The transmission of intestinal parasites is generally considered to be “mediated by the environment” which suggests that they are particularly sensitive to the changes that occur in it. The aim of the present study was to evaluate the environmental variables that act as risk factors for intestinal parasitosis in children and youths in Argentina. The association between environmental variables related to temperature, precipitation and soil and parasitosis found in children and youths from different provinces was evaluated, including land use/cover classes obtained from satellite images. Of the total population analyzed, 66.9% of the participants were parasitized. The total number of identified parasite species was 17 and the most prevalent were Blastocystis sp. (42.2%), Enterobius vermicularis (33.6%) and Giardia lamblia (17.0%). Infection by protozoa, and by G. lamblia in particular, was greater when the mean summer temperature was higher (OR=1.2 for both). Blastocystis sp. and geohelminths were greater due to an increase in isothermality (OR=1.1 and 1.2, respectively). The risk of infection with Ascaris lumbricoides was associated with an increase in the temperature in the wettest quarter (OR=1.2). Hookworm infection was associated with an increase in the normalized difference vegetation index (OR=32.5). Most of participants infected with hookworms lived in areas with abundant arboreal-shrubby and agropastoral use vegetation. The heterogeneous distribution of enteric parasites is indicative of the wide environmental variability of Argentina.
La transmisión de parásitos intestinales se considera, en general, «mediada por el ambiente», lo que sugiere que dichos organismos son particularmente sensibles a los cambios que ocurren en aquel. El objetivo del presente estudio fue evaluar las variables ambientales que actúan como factores de riesgo de parasitosis intestinal en niños y jóvenes de la Argentina. Se evaluó la asociación entre las variables ambientales relacionadas con la temperatura, la precipitación y el suelo, incluyendo las clases de uso/cobertura del suelo obtenidas de imágenes satelitales, y las parasitosis halladas en niños y jóvenes de diferentes provincias. El 66,9% de los individuos incluidos en el análisis estuvo parasitado. Se observaron 17 especies parásitas; las más prevalentes fueron Blastocystis sp. (42,2%), Enterobius vermicularis (33,6%) y Giardia lamblia (17%). La infección por protozoos y, en particular por G. lamblia, fue de mayor magnitud cuando se incrementó la temperatura media de verano (OR=1,2 para ambos). El riesgo de infección por Blastocystis sp. y geohelmintos fue mayor con un aumento de la isotermalidad (OR=1,1 y OR=1,2, respectivamente). El riesgo de infección por Ascaris lumbricoides estuvo asociado con un aumento de la temperatura en el trimestre más húmedo (OR=1,2). La infección por anquilostomideos estuvo asociada con un incremento en el índice de vegetación de diferencia normalizada (OR=32,5). La mayoría de los parasitados con anquilostomideos habitaban áreas con abundante vegetación arbórea-arbustiva y de uso agropastoril. La distribución heterogénea de parásitos intestinales refleja la amplia variabilidad ambiental presente en la Argentina.
Intestinal parasitosis caused by protozoa and helminths (e.g. Giardia lamblia, Blastocystis sp., Ascaris lumbricoides, hookworms – Ancylostoma duodenale and Necator americanus) are the most common infections among neglected infectious diseases23. It is estimated that more than 3500 million people, mainly children from developing countries, are infected with intestinal parasites. Parasitic infections can cause diarrhea and intestinal malabsorption, and in the most severe cases may affect the physical growth and intellectual development of children, as well as the labor productivity of adults12,30.
The transmission of intestinal parasites is generally considered to be “mediated by the environment”, which suggests that they are particularly sensitive to the changes that occur in it11. In this context, several studies showed an association between intestinal parasitosis and environmental factors (e.g. temperature, precipitation, vegetation indices)4–6,16,27. In particular, it is not surprising to observe that the prevalence of parasitic infection varies among locations due to the environmental diversity present in Argentina, with an evidently decreasing trend from north to south and from east to west3,8,9,18–20,28.
Despite the available information that explains the differences in the prevalence of enteroparasitosis, there are limited studies on the relationship with environmental variables4. For this reason, the present study aimed to evaluate the environmental variables that represent risk factors for parasitic infection in children and youth populations in Argentina.
