infection is dependent on factors acting at the family and household level28,51,52,69. Moreover, this predisposition is higher in families whose members not only live in the same residence, but also share the same genetic factors. Several studies have highlighted that these factors influence susceptibility to hookworm infections8,51. The combined effect of household and genetic factors explains much better the predisposition to heavy or light hookworm infections52. A high prevalence of intestinal parasitic infection was observed among household contacts of primary cases in cross-sectional surveys, where hookworm infection was the predominant parasitic infection21.
The agreement between PCR and screening tests was moderate to substantial and the NPV was higher for PCR. This result shows that the microscopy analysis and the PCR method evaluated here may be used as complementary tools, being the PCR method successful to detect hookworms in microscopic false-negative samples and provide species-specific identification. The results of the comparison among techniques showed good agreement.
In this study, PCR showed higher sensitivity than the screening tests (FECT and flotation) and detected 12 positive samples more than these techniques. Anamnart et al.1 pointed out that the formalin solution and the gauze pore size (employed to filter the samples) affected the recovery of hookworm eggs. This last consideration may explain the low sensitivity of the screening tests, in comparison with the PCR to detect hookworm infections.
Based on these results, we propose a diagnostic strategy which considers the simultaneous use of FECT and flotation techniques in parallel to perform an initial screening test, followed by a PCR assay to differentiate the hookworm species. PCR may be performed not only on egg-positive samples but also on samples from individuals who tested negative by microscopic examinations but with hookworm-positive household members. This diagnostic strategy includes the preservation of a series of fecal samples in 70% ethanol. This method of sample collection allows to perform morphological and molecular techniques without the need to ask people for a fresh sample to confirm the diagnosis. In this sense, unpreserved stool samples are preferred for molecular studies, but they have to be stored and transported frozen. Although ethanol is not classified as a parasitology fixative, mixing it with stool samples allows transportation and storage at room temperature for at least 3 months67. Our diagnostic strategy is in agreement with that of Knopp et al.39, who recommended the examination of multiple stool samples and the use of different techniques for the accurate diagnosis of worms, particularly in areas undergoing repeated antihelminthic drug administration.
All hookworm infections in this study were caused by N. americanus and this result was consistent with previous worldwide studies which highlight this parasite as the predominant human hookworm species43,45. On the other hand, A. duodenale is more focally endemic in India, China, Africa, and a few regions of the Americas43. With respect to A. ceylanicum, our study was unable to detect this parasite. However, this helminth was reported as the second most prevalent hookworm species infecting humans in Asia, and was only found in dogs in South America in 197634,49,53.
In Argentina, the few studies which made differential diagnosis also found N. americanus as a prevalent species. Borda et al.6 studied the prevalence of intestinal parasitism in children from the Province of Corrientes, and recorded a prevalence of 12% for N. americanus, 1% for A. duodenale and 4% for mixed infections using coproculture techniques. Similarly, Cimino et al.12 studied human intestinal parasites in an asymptomatic population from the Province of Salta and detected a prevalence of 36.4% for N. americanus and 19.1% for A. duodenale. Exceptionally, Echazú et al.19 reported that A. duodenale accounted for 86% (63/73) of the hookworm cases identified by species in a study about treatment of soil-transmitted helminths in the Province of Salta.
Several studies about the age-specific epidemiology of hookworm infection reported that although children are commonly infected, the prevalence and infection intensity is highest in adults36,43. In this study, these helminths were found in both children and adults. In Buenos Aires, adults were the most infected. They were probably chronically parasitized individuals who acquired the infection from their native countries, in this case Paraguay, which is a hookworm endemic area59. In this regard, these parasites can reside for many years in the small intestine of their hosts and it is known that elderly individuals in hookworm-endemic areas harbor the heaviest burdens8,30. On the other hand, children were the most infected in Misiones. In general, the risk of hookworm infection in children has been associated with wearing shoes, hand wash practice and personal hygiene20. Hence, children from Misiones likely got infected due to their poorly developed hygiene habits and susceptibility to enteric infections
Despite the high prevalence of hookworms recorded in the Province of Misiones in previous studies23,46,56,78, a lower value (38.7%) was found in the rural area studied. In accordance with our results, Cociancic et al.15 reported a low prevalence of hookworm infection (16.0%) in Misiones. This decrease in the frequency of infection may be due to the administration of preventive chemotherapy to control soil-transmitted helminth infections in children, recommended by the WHO73. To date, there are no molecular studies available on the differential diagnosis of hookworm in this province. Similarly, there are scarce and no current studies that report hookworm infections in Buenos Aires37. It is known that hookworms are dependent on environmental factors, being the soil and climate conditions of Buenos Aires less favorable for the survival of hookworm eggs. However, this study was focused on the immigrant population from Paraguay, with an average of 8 years of residence in Buenos Aires, taking into account that hookworm infection remains one of the most common chronic infections in humans. In fact, it was estimated that A. duodenale can live in the human intestine on average from 1 to 3 years, and N. americanus from 3 to 10 years, with a maximum lifespan of 18 years10. In this regard, Waks70 also investigated hookworm infection in an immigrant population who lived in overcrowded and unsanitary conditions in Buenos Aires. In this study, it was found that 4.7% of 1218 subjects were infected with N. americanus. Similarly, we also found a low prevalence of hookworm infection (4.4%) in the immigrant population studied from La Plata city in Buenos Aires. Gamboa et al.23 also reported sporadic cases of hookworm infections in suburban areas of La Plata (3 out of the 312 individuals studied); however, they did not provide information about the geographical origin of the population under study. On the other hand, Castex and Greenway10 reported four human cases of infections caused by A. duodenale in this province, proposing that these cases were indigenous because the people studied had no history of residence in endemic areas.
