Streptococcus pneumoniae is an important causal agent of pneumonia, meningitis, sepsis, bacteremia, and otitis media. Penicillin resistance rates in S. pneumoniae have remained stable in Argentina in the last years. In the late ‘90s more isolates with MIC of penicillin ≥2μg/ml were observed; however, their frequency has decreased in recent years. The phenotypic expression of penicillin resistance is due to a modification in penicillin-binding proteins associated with a mosaic structure in the coding genes. The expansion of successful resistant clones varies among the different regions and is influenced by the use of antibiotics, vaccines, particularly conjugated ones, as well as population density. Parenteral treatment with high doses of penicillin G continues to be effective for the treatment of pneumonia and bacteremia, oral aminopenicillins for otitis media and sinusitis and third generation cephalosporins for meningitis.
Streptococcus pneumoniae es un importante agente causal de neumonía, meningitis, sepsis, bacteriemia y otitis media. En la Argentina, las tasas de resistencia a penicilina en Streptococcus pneumoniae se mantuvieron estables durante los últimos años. Hacia finales de los años noventa, un mayor número de aislamientos presentaban CIM de penicilina ≥2μg/ml, pero su frecuencia disminuyó en los últimos años. La expresión fenotípica de resistencia a penicilina se debe a una modificación en las proteínas de unión a la penicilina, asociada a una estructura en mosaico en los genes codificantes. La expansión de clones resistentes exitosos varía entre diferentes regiones y está influida por el uso de antibióticos y de vacunas, particularmente de las conjugadas, así como por la densidad de la población. El tratamiento por vía parenteral con altas dosis de penicilina G continúa siendo eficaz para el tratamiento de las neumonías y bacteriemias, con aminopenicilinas por vía oral para otitis media y sinusitis, y con cefalosporinas de tercera generación para meningitis.
Streptococcus pneumoniae is a human pathogen whose reservoir is the nasopharynx. It is the main causative agent of community pneumonia in children under 5 years of age and older adults. It is also responsible for other pathologies such as meningitis, sinusitis and acute otitis media.
Basically, all strains of S. pneumoniae have a polysaccharide capsule, which is the basis for serotyping. There are one hundred diverse capsular types14,15.
The use of pneumococcal conjugate vaccines in children including a limited number of serotypes (PCV7, PCV10 and PCV13) has modified the epidemiology of pneumococcal infections. Since the inclusion of PCV7 in the year 2000 in vaccination programs, the incidence rate of pneumococcal disease caused by the serotypes present in the vaccine has decreased significantly in many countries13,34. In some countries, the widespread use of vaccines has been associated with a replacement of serotypes included in the vaccine by non-vaccine serotypes, both in carriage and invasive disease, with the consequent modification in the antibiotic resistance profile11,43.
The aim of the present review is to describe the mechanisms and the prevalence of β-lactam resistance in S. pneumoniae and their impact on treatment in the current scenario of vaccination especially focusing on the situation in Argentina.
Origin of β-lactam resistance in S. pneumoniaeS. pneumoniae has been susceptible to penicillin since the discovery of this antimicrobial in the early 1940s until 1965, when the first penicillin-non-susceptible S. pneumoniae strain was described in the USA25.
At the end of the ‘70s, strains of S. pneumoniae displaying resistance to penicillin were described in South Africa26 and Spain27. In the early ‘90s, the emergence and spread of penicillin-resistant clones of S. pneumoniae across Europe, Asia, North America and Latin America was associated with an increase in antibiotic consumption3,19. In Argentina the first penicillin-resistant pneumococci were isolated at the “Sor María Ludovica” Children's Hospital in La Plata, Buenos Aires in 19812. According to a review of Lopardo and Fossati, penicillin resistance increased from 17.0% in 1994 to 43.2% in 1996 and has remained between 30% and 40% so far29. However, notable changes were observed in the percentages of strains with MIC ≥2μg/ml, with peaks recorded in the late ‘90s and almost absent in recent years. In general, susceptibility to third generation cephalosporins behaved in parallel to that of penicillin regarding their MICs. However, considering the relative difference between its cut-off points, it can be observed that the percentage of non-susceptible third generation cephalosporins is currently negligible in most Argentine provinces29. Based on SIREVA II data, penicillin resistance was 39.7% and ceftriaxone was 2,6% in 2019 in S. pneumoniae isolated from children under 6 years of age with invasive disease, and they remained stable in the last years. In cases of meningitis in this age group, resistance to penicillin was 34.5% and 0% to cefotaxime1.