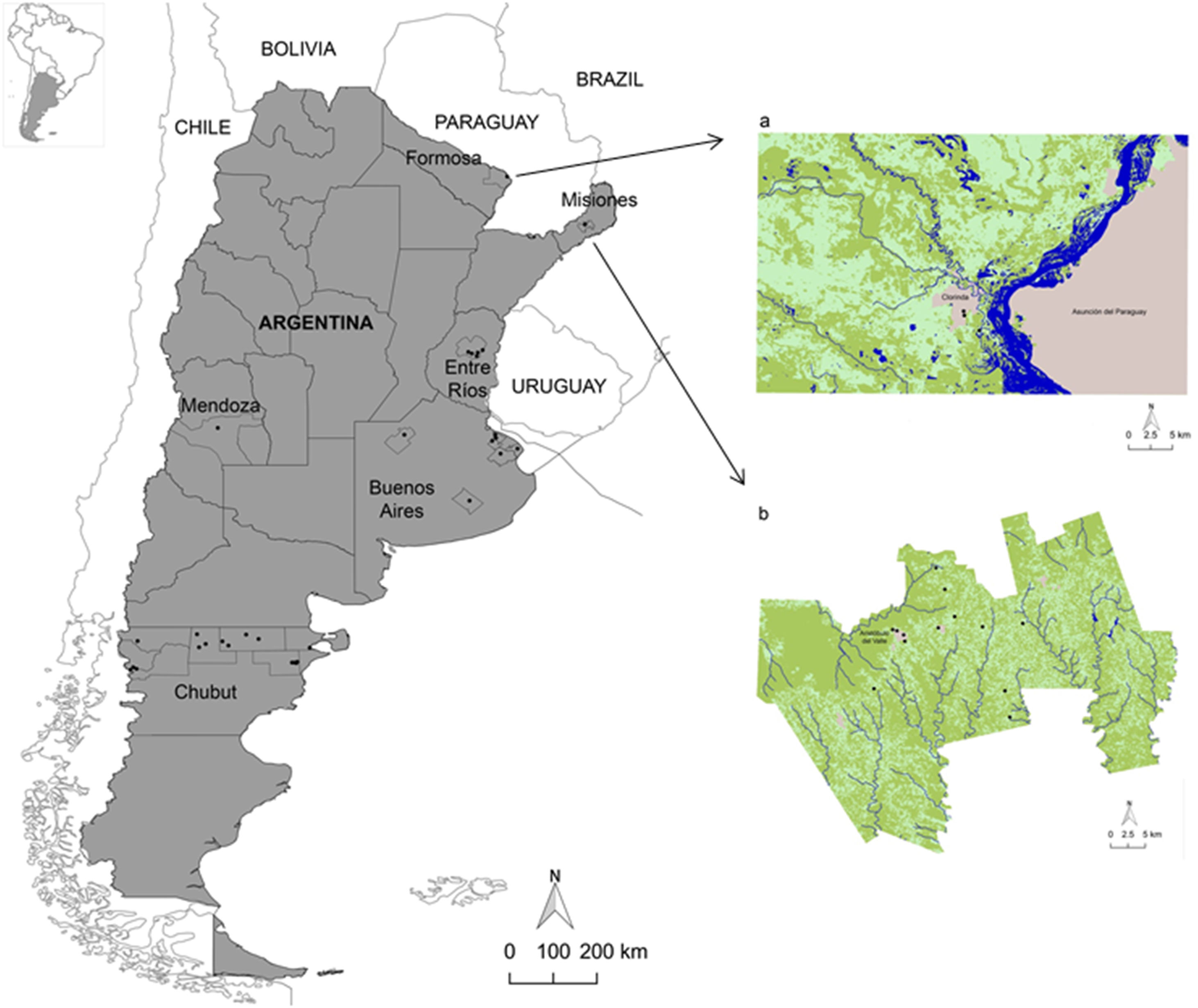
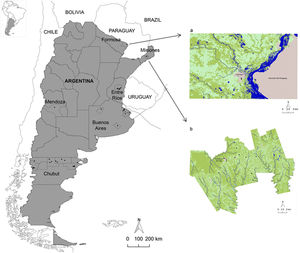
Materials and methodsStudy areaThe study was carried out in six provinces of Argentina: Misiones, Formosa and Entre Ríos (north), Buenos Aires and Mendoza (center) and Chubut (south)3. Populations from urban, periurban and rural areas of Cainguás department (Misiones), Pilcomayo department (Formosa), Villaguay department (Entre Ríos), Berazategui, Brandsen, La Plata, Lincoln, Punta Indio and Tandil departments (Buenos Aires), San Rafael department (Mendoza) and Cushamen, Futaleufú, Gaiman, Gastre, Languiñeo, Telsen and Biedma departments (Chubut) were analyzed (Fig. 1).