The samples that tested positive only by PCR were sequenced and subjected to phylogenetic analysis. This last analysis showed that all the sequences of N. americanus included in this study were grouped into a monophyletic clade. We found sixteen different nucleotides when all sequences generated using the primers designed in house were compared, and a maximum of 11 nucleotides when the pairwise analysis was performed. In this sense, when Hu et al.31 compared the complete sequence of the cob gene between N. americanus from Togo with another from China (1113 nucleotides), 6% of divergence was found. We obtained slightly homologous amplification products within the cob gene and likely represent polymorphisms in the sequence among isolates from Argentina. These results suggest that polymorphisms may occur in even the smallest endemic and non-endemic communities. As a consequence, these molecular data provide a basis for further studies in population genetics and possible functions that such polymorphisms may have on species-specific modes of disease pathogenesis and resistance to treatment.
These results show that it is important to include sensitive diagnostic tools to contribute to the monitoring of hookworm prevalence. Bergquist et al.5 mentioned that a diagnostic approach useful in practice is almost always a compromise between quality and quantity since the techniques needed for a large-scale application must be based on cost-effectiveness (i.e., time and resources required per test), precision, simplicity and robustness. In that regard, PCR-based methods are specific-sequence directed while microscopy remains an important diagnostic tool as it can identify other parasites present in the fecal sample for which no PCR is available and thus, to perform a broader intestinal parasite diagnostic33.
ConclusionIn conclusion, in the present study, the aim was to generate better hookworm prevalence estimates by performing both microscopic and molecular techniques in endemic populations residing in two areas with different susceptibility to hookworm infections. Although the province of Misiones is an endemic region for these infections, the prevalence has decreased in comparison with previous studies. On the other hand, the results obtained for the province of Buenos Aires indicate the persistence of the infection in immigrants from countries where hookworms are highly prevalent. Consequently, we highlight the importance of diagnosing these populations in non-endemic areas. N. americanus was the only species detected in both provinces. The higher sensitivity of the PCR allowed to identify human hookworm in samples testing negative by microscopic examination. This is the first study that contributes to the record of N. americanus in two different populations from different geographic areas in Argentina based on molecular data. Moreover, sequences of the cob gene provided for Argentinian isolates of N. americanus contributed to the scarce molecular information about this parasite in these regions.
Ethical standardsThe authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national research committee (Comité Consultivo de Bioética de la Universidad Nacional de La Plata 100-20120/18) and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008. All the participants included in this study gave consent for the scientific examination of their fecal samples and to have their parasitological results published. Furthermore, all individuals who tested positive for hookworm infections were individually notified.
Financial supportFinancial support was given by PUE (22920160100036-CONICET), Universidad Nacional de La Plata (UNLP 11/N881) and the Agencia Nacional de Promoción Científica y Tecnológica (ANPCyT) (PICT-2016-0610).
Author's contributionsAS conceived the study. AS and SAR participated in study design and molecular analyses, analyzed and interpreted the data. AS performed the sampling, parasitic examinations and wrote the draft of the manuscript. MLZ performed the sampling and parasitic examinations and contributed to discussing the results. GTN helped with parasitic examinations, coordinated the study and contributed to discussing the results. All authors read, revised and approved the final manuscript.
Conflict of interestThe authors declare that they have no conflicts of interest.
We are grateful to Richard Bradbury (CDC, USA) for providing hookworm DNA samples. We thank Estella Batalla, Héctor Gabriel Avila and Walter Ferrari for their assistance in molecular techniques. Likewise, we are also thankful to Martín Acosta Albarracín and Alan Zerbi for their technical assistance in the laboratory. We especially thank Rosana Wiscovitch for her critical reading of the report manuscript and Lucas Filipetti for the English revision. We gratefully acknowledge Consejo Nacional de Investigaciones Científicas y Técnicas, Agencia Nacional de Promoción Científica y Tecnológica (ANPCyT) and the Universidad Nacional de la Plata for financial support.