Patent ductus arteriosus (PDA) is a relatively common congenital heart disease and the alternatives for the treatment of PDA > 2.5 mm are surgery or percutaneous occlusion with plugs. The latter, although considered the method of choice, are not provided by the Brazilian National Health System (Sistema Único de Saúde –SUS). Our objective was to compare the incremental cost-effectiveness ratio (ICER) of both strategies.
MethodsSystematic review of clinical outcomes and development of a decision-making algorithm to evaluate the ICER of Amplatzer™ Duct Occluder (ADO) vs. surgical treatment for the closure of PDA. Costs for both methods were calculated based on the reimbursement figures paid by the SUS in 2010 and the cost of the percutaneous kit (device + support materials) was estimated at R$ 10,000.00. We used as a threshold the willingness to pay the equivalent of three times the Brazilian Gross Domestic Product, i.e., R$ 57,000.00 per year of life saved.
ResultsBoth techniques were safe and effective with less morbidity and shorter hospitalization time for percutaneous closure. Adjusted life expectancy was similar in both groups, and slightly better for the ADO group. Total cost was calculated as R$ 8,507 for surgery and R$ 11,000.00 for ADO. ICER was calculated as R$ 71,380.00 per year of life saved. A threshold analysis showed that a reduction of R$ 492.65 in the cost of the ADO kit would reduce the ICER to an acceptable value for the incorporation of this technology by the SUS.
ConclusionsPercutaneous occlusion was associated with less morbidity and shorter hospital stay with similar incremental effectiveness when compared to the surgical treatment. With the direct costs used in this study and considering that the entire population with PDA is treated with the ADO, percutaneous occlusion was less cost-effective. However, a slight reduction in the costs of the percutaneous kit would result in a aceptable ICER threshold for possible incorporation by the SUS.
DESCRIPTORSDuctus arteriosus, patent, Heart defects, congenital, Surgery, Prostheses and implants, Cost-benefit analysis.
Custo-Efetividade Incremental do Tratamento Cirúrgico vs. Percutâneo da Persistência do Canal Arterial com o Amplatzer® Duct Occluder em Crianças: Revisão Sistemática
IntroduçãoA persistência do canal arterial (PCA) é uma cardiopatia congênita relativamente comum e as alternativas para o tratamento de canais > 2,5 mm são a cirurgia ou a oclusão percutânea com próteses do tipo rolha. Essas últimas, apesar de consideradas o método de escolha, não estão previstas pelo Sistema Único de Saúde (SUS). Nosso objetivo foi comparar a razão de custo-efetividade incremental (RCEI) de ambas as estratégias.
MétodosRevisão sistemática em relação a desfechos clínicos e criação de modelo de decisão para avaliação da RCEI do Amplatzer® Duct Occluder (ADO) em comparação ao tratamento cirúrgico, para o fechamento da PCA. Os custos para ambos os métodos foram aqueles reembolsados pelo SUS em 2010, e o custo do conjunto (dispositivo + materiais de apoio) foi estimado em R$ 10.000,00. Foi considerado como limiar uma disposição para pagar equivalente a três vezes o Produto Interno Bruto brasileiro, resultando em R$ 57.000,00 por ano de vida salvo.
ResultadosAs duas técnicas foram seguras e eficazes, com menor morbidade e tempo de internação no fechamento percutâneo. A expectativa de vida ajustada foi similar nos dois grupos, sendo um pouco melhor para o ADO. O custo total foi calculado em R$ 8.507,00 para cirurgia e em R$ 11.000,00 para o ADO. A RCEI foi calculada em R$ 71.380,00 por ano de vida ganho. Uma análise de limiar demonstrou que a redução do valor do conjunto completo do ADO em R$ 492,65 traria a RCEI para o limiar aceitável para incorporação ao SUS nos dias de hoje.
ConclusõesO tratamento percutâneo apresentou morbidade e tempo de internação menores, além de efetividade incremental semel- hante áquela do tratamento cirúrgico. Com os valores diretos estipulados neste estudo e partindo do pressuposto de que toda a população de pacientes com PCA seria tratada com o ADO, o fechamento percutâneo foi menos custo-efetivo. Entretanto, com pequena redução nos valores do conjunto, o procedimento percutâneo estaria dentro de um limiar aceitável da RCEI para possível incorporação.
DESCRITORESPermeabilidade do canal arterial, Cardiopatias congênitas, Cirurgia, Próteses e implantes, Análise custo-benefício.
The ductus arteriosus is a structure present and indispensable during intrauterine life, carrying 55 to 60% of fetal cardiac output.1 After birth, a variety of mechanisms promote its occlusion, which usually is complete within 2 to 3 weeks of age.1 After this period, the persistence of blood flow in the channel corresponds to 7 to 11% of all congenital heart defects, and can be found alone or associated with other complex cardiac diseases.2 This incidence increases with prematurity, reaching 80% in those neonates weighing less than 1,200 g.1
Patent ductus arteriosus (PDA) persistence generates a picture of pulmonary over-circulation dependent on certain factors, including the diameter and length of the ductus arteriosus, and differences in pressure and resistance between the aorta and the pulmonary trunk.1 In prematurity, an exacerbated pulmonary blood flow is not well tolerated, and early treatment becomes a necessity. In term newborns, the evolution depends greatly on the size of the ductus arteriosus, and is generally more benign.
Excessive pulmonary blood flow can result in congestive heart failure with low weight gain and repeated respiratory infections. Later, this problem can cause changes in distal pulmonary capillary vasculature, evolving to presentations of fixed pulmonary arterial hypertension.1,2 Other complications, such as infections in the ductus arteriosus (endarteritis) are rare events (1:13,500 in children and 1:3,300 in adults) with declining rates, mainly due to early diagnosis and treatment.2
Clinical examination with auscultation of the heart murmur is the first step in the diagnosis, which is complemented by color Doppler echocardiography. The visualization of a flow through the ductus arterio-sus and the hemodynamic repercussions of the defect, manifested by an increase of the left cardiac chambers, suggest the need for occlusion.