Geographic location of Argentina and the provinces analyzed. Distribution of hookworms (Ancylostoma duodenale/Necator americanus) in relation to the land use/cover classes of the study area in Formosa (a) and Misiones (b). Figure 1a shows the Clorinda city and surroundings (Formosa province), including Asunción del Paraguay city (Paraguay), using an unsupervised classification of Landsat 8 OLI image, and Figure 1b shows the Cainguás Department (Misiones province) using an unsupervised classification of Landsat 5 TM image. The land use/cover classes were: water (blue), arboreal-shrubby vegetation (dark green), vegetation of agropastoral use (light green) and urban area (gray). Note the presence of hookworms as black dots.
The provinces were selected due to their contrasting environmental characteristics. Misiones has a temperate tropical-subtropical climate without a dry season, an average annual temperature of 20°C and an annual rainfall ranging between 1600 and 2000mm. The soils are deep, well drained and with extensive vegetation cover. Formosa province has a warm subtropical climate and an evident seasonal thermal amplitude. The average annual temperature is 23°C and rainfall is uniform throughout the year, sometimes exceeding 1200mm. The soils are poorly drained. The study area of Entre Ríos province has average temperatures of approximately 20°C and abundant rainfall that varies between 1000 and 1400mm. The soils are poorly drained. The sites analyzed in Buenos Aires province have a mild-humid to sub-humid climate and warm summers. The annual average temperatures vary between 15 and 18°C and the rainfall is distributed throughout the year, fluctuating between 600 and 1100mm. The soils are rich in nutrients and organic matter and have an excellent agricultural aptitude. In Mendoza province, the climate is semi-arid, temperate-dry with an average annual temperature of 15°C and marked thermal amplitudes. The average annual rainfall is approximately 300mm. Chubut province has a mild to cold and humid climate with strong winds and frequent snowfall, winter rainfall and frost. The average annual temperature is of the order of 10°C and the soils are stony and poor in organic matter.
Parasitological analysisThe study included 3937 individuals (Buenos Aires n=1411, Chubut n=377, Entre Ríos n=268, Formosa n=114, Mendoza n=752, Misiones n=1015). The participants were under 14 years of age and of both sexes (50.5% girls and 49.5% boys). The populations were analyzed between 2005–2008 and 2010–2016 in Buenos Aires; 2010–2013 and 2017 in Chubut; 2010–2012 in Entre Ríos; 2014 in Formosa; 2008–2011 in Mendoza; and between 2005–2008 in Misiones.
Meetings with children, youths and parents were held in different institutions (public schools, community canteens, health-care centers and non-governmental institutions) in order to inform them about the biology of intestinal parasites and strategies to prevent them. Free parasitological tests were offered. Children and youths who had given written and oral consent were included in the study. Each consenting family was provided with two vials for each participant for stool samples and anal swabs for diagnosing intestinal parasites. Samples were collected during 5–7 successive days. They were asked to fill the vial with a nut-sized stool sample each day. Anal swabs were specifically obtained each morning before getting up by rubbing the perianal margins with sterile gauze and the samples were placed in the vial immediately afterwards. Coproparasitological tests were performed using formalin-ethyl acetate concentration, Willis, Sheather and/or FLOTAC Pellet with saturated sodium chloride and zinc sulphate flotation solutions10,13,30. The anal swab technique was used as a specific method for the detection of Enterobiusvermicularis24. The anal-swab vials were agitated vigorously, and the suspensions were centrifuged for 10min at 400×g. Staining with Lugol and Ziehl-Neelsen was used when necessary. Every sample was examined using an optical microscope at 100×, 400× and 1000× magnifications. The identification of parasitic elements was based on their measures and morphological characteristics.
Environmental data extractionHouseholds and institutions to which the analyzed participants belonged (educational institutions, communal and health centers) were georeferenced with geographical coordinates using Google Earth.
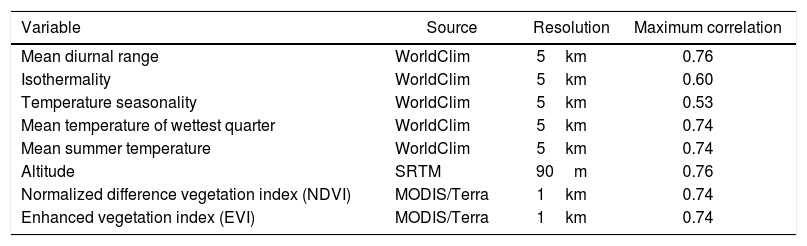
A total of 27 environmental variables were considered based on their perceived biological relevance for survival and dispersal of intestinal parasites. The examined variables were the 19 bioclimatic variables and the precipitation, and the maximum, average and minimum temperature variables from WorldClim for the current climatic conditions (average climate data from 1970 to 2000), soil pH from ISRIC World Soil Information, altitude from the NASA Shuttle Radar Topographic Mission (SRTM), and normalized difference (NDVI) and enhanced (EVI) vegetation indices from MODIS/Terra.