Ten years ago, 15–30% of S. pneumoniae worldwide were multidrug-resistant, defined as strains resistant to three or more classes of antimicrobials30. These profiles are much less common after the introduction of conjugate vaccines24. Some of the serotypes included in conjugate vaccines have nearly disappeared and have largely been replaced by non-vaccine serotypes (NVTs) in countries with routine vaccination, as is the case of the multidrug-resistant 19A serotype after PCV7 implementation11,35. NVTs increased in Argentina after PCV13 introduction in 2012, mainly associated to serotypes 24, 12F and 23B. Serotype 24 represents the main NVT in <2 year-old-children with invasive pneumococcal disease and is strongly associated with MDR12. As for serotype 19A, penicillin resistance was 65% but MDR accounted for only 9% of the isolates. MDR was associated with minor clonal types and not with the main clones detected: ST1131 (a single locus variant of ST172), which represented 54.5% and ST8121 (a single locus variant of ST199), which represented 11.4% of pneumococcal resistant population11.
Penicillin-binding proteins and other proteins involved in β-lactam resistancePenicillin-binding proteins (PBPs), the target of β-lactam antibiotics are membrane-bound enzymes catalyzing late steps in peptidoglycan biosynthesis. S. pneumoniae contains six PBPs: class A high molecular mass (HMM) PBP1a, PBP1b and PBP2a; class B HMM PBP2x and PBP2b; and low molecular mass (LMM) class 3 PBP3. Class A enzymes are multimodular proteins exhibiting transglycosylase and transpeptidase activity. Class B PBPs (PBP2x and PBP2b) function as transpeptidases, and PBP3 has a D,D-carboxypeptidase activity, involved in the maturation of peptidoglycan. The three class A enzymes are individually dispensable, while PBP1a or PBP2a are required for in vitro growth. PBP3 is not essential; however, mutants defective in PBP3 exhibit aberrant morphologies. Class B PBPs are essential: PBP2b is important for elongation and PBP2x is critical in division37.
β-Lactam resistance in S. pneumoniae is due to the acquisition of mutations within the pbp2b, pbp2x, and pbp1a genes, causing altered PBP2b, PBP2x, and PBP1a, with decreased affinity for β-lactams. Exogenous DNA provided by β-lactam-resistant Streptococcus strains sharing the same niche is incorporated into the chromosome by homologous recombination. Therefore, S. pneumoniae resistant strains exhibit PBPs with a mosaic structure, as a result of intraspecies and interspecies gene transfer, especially from species such as Streptococcus mitis and Streptococcus oralis, cohabiting the nasopharynx as their ecological niche6. Consequently, the sequences of resistant S. pneumoniae isolates exhibit blocks of sequences of different lengths that can differ up to 20% at the DNA level and up to 10% at the amino acid level compared to the corresponding regions in penicillin-susceptible isolates. The altered sequence reduces the affinity of the transpeptidases for the β-lactams without affecting their enzymatic function, conferring an advantage in the presence of the antibiotic22. By contrast, the nucleotide sequence of pbp genes in susceptible isolates is highly conserved. The absence of homologous recombination between commensal streptococci and S. pneumoniae in pbp genes in susceptible strains may be due to reduced fitness of strains displaying a mosaic structure in the absence of antibiotic selection pressure22.
Additionally, non-pbp genes such as the murMN operon, have been described to play a role in penicillin resistance. This operon encodes transferases that add an L-Ala-L-Ala cross-bridge extension to the L-Lys residue of the peptidoglycan stem. The mechanism involved in the contribution of these extensions to β-lactam resistance was not completely clarified10.
Molecular epidemiology of global resistant clonesSince the 1970s, it has been described that resistant isolates tend to be members of a small number of lineages, some of which have spread around the globe. The expansion of these successful S. pneumoniae resistant clones varies considerably between different geographic regions and is influenced by patterns of antibiotic use, population density and by the use of vaccines. This feature was revealed for the first time with the expansion of a 23F serotype clone with resistance to several antibiotics, which was disseminated from Spain in the 1980s33. In 1997 a molecular epidemiology network was established for S. pneumoniae: The Pneumococcal Molecular Epidemiology Network (PMEN, www.pneumogen.net), supported by the International Union of Microbiology Societies, in order to standardize the nomenclature and classification of resistant pneumococcal clones throughout the world. The nomenclature of the clones was defined according to the country where the strain was firstly identified, followed by the serotype of the initial strain and the clone number assigned by the network. For example, the Spain 23F-1 clone, was initially serotype 23F strain, then variants of this clone have been registered in several parts of the world including strains expressing serotypes 19F, 14, 19A, 9N, 3 and serogroup 632. These variants usually arise by recombination at the cps locus, which is defined as capsular switching.