The treatment of PDA should be individualized, according to patient age. In premature infants, neo- nates, and infants with up to 5 kg, surgical treatment is usually the choice. Percutaneous occlusion in these patients, although feasible, is associated with higher risks of failure and complications, such as left pulmonary artery stenosis and pseudo-coarctation.3,4 In children over 5 kg, adolescents, and adults, percutaneous closure is usually the most appropriate option, due to its high efficiency, very low morbidity (less than surgery), and limited hospitalization time.5 Spring-like prostheses (coils) are employed in smaller channels (<2.5 mm), and channels with constriction in the pulmonary side (Krichenko type A, D, and E).6 Plug-type prostheses are indicated for major, window-type (B), or tubular-type (C) channels.
Several Brazilian groups have been performing percutaneous closure of PDA with various types of devices in patients with supplementary health insurance plans and in those included in research protocols, with excellent results.47-10 Taking in consideration that, in the Brazilian Unified Health System (Sistema Único de Saúde – SUS), only coils are approved for clinical use, the percutaneous closure of major channels is unviable, requiring a surgical solution. The main factor that limits the incorporation of this technology by the SUS is the value of the prostheses. Conversely, it is speculated that the higher morbidity and the longer in-hospital length of stay for surgery patients3 may financially burden the hospital system, due to the higher occupancy rate of beds and of the operating room, and also due to the treatment of possible complications.
To date, no Brazilian studies taking into account the costs were published, when comparing both methods of PDA treatment. Thus, the present study aimed to conduct an economic analysis of incremental cost-effectiveness, in order to compare surgical versus percutaneous closure of this common congenital heart defect in conditions amenable to the use of both strategies, through the perspective of the SUS.
METHODSSystematic revisionSearch strategy and bibliographic databasesThis systematic review used MEDLINE and the Cochrane Central bibliographic databases, accessed on PubMed and Wiley Library Online portals, respectively. The search strategy on PubMed included terms referring to PDA, interventions (surgical and percutaneous repair), and types of desired studies. A preliminary search showed that randomized clinical trials comparing percutaneous and surgical treatment in this disease have not been conducted. Thus, it was decided to include, in the search strategy, terms which also addressed to observational studies. A limitation to articles with abstracts that had been published in the previous 20 years was established with respect to the time of the search, which was performed on September 9th, 2011. For Cochrane Central, the strategy included only aspects related to diseases and therapeutic possibilities, considering that this database contains only clinical trials, and thus search terms relating to outlines were not necessary.
Study eligibility criteriaStudies evaluating the percutaneous or surgical closure of PDA in children and adolescents were included. The prostheses used in percutaneous procedures should necessarily be of the coil type (Gianturco) or the Amplatzer Duct Occluder® (ADO – St. Jude Medical). The choice for these types of prostheses was rooted in the fact that both were approved by Federal and Drug Administration (FDA); are in common use in the pediatric interventional practice; and have extensive safety and efficacy, documented in the literature. Studies related to occlusion of PDA with coils were not used for economic analysis, since these devices are already covered by the SUS. The surgical procedure should have been performed by conventional technique, i.e. excluding procedures such as thoracoscopy or other that are not part of the SUS routine for treatment of PDA.
Exclusion criteria were: studies with fewer than 50 patients; mean (or median) age of patients > 14 years old; mean patient weight < 6 kg; data without division by type of prosthesis (e.g., pooled data from Gianturco coils and ADO prostheses); and patient inclusion prior to 1991.
Process of selection of studiesTitles and abstracts of citations returned by the search strategy were evaluated by two reviewers. Studies that met the eligibility criteria or whose titles or abstracts did not allow a proper assessment of inclusion and exclusion criteria were selected for evaluation of their full texts. This procedure was also performed by two reviewers. Data from the included studies was extracted and entered into electronic spreadsheets.
Outcomes of interestThe following were considered as outcomes of interest: perioperative and overall (within one year) mortality; proportion of patients who required a second procedure (the same or another procedure, e.g., patients undergoing percutaneous treatment, later requiring surgery); and proportion of patients with late residual flow, encompassing patients who at no time were treated (e.g., failure of prosthesis implantation) or who were treated without full closure of the defect, and who remained so during follow-up. The number of patients with major complications, defined by the authors of each analyzed paper, including (but not limited to) those described in previous studies, was also considered.5 Finally, the mean hospitalization time was computed.
For late residual flow outcomes, only those of moderate to severe grade were considered, according to the authors’ classification; studies that did not report late flow intensity were considered as having such information missing. If the article did not report, during follow-up, the conduct adopted for patients with prosthesis implant failure after percutaneous treatment, it was assumed that they underwent subsequent surgical correction, with procedure effectiveness similar to that of patients with surgery as their first therapeutic choice.
Data analysisThe main purpose of this systematic review was the gathering of parameters for the cost-effectiveness model (and not for a safety and efficacy evaluation meta-analysis). To the extent that much of this information was entered into the model as probabilities (and not as relative risks), it was decided to analyze the data relating to the outcomes of interest separately for the different strategies –surgical and percutaneous. The proposed initial approach was a single-arm meta-analysis (i.e., a meta-analysis for single groups).10 However, the mathematical formula in this approach cannot be used when no events occur, which was observed in several studies, considering the analyzed outcomes.