In addition, Landsat images provided by CONAE and USGS were evaluated. The images were selected according to the sampling year and subsequently, they were calibrated atmospherically and radiometrically, and classified by an unsupervised method by k-means7,17. The land use/cover classes obtained were validated using overall accuracy and the Kappa index22, reaching values higher than 95% and 0.8, respectively. Quantum GIS and SoPI software were used.
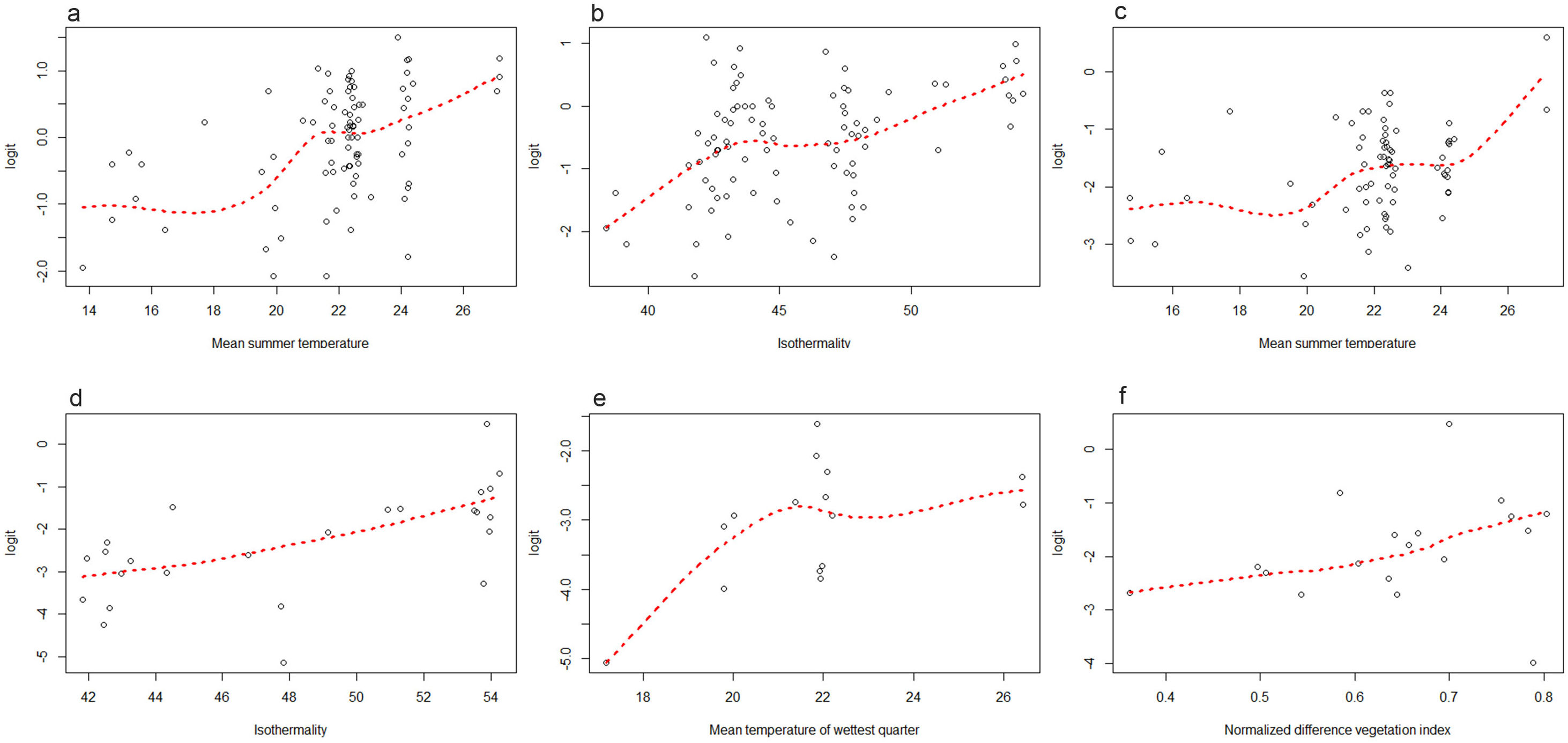
Statistical analysisThe Pearson's test was performed to assess the correlation between the environmental variables, using a correlation coefficient >0.75. Data source, spatial resolution and maximum correlation values of the selected variables are summarized in Table 1. Subsequently, the linearity of each environmental variable with respect to the parasitic logit was analyzed using dispersion diagrams. The variables that showed a linear relationship were evaluated by generalized linear models, considering those that presented a lower Akaike information criterion as better models. The variables that showed a non-linear relationship were analyzed using generalized additive models and it was considered a good model if it presented an explained return greater than 70%. Odds ratio (OR) values and their respective 95% confidence intervals (95% CI) were calculated. The goodness of fit of the final model was evaluated using the Hosmer–Lemeshow test and a p value >0.05 indicated an adequate model. All statistical analyses were performed using the R software.
