In Argentina there are no reports on Aspergillus fumigatus fumagillin-producing strains. In this study we describe the isolation and mycotoxin production capacity of ten A. fumigatus strains isolated from farm and clinical samples. Farm strains were isolated from milk samples taken from dairy cows in Córdoba province, some of which were associated with subclinical mastitis. A culture medium was defined to optimize fumagillin production and a detection method was developed by HPLC chromatography. It is known that in addition to the host immune status, strain virulence is a fundamental characteristic that will determine its pathogenicity and, in this sense, fumagillin is considered to be among the virulence factors. In the present work, all the strains tested for the production of fumagillin were able to synthesize it, highlighting that the strain A. fumigatus RC2243, from a milk sample from a cow with clinical mastitis, was the most productive. The existence of fumagillin-producing strains represents a potential risk of mycotoxins being transferred to raw milk, constituting a public health risk.
En Argentina no existen reportes sobre cepas de Aspergillus fumigatus productoras de fumagilina. En este trabajo se describe el aislamiento y la producción de dicha micotoxina por 10 cepas, provenientes del medioambiente rural y aisladas de muestras clínicas. Las cepas de origen rural fueron aisladas de vacas lecheras en tambos de la provincia de Córdoba, y algunas de esas cepas se asociaron a casos de mastitis subclínica. Se definió la composición de un medio de cultivo para optimizar la producción de fumagilina y se desarrolló un método de cromatografía HPLC para su determinación. Es conocido que, además del estado inmunitario del huésped, la virulencia de la cepa es una de las características fundamentales que determinan su potencial patogénico y, en este sentido, la fumagilina es considerada un factor de virulencia. En el presente trabajo todas las cepas estudiadas fueron capaces de sintetizarla y la cepa A. fumigatus RC2243, proveniente de leche de una vaca con mastitis subclínica, se destacó como la cepa más productora. La existencia de cepas productoras de fumagillina representa un riesgo potencial por el pasaje de dicha micotoxina a la leche, lo cual constituye un problema para la salud pública.
Aspergillus fumigatus is a worldwide distributed saprophytic fungus and an opportunistic pathogen for human and animals. This fungus is of great importance to public health because it is capable of producing tremorgenic mycotoxins and inducing neurological syndromes in contaminated food handlers, both in maize silage and other contaminated animal feeds as well as in rural workers5,8. Aspergillus fumigatus pathogenicity depends not only on the host immune status but also on the virulence of the fungal strain, which is not given by a single factor of essential virulence, but is under polygenetic control. It is known that A. fumigatus produces several immunosuppressive mycotoxins including gliotoxin, fumagillin, helvolic acid, fumitoxins, fumiclavines A and C, fumitremorgins, verruculogen, among others. All these mycotoxins inhibit the function of leukocytes in terms of migration, superoxide production and fungicidal activity9. Among them, fumagillin has the ability to inhibit endothelial cell proliferation acting as an anti-angiogenic factor, and suppress neutrophil function2. Our research group has demonstrated that there were no differences between animal and human isolated strains from aspergillosis cases8. In this way, they may become pathogenic to cows and also to farm handlers. In Argentina, there are currently no reports on the production capacity of fumagillin in native strains. The presence of A. fumigatussensu stricto able to produce other mycotoxins such as fumagillin in raw cow milk could be a very important risk factor since milk and its by-products are destined for human consumption, therefore the aim of this study was to describe the fumagillin production ability of isolated Argentinean A. fumigatussensu stricto strains from raw cow milk and clinical samples.
Strains correspond to a broad sampling conducted between March and September 2017; the animals sampled came from 44 dairy establishments located in Córdoba province, in the central region of Argentina. Each one of these establishments owned between 100 and 250 milking cows with daily average milk production of 10–20l/cow. Every dairy establishment was visited once during the morning milking. In each herd, between 20% and 40% of the milking animals (Holando-Argentino breed) were randomly sampled in accordance with the instructions recommended by the National Mastitis Council (2004)6. A total of 901 individual milking cows were sampled. The udders were disinfected with ethanol 70%, dried with individual paper towel and the first jet of milk discarded. As a result of this sampling, several fungal strains were isolated and identified. Seven of them, corresponding to A. fumigatus isolates were deposited in Universidad Nacional de Río Cuarto, Córdoba, Argentina Collection Center (RC). Three strains isolated from human pulmonary aspergillosis were also added to the collection. All the strains were confirmed as A. fumigatussensu stricto species by morphological and molecular characterization, by sequencing the benA gene of selected strains and by a maximum parsimony analysis8.
Strains were subcultured on malt extract agar (MEA) and incubated at 25°C for 7 days in darkness. Three agar plugs of 6mm diameter (approx. 85mm2 of surface) were removed from different points of the colony to represent its variability, then weighed and introduced into a 250ml Erlenmeyer flask containing 50ml of YES medium (sucrose 150g/l, yeast extract 20g/l modified with 0.5g/l of SO4Mg·7H2O, in distilled water) at 25°C for 7 days.