In such cases, a possible procedure is to add 0.5 to the number of events and to the total number of patients; however, this strategy is not sufficiently accurate in cases with a very large rate of studies with zero events (>20%), because there is an overestimate of the rate of occurrence of events. In such cases, the extraction of a weighted average is suggested, in order to obtain the average incidence of events in the studies – a procedure that was adopted in the present work.11
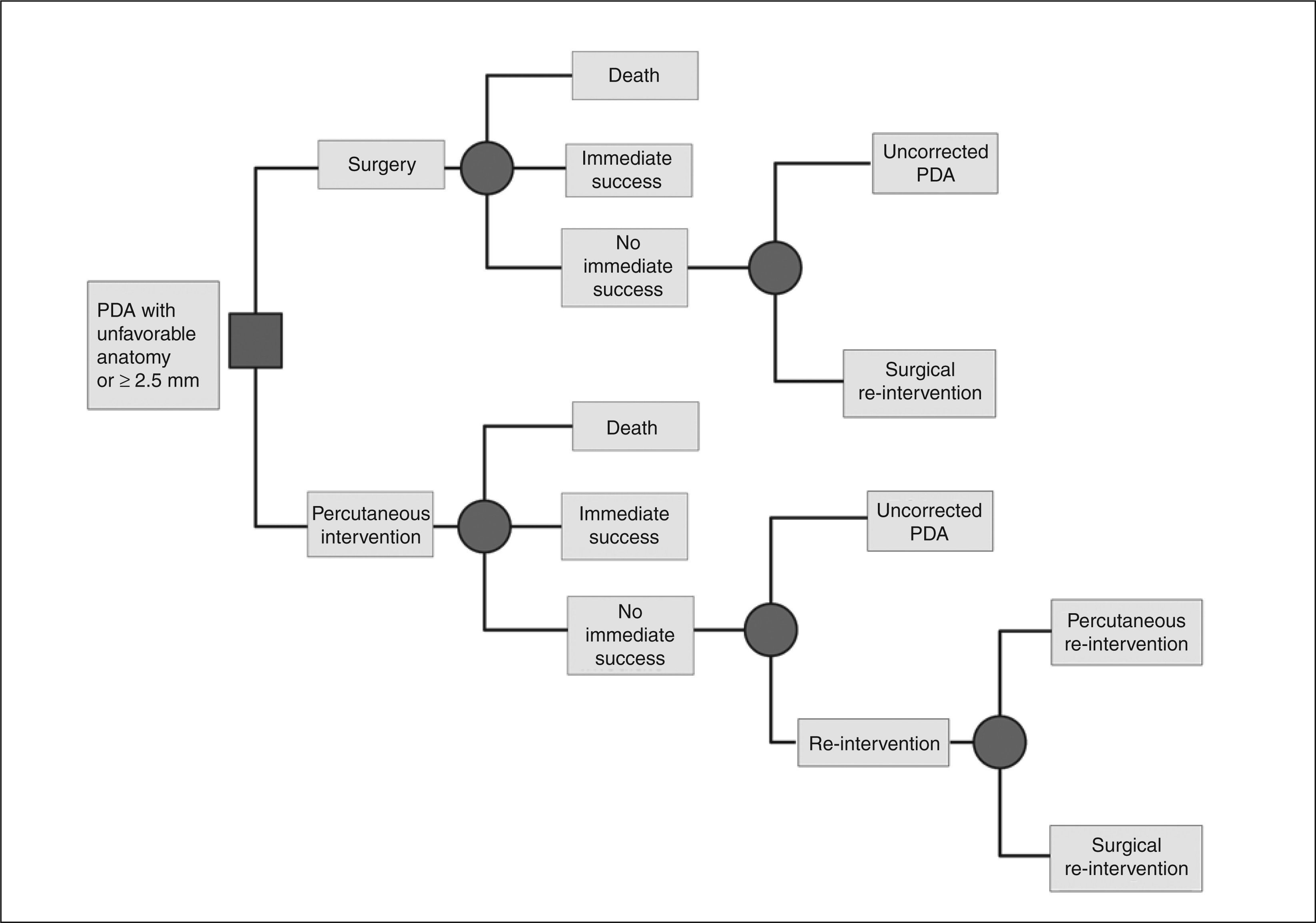
Economic analysis – model descriptionA decision-making model was constructed to estimate the life expectancy and the cumulative costs of strategies for surgical and percutaneous closure of patent ductus arteriosus in the perspective of the SUS. The model compared the costs and benefits over the long-term (for life) of the two therapeutic strategies for closure of PDA in pediatric patients, defined as ≤ 14 years old. The first strategy consisted of surgical closure, by ligature or section, through lateral (classical or extra pleural) thoracotomy; this treatment is currently funded by the SUS. The second strategy consisted of percutaneous closure of PDA using the ADO prosthesis. The analysis considered only cases with favorable diameter and anatomy for closing with plug-type prostheses.6 This restriction assumed that PDAs with diameter < 2.5 mm are treated with Gianturco coils, which are reimbursed by the SUS. A schematic representation of the model is shown in Figure 1.
In the surgical treatment strategy, all patients initially underwent PDA correction by ligation or section of the ductus arteriosus. The mortality rate associated to the procedure was analyzed. The immediate success was considered as a complete closure or presence of minimal residual shunt, as reported in the long-term follow-up (6 to 12 months) of reviewed literature. Patients with significant residual shunt (with hemodynamic repercussions) after a first operation could undergo a second surgery or a percutaneous intervention. Additionally, in the model, the possibility of surgical treatment failure was considered, defined as the late (6 to 12 months after surgery) presence of a persistent shunt which had not been corrected for any clinical reason, or whose subsequent closing attempts also have resulted in failure.
In the percutaneous intervention strategy with the ADO prosthesis, all patients were initially treated by this method. The mortality associated with the method was analyzed. The definition for immediate success was the same as that adopted for the surgical strategy. Those patients in whom closure of PDA was not observed after a first intervention were eligible for a second percutaneous procedure, for closure of the defect (usually with implantation of a new device). Alternatively, the second intervention could consist of surgical treatment. For purposes of the model, it was assumed that all patients undergoing re-intervention of any kind would achieve therapeutic success, although the probabilities of failure of a second intervention had been considered in those general probabilities derived from the literature and used in the analysis. Those lesions that remained with a permanent residual flow were also computed.