Overview of environmental variables used to model the risk of intestinal parasitosis in children and youth from Argentina.
| Variable | Source | Resolution | Maximum correlation |
|---|---|---|---|
| Mean diurnal range | WorldClim | 5km | 0.76 |
| Isothermality | WorldClim | 5km | 0.60 |
| Temperature seasonality | WorldClim | 5km | 0.53 |
| Mean temperature of wettest quarter | WorldClim | 5km | 0.74 |
| Mean summer temperature | WorldClim | 5km | 0.74 |
| Altitude | SRTM | 90m | 0.76 |
| Normalized difference vegetation index (NDVI) | MODIS/Terra | 1km | 0.74 |
| Enhanced vegetation index (EVI) | MODIS/Terra | 1km | 0.74 |
WorldClim-Global Climate data (http://www.worldclim.org/).
SRTM-NASA Shuttle Radar Topographic Mission (http://srtm.csi.cgiar.org/).
MODIS/Terra-Moderate Resolution Imaging Spectroradiometer from CONAE (http://catalogos.conae.gov.ar/catalogo/) and US Geological Survey (https://earthexplorer.usgs.gov/).
The study was performed without affecting the physical, psychological and moral integrity of the participants and protecting their identity. This research was approved by the Comité de Ética de la Escuela Latinoamericana de Bioética (CELABE) under Resolution No. 003/2016, Record No. 73. The study was conducted in accordance with the principles proclaimed in the Universal Declaration of Human Rights (1948), the ethical standards established by the Nüremberg Code (1947), the Declaration of Helsinki (1964) and its successive amendments. Special attention was also paid to Article 5 of the Regulation Decree of National Law 25.326.
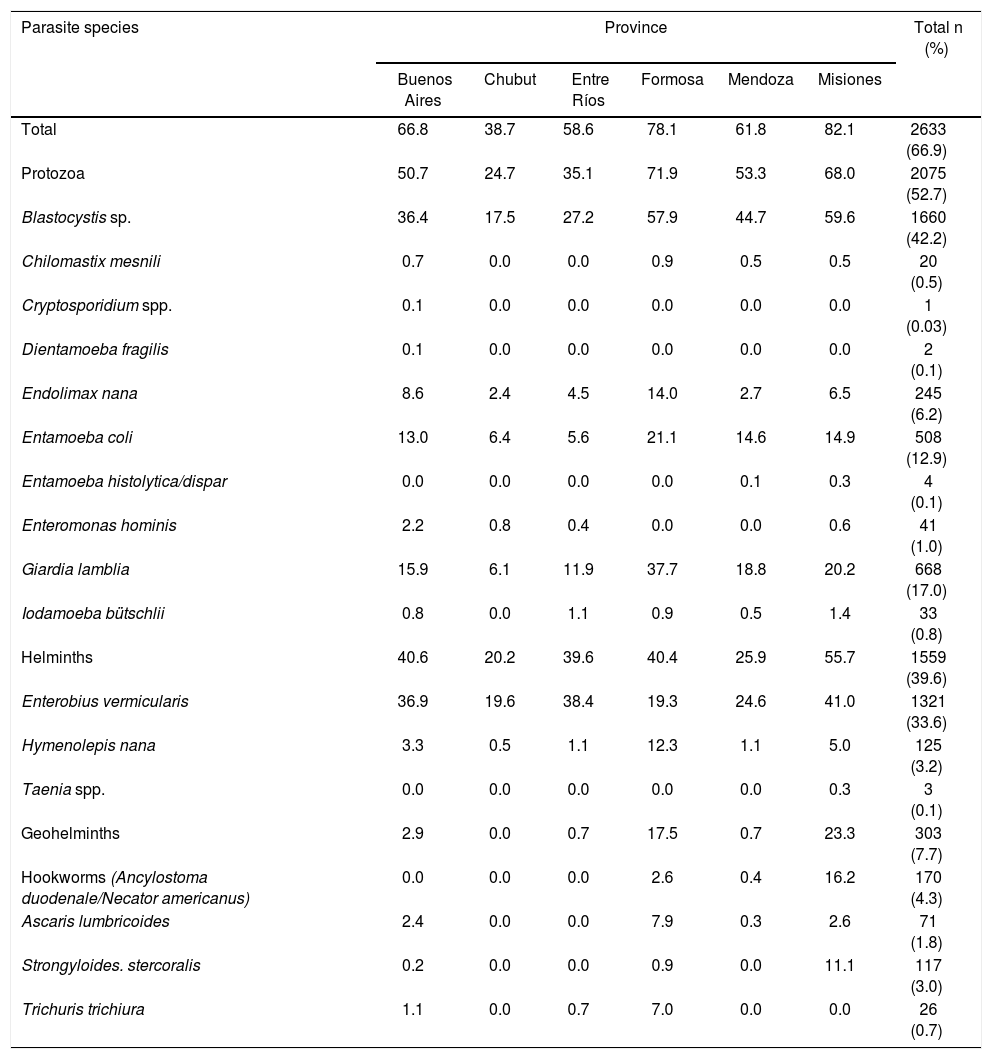
ResultsOf the total population analyzed, 66.9% (2633/3937) were parasitized by at least one parasite species. Protozoan infection was more frequent than helminth infection (52.7% and 39.6%, respectively). The total number of identified parasite species was 17 and the most prevalent ones were Blastocystis sp. (42.2%), Enterobius vermicularis (33.6%) and Giardia lamblia (17.0%). Moreover, 7.7% were parasitized with at least one geohelminth species, hookworms and Strongyloides stercoralis (4.3% and 3.0%, respectively) being the most frequent. Entamoeba coli was the most prevalent non-pathogenic species (12.9%) (Table 2).