The selected base medium for fumagillin production was developed by Peterson and Goldstein (1957) (saccharose 30g/l, starch 15g/l, NaNO3 3g/l, K2HPO4 1g/l, MgSO4·7H2O 0.5g/l, KCl 0.5g/l, FeSO4·7H2O 0.01g/l) modified by the addition of carboxymethylcellulose (CMC) 10g/l to obtain small pellets10. Cultures were grown at 30°C for 6 days in an orbital shaker at 220rpm (Heidolph Rotamax 120, Heidolph Instruments GmbH, Schwabach, Germany). A 5ml aliquot of broth was transferred to centrifuge tubes, with 5ml of methanol; the extraction process was carried out at 30°C and 220rpm for 3h. The obtained suspension was centrifuged at 3000×g for 10min, supernatant was filtered through a membrane filter (0.45μm) and dried under nitrogen stream. The extracts were resuspended in 1ml of acetonitrile for HPLC analysis.
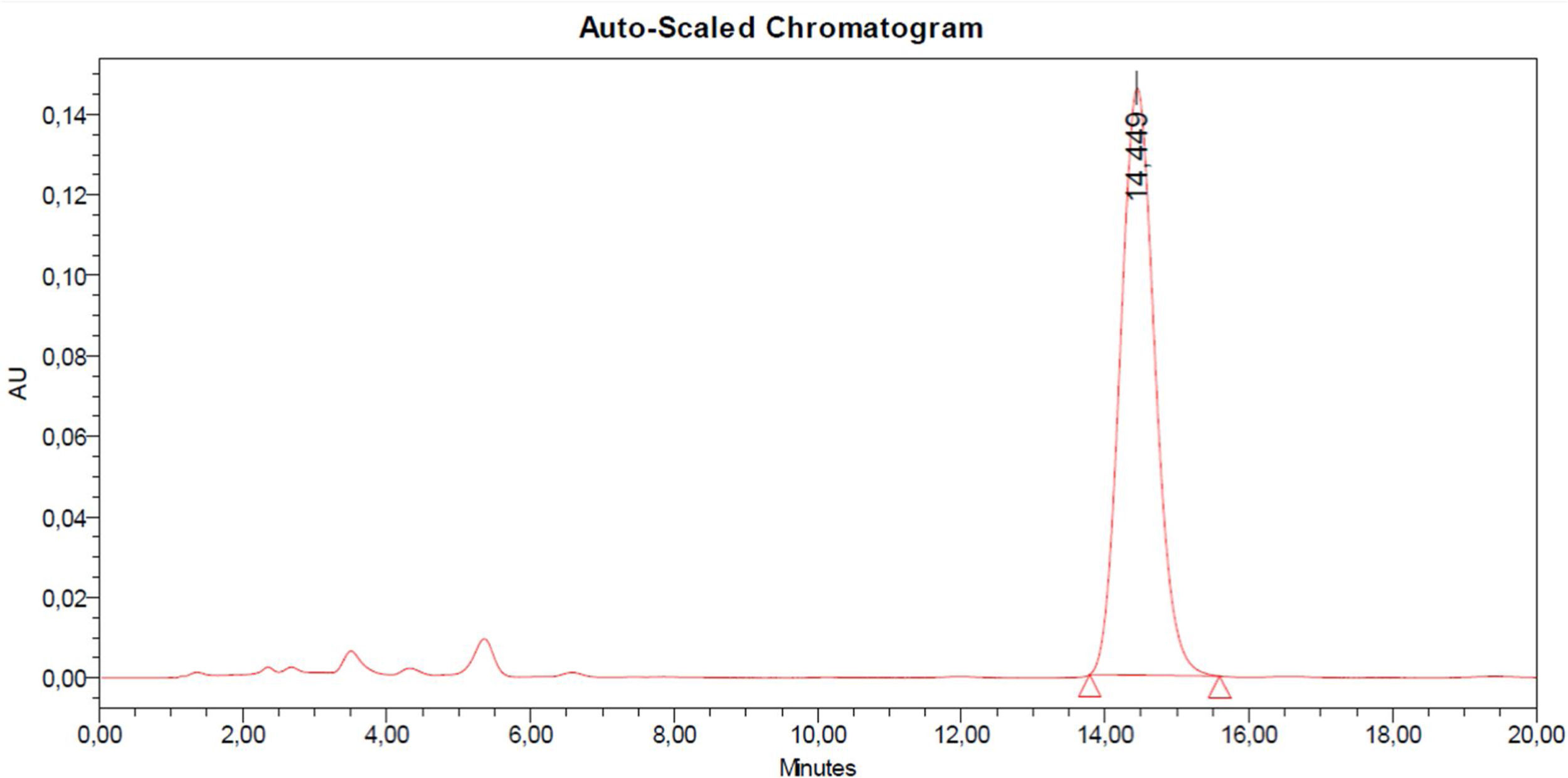
For obtaining the calibration curve, 1mg pure fumagillin (Sigma USA) was dissolved in 1ml acetonitrile HPLC grade (Sintorgan S.A.). A stock solution of fumagillin 10μg/m and the working solutions of 100, 50, 20, 10, and 5ng/ml were prepared. A Waters e2695 HPLC was used, with a 20μl loading loop and equipped with a diode array detector Waters 2998; separations were carried out on a C18 Phenomenex Luna RP C18 column (150mm×4.6mm, 5μm particle size, Phenomenex Inc, CA, USA) at 25°C. An isocratic mode was used, as the mobile phase, acetonitrile (Sintorgan S.A., Argentina) and water (Sintorgan S.A., Argentina) with 1% acetic acid were used in a 50/50 v/v ratio. The flow for the mobile phase was 1ml/min and the wavelength used was 351nm. Retention time was 15min. The fumagillin concentrations were determined from the areas obtained as a function of the calibration curve. All the strains investigated were able to produce fumagillin at levels between 4.20 and 16.79μg/ml (Table 1). A chromatogram of the fumagillin peak corresponding to the injection of 20μl of pure standard (10μg/ml) can be observed at 14.5min (Fig. 1). The analyzed strains from raw cow milk samples showed a variable production capacity of fumagillin. The strain A. fumigatus RC2243, from a cow milk sample with clinical mastitis, showed the highest production capacity among all the tested strains. Strains from healthy cow samples yielded intermediate values between 9.23 and 9.97μg/ml, while the lowest level was shown in A. fumigatus RC2251 strain, isolated from a subclinical mastitis sample. With the purpose of comparing the toxic potential of strains from different origins, three strains of A. fumigatussensu stricto from human pulmonary aspergillosis were included in the study. Among these strains, one of them had the highest production capacity (12.74μg/ml) while the others had moderate fumagillin production capacity. Pellegrino et al. (2013)7 conducted a mycological survey of the milk samples from which the strains of the current study were isolated and found that A. fumigatus was the second more frequently isolated species of Aspergillus after A. flavus. Moreover, they demonstrated the gliotoxinogenic ability of the strains and its association with high somatic cell counts in milk samples. The results obtained in the present work constitute a first step in the knowledge of the production