Definition of base-case and estimates of survivalFor the model of decision analysis to be representative of the medical practice, regarding the treatment of PDA in children, the present analysis defined 6 years old as the median age of intervention. The life expectancy for individuals who have reached the age of 6 years was obtained from the general Mortality Table for the Brazilian population in 2010, organized by the Instituto Brasileiro de Geografia e Estatística (IBGE); the life expectancy for individuals with PDA remaining uncorrected for life was derived from the cited table, and from those mortality estimates by age group of patients with PDA described by Campbell.12 The analyzes considered only adjusted values,
applying a discount rate of 5% per year in the estimates of life expectancy, according to the guidelines of the Brazilian Ministry of Health.13
The estimate of the long-term survival expectancy was performed with the adoption of the following assumptions: the survival of patients with untreated PDA is significantly lower than that of patients with successfully treated PDA; the life expectancy of patients with successfully treated PDA is equal to that of the general population; this life expectancy is earned, regardless of the method employed for PDA closure, whether by percutaneous intervention or surgical treatment, provided there is no hemodynamically significant residual defect; the current life expectancies for different age groups, calculated from the Mortality Table by IBGE, 2010, represent the life expectancy in the absence of PDA, considering the very low incidence of this condition (0.006-0.047%) and that virtually all diagnosed cases are currently treated; the only explanation for a difference in survival of patients with PDA percutaneously or surgically treated would come from the different rates of immediate success in the defect closure with one of the methods.
Mortality Table and extrapolation to individuals with patent ductus arteriosusAs already detailed, to calculate the expected survival for patients with PDA (uncorrected, or with late residual shunt with hemodynamic repercussions), this analysis used the estimate from the Mortality Table by IBGE in 2010. In that year, the life expectancy for an individual in the general population who had reached the age of 6 years was 69.5 years.
In order to obtain a comparable estimate of the life expectancy of patients with uncorrected PDA versus that of the general population, a mortality table based on the integration among the estimates from the literature and the mortality table of the Brazilian population was projected. The method used was an adaptation of that described by Pharoah and Hollingworth.14 Briefly, the method consists in calculating the fraction of mortality by age group attributable to a particular health condition. In the present study, the adaptation consisted in assume that, for the Brazilian population, the current mortality rate of the general population by age group is not significantly influenced by the presence of untreated PDA in the population, taking in consideration: (a) the low estimated prevalence for this condition, and (b) the fact that virtually all diagnosed cases of PDA with hemodynamic repercussion are currently treated in Brazil.
CostsThe costs were obtained from SUS reimbursement values in 2010, related to the procedures of surgical closure of PDA (R$ 8,432.00) and cardiac catheterization (R$ 800.00 – diagnostic hemodynamic study, without the costs of covered devices, in the case of Gianturco coils). The ADO prosthesis was used in the base-case of the analysis because this device is not covered by SUS, and also due to the extensive experience, safety, and efficacy of this device, documented in various case series.4 The cost of the ADO prosthesis used in the analysis was set at R$ 10,000.00, with R$ 8,000.00 for the prosthesis and R$ 2,000.00 for the releasing system. These values correspond to those used in a study that evaluated the safety and efficacy of both treatment methods, conducted in one of the Brazilian Ministry of Health charity hospitals of excellence and funded by this Ministry as part of a project to assess new technologies.5
Considering that all costs included in the analysis fall within the present time (i.e., at the time of intervention), and that the long-term costs appear to be similar among patients treated with surgery or by percutaneous intervention, neither adjustment of costs was performed for inflation or discount rates, nor indirect costs related to procedures, such as expenses with the blood bank (serology and other pre-transfusional examinations), and loss of workdays by parents or guardians were included.
The model was developed with the program Tree- Age, version 9.12. Sensitivity analyzes were conducted, considering alternative parameters of effectiveness, prosthesis costs, probability of success, and procedure-related death. Thresholds were estimated (threshold analyzes) to determine whether the new technology evaluated, from the perspective of the SUS (percutaneous closure of PDA with ADO prosthesis), was cost-effective. To allow a better interpretation, a willingness to pay a threshold value of R$ 57,000.00 (three times the per capita Gross Domestic Product [GDP] in 2010) per QALY (quality- adjusted life year, i.e., quality of life adjusted for year of life gained). This study was not intended to perform a deeper statistical approach; however, in general, the concept of QALY relates to an increase in quality of life and survival time, through incorporation of a new procedure or a different treatment.15
RESULTSSystematic reviewAfter reviewing titles and abstracts of articles found by the systematic review, 77 papers remained and were analyzed in full text. Thirty-three were excluded: 11 for having fewer than 50 patients; seven due to studying prostheses or surgeries different from those defined in the eligibility criteria; 5 for presenting data not separated by type of procedure or prosthesis; 3 representing re-analyses of data already published; 2 for including patients prior to 1991; 2 for including patients with a mean weight < 6 kg; 1 for including patients with a mean age > 14 years old; 1 for not presenting any outcome of interest; and one for data judged to be unreliable, since the study showed a subset of pooled cases with poorly defined selection criteria.
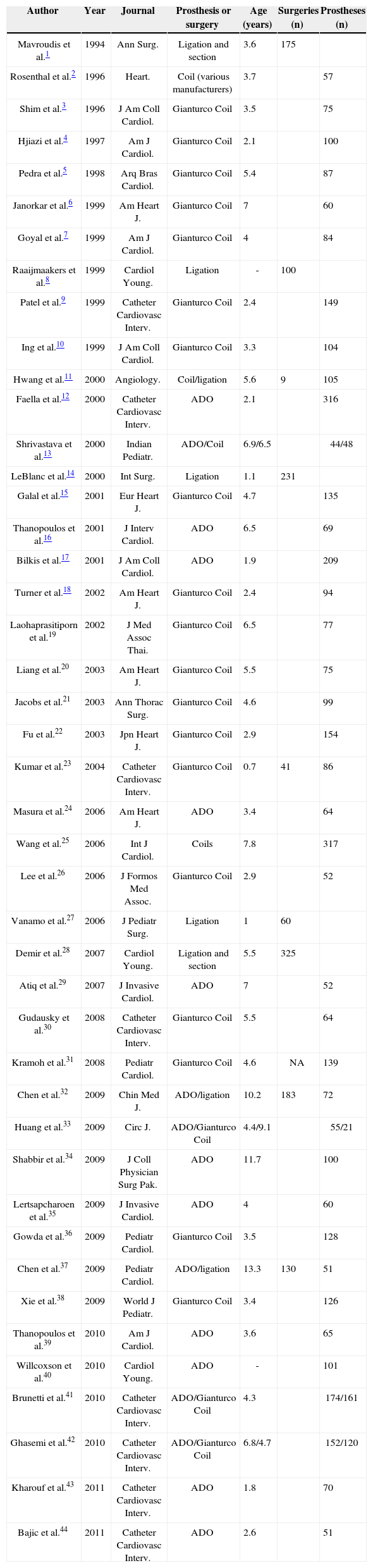
Of the 44 articles included (Table 1 and Appendix 1), nine had more than one group of patients. Twenty- seven articles were related to occlusion procedures with coils, and 22 used almost 100% Gianturco coils.