Prevalence of parasite species found in the analyzed provinces.
| Parasite species | Province | Total n (%) | |||||
|---|---|---|---|---|---|---|---|
| Buenos Aires | Chubut | Entre Ríos | Formosa | Mendoza | Misiones | ||
| Total | 66.8 | 38.7 | 58.6 | 78.1 | 61.8 | 82.1 | 2633 (66.9) |
| Protozoa | 50.7 | 24.7 | 35.1 | 71.9 | 53.3 | 68.0 | 2075 (52.7) |
| Blastocystis sp. | 36.4 | 17.5 | 27.2 | 57.9 | 44.7 | 59.6 | 1660 (42.2) |
| Chilomastix mesnili | 0.7 | 0.0 | 0.0 | 0.9 | 0.5 | 0.5 | 20 (0.5) |
| Cryptosporidium spp. | 0.1 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 1 (0.03) |
| Dientamoeba fragilis | 0.1 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 2 (0.1) |
| Endolimax nana | 8.6 | 2.4 | 4.5 | 14.0 | 2.7 | 6.5 | 245 (6.2) |
| Entamoeba coli | 13.0 | 6.4 | 5.6 | 21.1 | 14.6 | 14.9 | 508 (12.9) |
| Entamoeba histolytica/dispar | 0.0 | 0.0 | 0.0 | 0.0 | 0.1 | 0.3 | 4 (0.1) |
| Enteromonas hominis | 2.2 | 0.8 | 0.4 | 0.0 | 0.0 | 0.6 | 41 (1.0) |
| Giardia lamblia | 15.9 | 6.1 | 11.9 | 37.7 | 18.8 | 20.2 | 668 (17.0) |
| Iodamoeba bütschlii | 0.8 | 0.0 | 1.1 | 0.9 | 0.5 | 1.4 | 33 (0.8) |
| Helminths | 40.6 | 20.2 | 39.6 | 40.4 | 25.9 | 55.7 | 1559 (39.6) |
| Enterobius vermicularis | 36.9 | 19.6 | 38.4 | 19.3 | 24.6 | 41.0 | 1321 (33.6) |
| Hymenolepis nana | 3.3 | 0.5 | 1.1 | 12.3 | 1.1 | 5.0 | 125 (3.2) |
| Taenia spp. | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.3 | 3 (0.1) |
| Geohelminths | 2.9 | 0.0 | 0.7 | 17.5 | 0.7 | 23.3 | 303 (7.7) |
| Hookworms (Ancylostoma duodenale/Necator americanus) | 0.0 | 0.0 | 0.0 | 2.6 | 0.4 | 16.2 | 170 (4.3) |
| Ascaris lumbricoides | 2.4 | 0.0 | 0.0 | 7.9 | 0.3 | 2.6 | 71 (1.8) |
| Strongyloides. stercoralis | 0.2 | 0.0 | 0.0 | 0.9 | 0.0 | 11.1 | 117 (3.0) |
| Trichuris trichiura | 1.1 | 0.0 | 0.7 | 7.0 | 0.0 | 0.0 | 26 (0.7) |