capacity of fumagillin from A. fumigatussensu stricto gliotoxin producer strains obtained from animal and human samples in Argentina. Boudra and Morgavi (2005)1 analyzed the toxicogenic capacity of A. fumigatus strains in different natural feed substrates (wheat, corn, barley, triticale, rye grass, orchard grass, and tall fescue); fumagillin production levels up to 25.9μg/ml were obtained after six days of development in a medium other than that used in the present study (minimal essential medium Eagle supplemented with fetal bovine serum and glucose). In another work, the growth and production of fumagillin by A. fumigatus NRRL 2436 was measured in Peterson-Goldstein medium modified with carboxymethylcellulose and similar fumagillin levels to ours were obtained (average of 13.4mg/l)10. The medium used in the present work was useful to perform a screening among strains in order to identify those with a capacity to produce fumagillin. This knowledge is relevant due to the well-known antiangiogenic effects of fumagillin that reduce the proliferation of endothelial cells and blood vessel formation4 probably related to the process of A. fumigatus pathogenesis in invasive aspergillosis. Guruceaga et al. (2018)3 showed the gene-expression and metabolite bioactivity of the fumagillin/pseurotin gene cluster during invasive lung aspergillosis after intranasal exposure to A. fumigatus conidia using a murine model. They concluded that fumagillin contributed to tissue damage during invasive aspergillosis, and allowed A. fumigatus progress by the lungs through angioinvasion and disruption of the lung parenchymal structure. Furthermore, it has been shown that fumagillin inhibits neutrophil function contributing to the reduction of the local immune response2. These facts confirm or increase the risk posed by the workers’ exposure to fumagillin contamination. Although fumagillin toxicity to humans is important because it poses a direct risk to the consumer, this mycotoxin is on the list of products approved for use in beekeeping in several countries including Argentina, according to SENASA regulations (Cert. No. 97.117) for the treatment of nosema disease (Nosema apis and Nosema ceranae) and is commonly used. In the present work, all the strains tested for the production of fumagillin were able to synthesize it, highlighting that the strain A. fumigatus RC2243, from a milk sample of a cow with clinical mastitis, was the most productive. It should be noted that the strains isolated and studied in this work came from the animal environment, specifically the dairy farm environment, all of which are able to produce at least two of the most important mycotoxins that have been identified in A. fumigatus. This increases the possibility of being transferred to milk and polluting the environment in which they develop, constituting a public health risk. It is necessary to conduct specific trials for investigating the efficiency of different mycotoxin adsorbents and biodegradation products against several toxins that are mainly detected in forage and avoid its transfer to the human food chain.
The authors declare that they have no conflicts of interest.
This work was supported by grants from FONCyT-PICT2033/15.