Key data of studies included in the cost-effectiveness analysis after applying the exclusion criteria (Appendix 1)
| Author | Year | Journal | Prosthesis or surgery | Age (years) | Surgeries (n) | Prostheses (n) |
|---|---|---|---|---|---|---|
| Mavroudis et al.1 | 1994 | Ann Surg. | Ligation and section | 3.6 | 175 | |
| Rosenthal et al.2 | 1996 | Heart. | Coil (various manufacturers) | 3.7 | 57 | |
| Shim et al.3 | 1996 | J Am Coll Cardiol. | Gianturco Coil | 3.5 | 75 | |
| Hjiazi et al.4 | 1997 | Am J Cardiol. | Gianturco Coil | 2.1 | 100 | |
| Pedra et al.5 | 1998 | Arq Bras Cardiol. | Gianturco Coil | 5.4 | 87 | |
| Janorkar et al.6 | 1999 | Am Heart J. | Gianturco Coil | 7 | 60 | |
| Goyal et al.7 | 1999 | Am J Cardiol. | Gianturco Coil | 4 | 84 | |
| Raaijmaakers et al.8 | 1999 | Cardiol Young. | Ligation | - | 100 | |
| Patel et al.9 | 1999 | Catheter Cardiovasc Interv. | Gianturco Coil | 2.4 | 149 | |
| Ing et al.10 | 1999 | J Am Coll Cardiol. | Gianturco Coil | 3.3 | 104 | |
| Hwang et al.11 | 2000 | Angiology. | Coil/ligation | 5.6 | 9 | 105 |
| Faella et al.12 | 2000 | Catheter Cardiovasc Interv. | ADO | 2.1 | 316 | |
| Shrivastava et al.13 | 2000 | Indian Pediatr. | ADO/Coil | 6.9/6.5 | 44/48 | |
| LeBlanc et al.14 | 2000 | Int Surg. | Ligation | 1.1 | 231 | |
| Galal et al.15 | 2001 | Eur Heart J. | Gianturco Coil | 4.7 | 135 | |
| Thanopoulos et al.16 | 2001 | J Interv Cardiol. | ADO | 6.5 | 69 | |
| Bilkis et al.17 | 2001 | J Am Coll Cardiol. | ADO | 1.9 | 209 | |
| Turner et al.18 | 2002 | Am Heart J. | Gianturco Coil | 2.4 | 94 | |
| Laohaprasitiporn et al.19 | 2002 | J Med Assoc Thai. | Gianturco Coil | 6.5 | 77 | |
| Liang et al.20 | 2003 | Am Heart J. | Gianturco Coil | 5.5 | 75 | |
| Jacobs et al.21 | 2003 | Ann Thorac Surg. | Gianturco Coil | 4.6 | 99 | |
| Fu et al.22 | 2003 | Jpn Heart J. | Gianturco Coil | 2.9 | 154 | |
| Kumar et al.23 | 2004 | Catheter Cardiovasc Interv. | Gianturco Coil | 0.7 | 41 | 86 |
| Masura et al.24 | 2006 | Am Heart J. | ADO | 3.4 | 64 | |
| Wang et al.25 | 2006 | Int J Cardiol. | Coils | 7.8 | 317 | |
| Lee et al.26 | 2006 | J Formos Med Assoc. | Gianturco Coil | 2.9 | 52 | |
| Vanamo et al.27 | 2006 | J Pediatr Surg. | Ligation | 1 | 60 | |
| Demir et al.28 | 2007 | Cardiol Young. | Ligation and section | 5.5 | 325 | |
| Atiq et al.29 | 2007 | J Invasive Cardiol. | ADO | 7 | 52 | |
| Gudausky et al.30 | 2008 | Catheter Cardiovasc Interv. | Gianturco Coil | 5.5 | 64 | |
| Kramoh et al.31 | 2008 | Pediatr Cardiol. | Gianturco Coil | 4.6 | NA | 139 |
| Chen et al.32 | 2009 | Chin Med J. | ADO/ligation | 10.2 | 183 | 72 |
| Huang et al.33 | 2009 | Circ J. | ADO/Gianturco Coil | 4.4/9.1 | 55/21 | |
| Shabbir et al.34 | 2009 | J Coll Physician Surg Pak. | ADO | 11.7 | 100 | |
| Lertsapcharoen et al.35 | 2009 | J Invasive Cardiol. | ADO | 4 | 60 | |
| Gowda et al.36 | 2009 | Pediatr Cardiol. | Gianturco Coil | 3.5 | 128 | |
| Chen et al.37 | 2009 | Pediatr Cardiol. | ADO/ligation | 13.3 | 130 | 51 |
| Xie et al.38 | 2009 | World J Pediatr. | Gianturco Coil | 3.4 | 126 | |
| Thanopoulos et al.39 | 2010 | Am J Cardiol. | ADO | 3.6 | 65 | |
| Willcoxson et al.40 | 2010 | Cardiol Young. | ADO | - | 101 | |
| Brunetti et al.41 | 2010 | Catheter Cardiovasc Interv. | ADO/Gianturco Coil | 4.3 | 174/161 | |
| Ghasemi et al.42 | 2010 | Catheter Cardiovasc Interv. | ADO/Gianturco Coil | 6.8/4.7 | 152/120 | |
| Kharouf et al.43 | 2011 | Catheter Cardiovasc Interv. | ADO | 1.8 | 70 | |
| Bajic et al.44 | 2011 | Catheter Cardiovasc Interv. | ADO | 2.6 | 51 |
Thus, for the economic data analysis, 9 articles with surgical data and 17 articles with data from the ADO prosthesis were included.