The highest prevalence of intestinal parasitosis was observed in Misiones (82.1%) and Formosa (78.1%), followed by Buenos Aires (66.8%), Mendoza (61.8%), Entre Ríos (58.6%) and Chubut (38.7%). Protozoan infection was more prevalent in Formosa (71.9%) and Misiones (68.0%), and less prevalent in Chubut (24.7%). Blastocystis sp. was more frequent in Misiones (59.6%) and Formosa (57.9%) and less frequent in Chubut (17.5%). The highest prevalence of infection by G. lamblia was observed in Formosa (37.7%) and Misiones (20.2%), and the lowest prevalence was found in Chubut (6.1%). Geohelminth infection was more prevalent in Misiones (23.3%) and Formosa (17.5%), and was absent in Chubut. Among the geohelminth species, Ascaris lumbricoides and Trichuris trichiura were more prevalent in Formosa (7.9% and 7.0%, respectively), and hookworms and S. stercoralis in Misiones (16.2% and 11.1%, respectively) (Table 2).
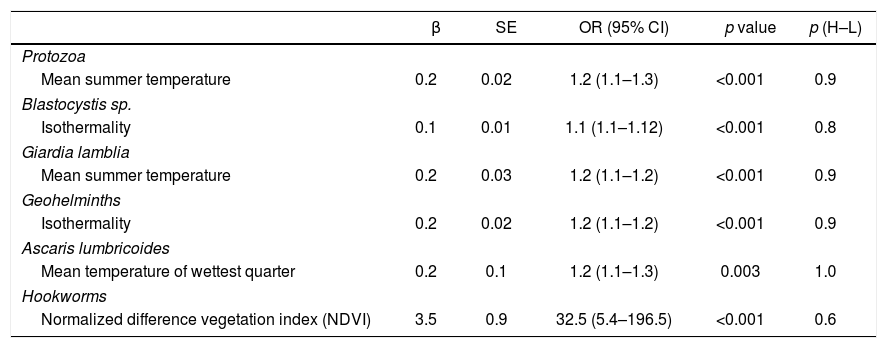
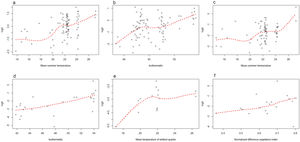
Infection by protozoa, and by G. lamblia in particular, was greater when the mean summer temperature was higher (OR=1.2 for both). On the other hand, it was observed that for each increase in isothermality, the risk of infection by Blastocystis sp. increased (OR=1.1). Isothermality was also associated with an increased risk of geohelminth infection (OR=1.2). The risk of infection with A. lumbricoides was associated with an increase in the mean temperature of the wettest quarter (OR=1.2). On the other hand, infection by hookworms was associated with an increase in NDVI (OR=32.5) (Table 3). Fig. 2 shows the parasitic logit with respect to each environmental variable. In Misiones province, 45.5% of the participants infected by hookworms lived in areas with abundant arboreal-shrubby vegetation, and 36.4% in areas with vegetation of agropastoral use. In contrast, a minor proportion of infected participants lived in urban areas (18.2%). However, in Formosa province, 66.7% of the infected participants lived in urban areas and 33.3% in areas of vegetation of agropastoral use (Fig. 1a and b).