Something similar occurred with the Spain 9V-3 clone which represented 82% of S. pneumoniae isolated in Argentina between 1993 and 1996 from children under 6 years of age with invasive pneumococcal disease. However, unlike what happened in France or Spain, in Argentina most of the strains of the Spain9V-3 clone expressed serotype 1436.
Moreover, molecular studies have shown that populations of penicillin-resistant pneumococci such as multidrug-resistant pneumococci are highly dynamic and this resistance is the combination of the dispersion of certain clones, loss or acquisition of resistance genes within those lineages and the propagation of resistance determinants to new lineages30. Recently, a Global Pneumococcal Sequencing Project (GPS, https://www.pneumogen.net/gps/index.html) was initiated aiming at the application of whole genome sequencing to create an international genome-based scheme for defining the pneumococcal population structure. This strategy allows to characterize and compare lineages across countries, giving international context to serotype, antibiotic resistance and geographical spread17.
Susceptibility tests and interpretation criteriaAs the identification of a penicillin-resistant isolate of S. pneumoniae may modify the empirical antibacterial therapy, the early determination of S. pneumoniae antimicrobial susceptibility is of great importance in the clinical setting18. Phenotypic susceptibility methods based on bacterial culture remain the gold-standard approach in clinical laboratories. According to the CLSI recommendations, the 1μg-oxacillin-disk-diffusion test is an effective screening method commonly used for the detection of penicillin resistance in pneumococci. Isolates with zones of inhibition ≥20mm in the 1-μg oxacillin-disk-diffusion test correlate with a penicillin MIC of ≤0.06μg/ml being reported as susceptible, and predict susceptibility for ampicillin (oral or parenteral), ampicillin–sulbactam, amoxicillin, amoxicillin–clavulanate, cefaclor, cefdinir, cefditoren, cefepime, cefotaxime, cefpodoxime, cefprozil, ceftaroline, ceftizoxime, ceftriaxone, cefuroxime, doripenem, ertapenem, meropenem, imipenem, loracarbef8. Penicillin, cefotaxime, ceftriaxone or meropenem minimum inhibitory concentrations (MICs) should be determined for isolates with oxacillin zone diameters ≤19mm because zones ≤19mm occur with penicillin-resistance, -intermediate or certain -susceptible strains8.
The oxacillin-disk-diffusion test is a useful tool in predicting penicillin resistance in cases of meningeal strains of S. pneumoniae, particularly in low- and middle-income countries, where MIC determination is not routinely available18.
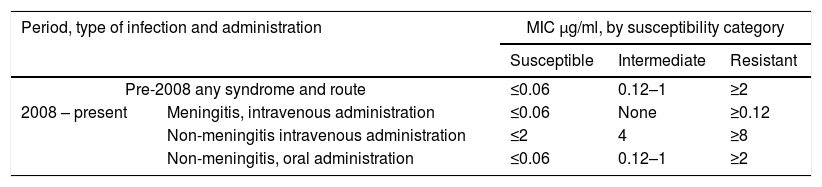
Until 2007 the breakpoints used for the interpretation of MICs for penicillin defined by the CLSI were ≤0.06μg/ml susceptible, 0.12–1μg/ml intermediate, ≥2μg/ml resistant. These values were based on concentrations that could be reached in cerebrospinal fluid. In 2008, as a result of the successful treatment of non-meningitis infections caused by strains categorized as intermediate, new breakpoints were defined by the CLSI7. Consequently, different breakpoints have to be applied for the parenteral use of penicillin depending on the presentation of the S. pneumoniae disease (non-meningitis or meningitis). For oral application, the old interpretive criteria were maintained (Table 1). Moreover, interpretive criteria of the European Committee on Antimicrobial Susceptibility Testing (EUCAST) also specify different breakpoints for meningitis and non-meningitis cases39,40.
Clinical and Laboratory Standards Institute penicillin breakpoints for Streptococcuspneumoniae8
| Period, type of infection and administration | MIC μg/ml, by susceptibility category | |||
|---|---|---|---|---|
| Susceptible | Intermediate | Resistant | ||
| Pre-2008 any syndrome and route | ≤0.06 | 0.12–1 | ≥2 | |
| 2008 – present | Meningitis, intravenous administration | ≤0.06 | None | ≥0.12 |
| Non-meningitis intravenous administration | ≤2 | 4 | ≥8 | |
| Non-meningitis, oral administration | ≤0.06 | 0.12–1 | ≥2 | |
MIC, minimum inhibitory concentration.