Surgical closureA total of 9 articles were selected, with a total of 1,254 patients analyzed. The patients’ final year of inclusion ranged from 1993 to 2007. The mean age of these patients was 5.1 years. The follow-up period ranged from four months to 6 years. The average size of PDA was between 1.5 and 4.0 mm in two studies and > 5 mm in three other studies; the other articles did not report this information.
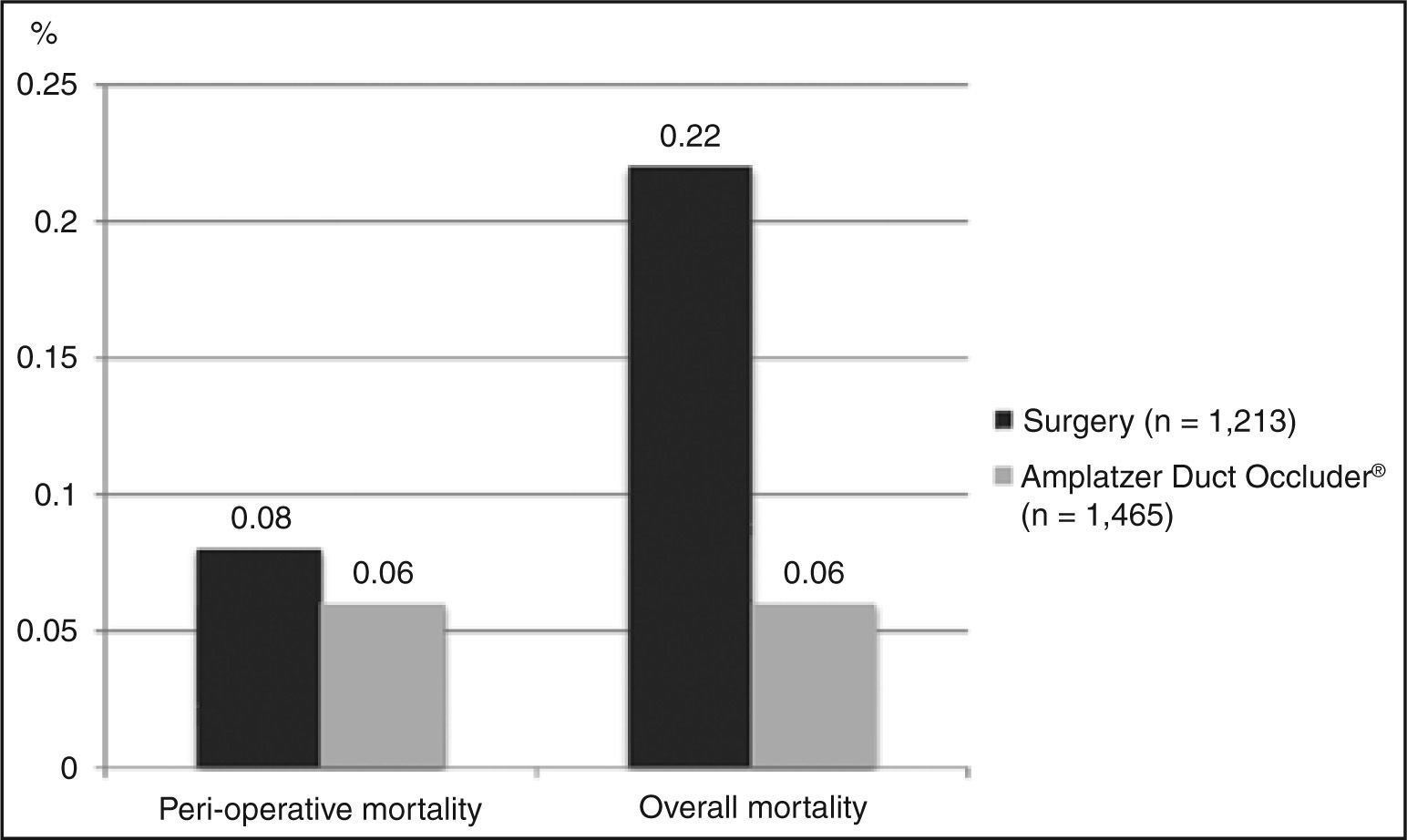
DeathEight studies included the perioperative death outcome, with a total of 1,213 patients. The weighted average obtained across these studies for this outcome was 0.08%. Ten studies reported overall deaths, with n = 1,319. The weighted average obtained was 0.22% (Figure 2).