Risk factors for infection with protozoa, Blastocystis sp., Giardia lamblia, geohelminths, Ascaris lumbricoides and hookworms (Ancylostoma duodenale/Necator americanus)a
| β | SE | OR (95% CI) | p value | p (H–L) | |
|---|---|---|---|---|---|
| Protozoa | |||||
| Mean summer temperature | 0.2 | 0.02 | 1.2 (1.1–1.3) | <0.001 | 0.9 |
| Blastocystis sp. | |||||
| Isothermality | 0.1 | 0.01 | 1.1 (1.1–1.12) | <0.001 | 0.8 |
| Giardia lamblia | |||||
| Mean summer temperature | 0.2 | 0.03 | 1.2 (1.1–1.2) | <0.001 | 0.9 |
| Geohelminths | |||||
| Isothermality | 0.2 | 0.02 | 1.2 (1.1–1.2) | <0.001 | 0.9 |
| Ascaris lumbricoides | |||||
| Mean temperature of wettest quarter | 0.2 | 0.1 | 1.2 (1.1–1.3) | 0.003 | 1.0 |
| Hookworms | |||||
| Normalized difference vegetation index (NDVI) | 3.5 | 0.9 | 32.5 (5.4–196.5) | <0.001 | 0.6 |
B: regression coefficient; SE: standard error; OR: odds ratio; CI: confidence interval; p(H-L): p value of Hosmer–Lemeshow.
The present study shows that the intestinal parasitosis found in the populations analyzed are associated with environmental factors related to the temperature and soil characteristics in the different study areas.
The risk of infection by protozoa and, by Giardia lamblia in particular, was associated with the mean temperature in the summer months. Other studies showed an increased risk of giardiasis when the temperature increased1,16. Warm temperatures can prolong the infective period of cysts and facilitate transmission through reservoirs and vectors or through intensified pathogen-host interactions. Likewise, the expected global increase in temperature and rainfall could favor the survival and dispersion of cysts, exposing new populations to parasitic infection. Likewise, an increase in temperature could also favor infection by other pathogens (e.g. Cryptosporidium spp.), which have the same mode of transmission and that have been positively associated with higher temperatures16.
The risk of infection by Blastocystis sp. and geohelminths was positively associated with isothermality. It is known that extreme temperature conditions could adversely affect the survival of parasitic forms present in the environment. On one hand, the cystic form of Blastocystis sp. can survive for one month at room temperature and two months at 4°C. Although this is the most resistant form of Blastocystis sp., this parasite is affected by extreme temperatures. In contrast, the vacuolar form frequently found in feces is highly sensitive to changes in temperature14,29. On the other hand, embryonic development of geohelminths occurs within defined temperature limits between 28 and 32°C, suggesting that extreme values could inhibit the development and therefore the infectivity of these species2.
It has been observed that as the mean temperature in the wettest quarter increased, the risk of infection with Ascaris lumbricoides was greater. Similarly, Chammartin and collaborators observed that the risk of infection was higher due to an increase in the mean temperature in the warmest quarter6. Similar results were found in Malaysia, where the mean soil surface temperature was positively associated with the infection; however, the minimum and maximum temperatures showed a negative association21. Likewise, Brooker and collaborators reported that in Uganda the highest prevalence was observed in areas with temperatures of 30°C and it decreased in areas where the temperature exceeded 38°C2. This result was attributed to the effects of extreme heat and insufficient humidity on egg survival and embryonic development.
The risk of infection with hookworms became higher as the NDVI increased. Similar results were found in Brazil and South Africa26,27. In contrast, a negative association was observed between the NDVI and hookworm infection; however, in China, a positive association with A. lumbricoides and Trichuris trichiura infection was reported15. However, other studies stated that geohelminthiasis is generally frequent in areas with forest cover because forests provide shade and prevent desiccation of the soil surface, thus protecting the larvae from solar radiation21,25.
In Argentina, a bibliographic review conducted by Juárez and Rajal (2013) reported that there is a coincidence between the parasite species found in both fecal and environmental samples12. This finding suggests that parasites that can cause intestinal disorders in humans can be isolated from samples of water and soil that are exposed to different environmental conditions. The intestinal parasites found in this study are indicative of the different climatic-environmental conditions present in Argentina. The present study generated knowledge about the environmental factors that are associated with an increased risk of parasitic infection. Thus, it strengthens the development of control and prevention strategies that would improve the quality of life of the most vulnerable populations.
FundingThis work was funded by the Universidad Nacional de La Plata (UNLP-11/N 679 y N759), Agencia Nacional de Promoción Científica y Tecnológica (PICT 1541) and Consejo Nacional de Investigaciones Científicas y Técnicas (PIP 734, PIO N13420130100004CO).
Conflict of interestThe authors declare that they have no conflicts of interest.
The authors are grateful to the local authorities, the educational community and the participants of this study for their cooperation. We are also thankful to Graciela Minardi for their help with the statistical analyses and to Evelia Oyhenart for the help given in the samples collection.