These changes allow clinicians to choose whether to use penicillin instead of broad-spectrum antimicrobials to treat penicillin-susceptible non-meningitis pneumococcal infections.
β-Lactams in the treatment of pneumococcal infectionsPenicillin has classically been the first-choice antibiotic for treating pneumococcal infections9. The emergence of resistance and the spread of resistant clones of different serotypes, such as 19A, following the introduction of conjugate pneumococcal vaccines challenged the management of these infections20,21.
Rates of penicillin-resistant S. pneumoniae have decreased significantly since 2008, when new penicillin susceptibility breakpoints were published9.
Current guidelines recommend empirical, broad-spectrum antibiotic therapy for acute bacterial infections with consideration of the common etiologic pathogens, probability of pneumococcal involvement, and antibiotic resistance trends in the local geographic area4,5,38,41. Use of narrow-spectrum agents, such as penicillin, is encouraged to prevent the spread of antimicrobial resistant S. pneumoniae and the spread of methicillin-resistant Staphylococcus aureus and Clostridium difficile, which can result from using broader-spectrum antimicrobials16,20,31.
High-dose penicillin G and many other agents administered parenterally continue to be efficacious for pneumonia and bacteremia. However, resistance has limited treatment options for meningitis and for infections treated with oral agents, particularly in children.
For bacteremic pneumonia in hospitalized children without underlying conditions, penicillin, ampicillin, or cefuroxime could be adequate antibiotics when infections are caused by isolates with penicillin MICs of ≤2μg/ml23. In children, oral monotherapy with amoxicillin, cefuroxime, or cefdinir should be effective after initial parenteral therapy23. As empirical treatment, amoxicillin at high doses should be used as first-line therapy for previously healthy, appropriately immunized children <5 years of age with mild to moderate community acquired pneumonia (CAP) suspected to be of bacterial origin, such as S. pneumoniae is the most prominent invasive bacterial pathogen. For community-acquired pneumonia in adult outpatients (under 65 years of age) without comorbidities, the Argentine Society of Infectology (SADI) recommends amoxicillin as an empirical antibiotic treatment; for patients ≥65 years of age or with comorbidities: amoxicillin–clavulanic acid/ampicillin–sulbactam; in hospitalized patients in the general ward: ampicillin–sulbactam with or without clarithromycin and for patients admitted to intensive care unit: ampicillin–sulbactam plus clarithromycin28.
Although third-generation cephalosporins such as ceftriaxone and cefotaxime have excellent activity against pneumococcus, SADI discourages their routine use for pneumonia due to the significant epidemiological impact they produce by selecting and inducing important bacterial resistance mechanisms. If the isolate has a penicillin MIC of 4μg/ml, clindamycin, linezolid or vancomycin are recommended; however, this recommendation might be questioned since most present-day invasive strains with this level of penicillin resistance are also clindamycin-resistant24,28.
Except for meningeal infections, in which antimicrobial concentration in the cerebrospinal fluid is essential, the intermediate resistance to β-lactams does not influence mortality, provided appropriate dosages are used. However, high-grade resistance can result in therapeutic failure. Penicillin resistance has not been related to more severe clinical forms or to a higher incidence of suppurative complications9.
The empiric treatment in cases of suspected pneumococcal meningitis currently includes intravenous ceftriaxone and vancomycin, pending culture results9,41–43 to overcome ceftriaxone-resistant disease. Addition of vancomycin bears a risk of adverse events. It had been previously suggested that in the epidemiologic setup of low ceftriaxone resistance (<1%), it may be suitable to treat suspected pneumococcal meningitis cases empirically only with ceftriaxone, without adding vancomycin to the treatment regimen41. Taking this fact into account, some authors recommend that assuming there is suitable ongoing surveillance, it may be appropriate to recommend only ceftriaxone as an empiric treatment for pneumococcal meningitis21. This decision should be based on surveillance data and local epidemiology.
Concluding remarksThe emergence of Streptococcus pneumoniae resistance to penicillin and the subsequent evolution of resistance to multiple classes of antibiotics are subjects of great concern for public health. The continuous surveillance of S. pneumoniae is required to obtain useful information that allows to explore alternative treatments and preventive strategies. The reduction of the inappropriate use of antibiotics, the improvement of pneumococcal vaccination programs and the development of other therapeutic options should be the goals.
Conflict of interestThe authors declare that they have no conflicts of interest.