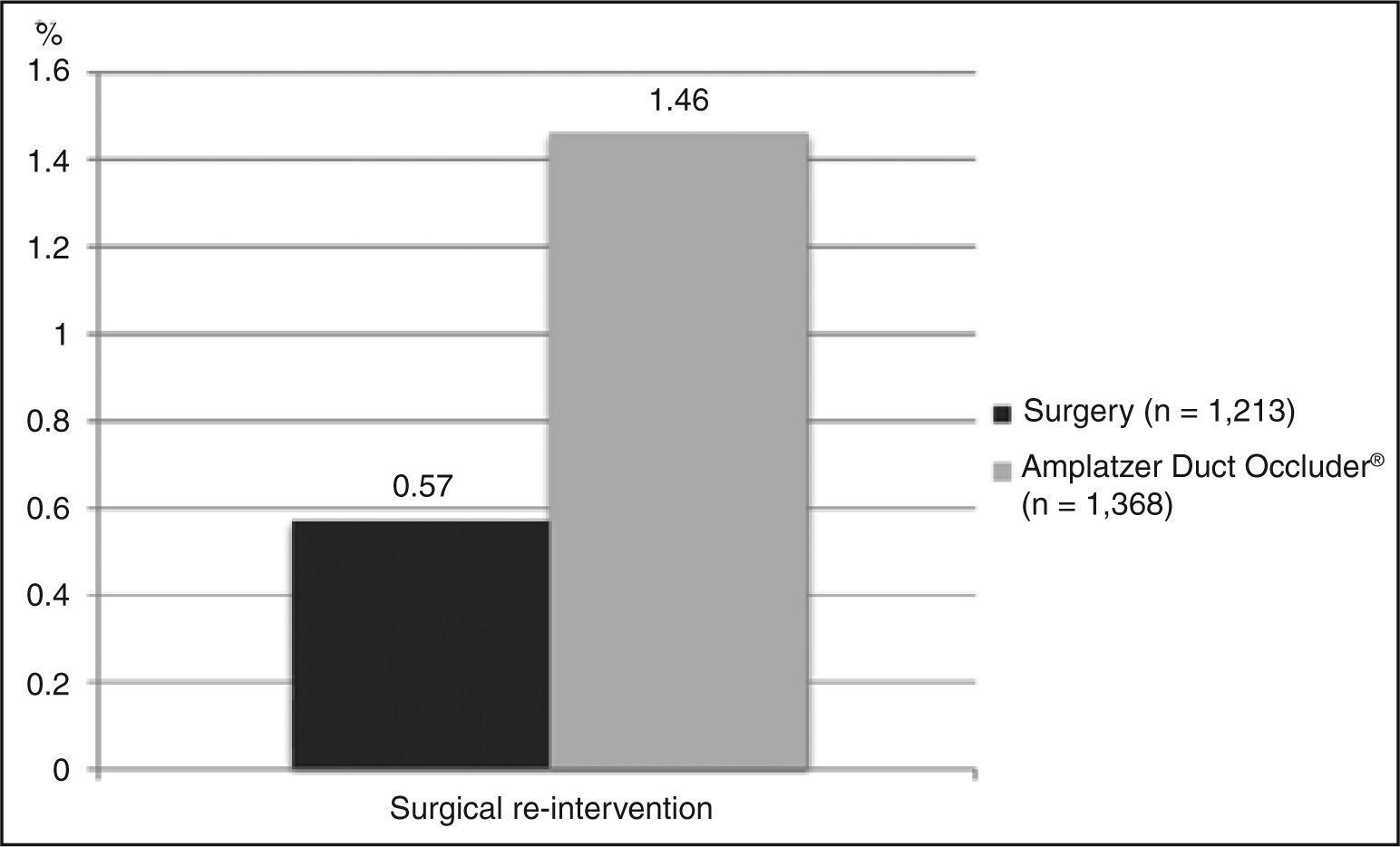
Need for a second procedureEight studies that provided data for requiring a second surgery, with a total of 1,213 patients evaluated, were included. On average, 0.57% of patients underwent a second surgical intervention. Importantly, these corrections were performed in another hospitalization, mostly for a later correction of residual shunts. As to the outcome of performing a percutaneous closure after surgical failure, the same eight studies were included. The weighted average obtained was 0.32%. The data relating to the need for a second procedure are shown in Figure 3.
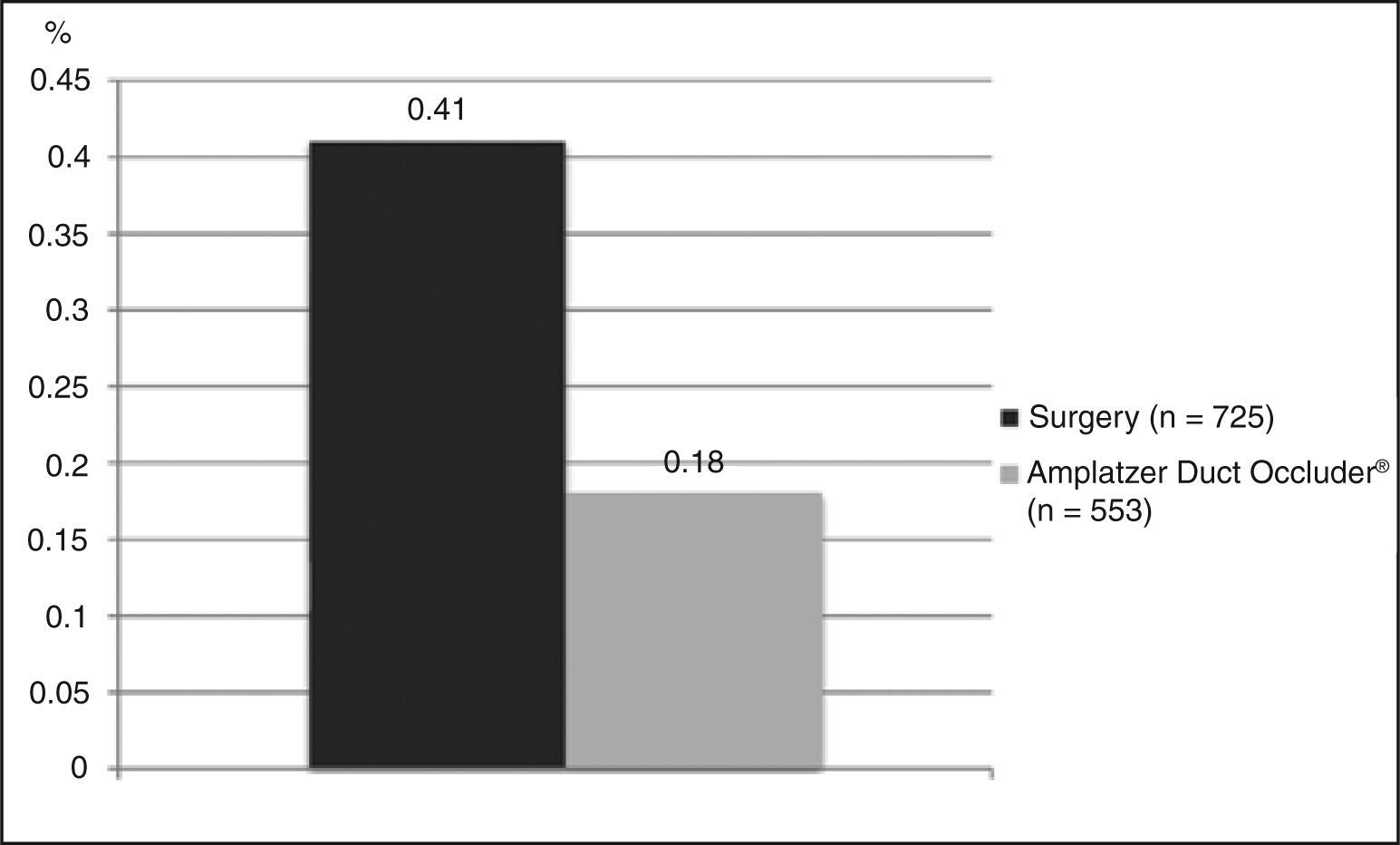
Residual flow and other complicationsOf those studies included, five had a complete dataset for the evaluation of moderate or large late residual shunts, with a total of 725 patients. The weighted average observed was 0.41% (Figure 4). It is important to keep in mind that this value reflects the number of patients who persisted with a shunt at the end of follow-up, i.e., after excluding patients who had their defects corrected by a possible second procedure. Another relevant detail is that four of these studies had ligation as their surgical technique; the fifth combined this technique (i.e., ligation) with section (but without rate improvement versus other studies, which was 0.6%).
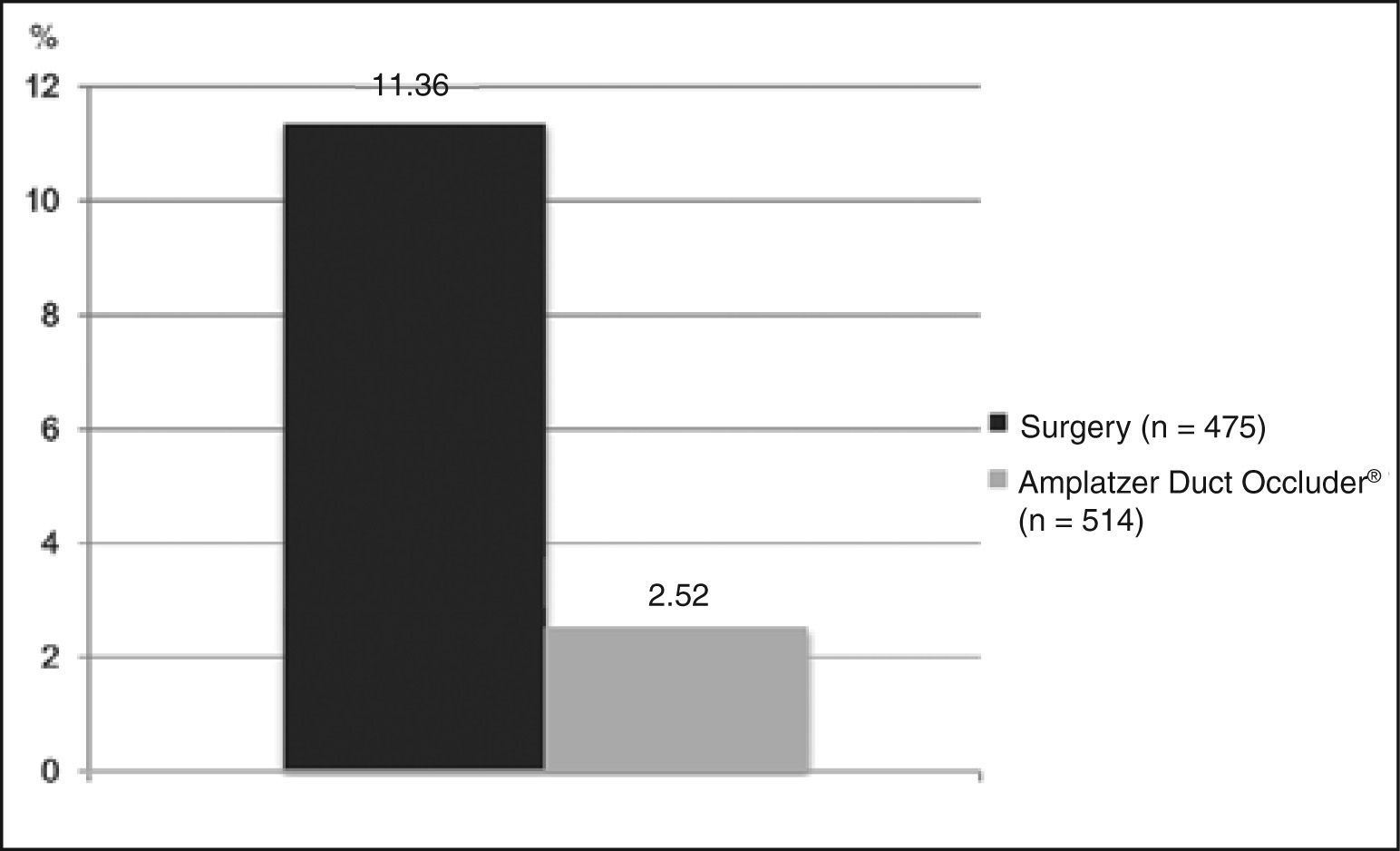
As to the outcome of major complications, four studies were included, with a total of 475 patients. The remaining studies had no clear data for this outcome, quite often mixing data regarding minor complications. The weighted average of major complications was 11.36% (Figure 5).
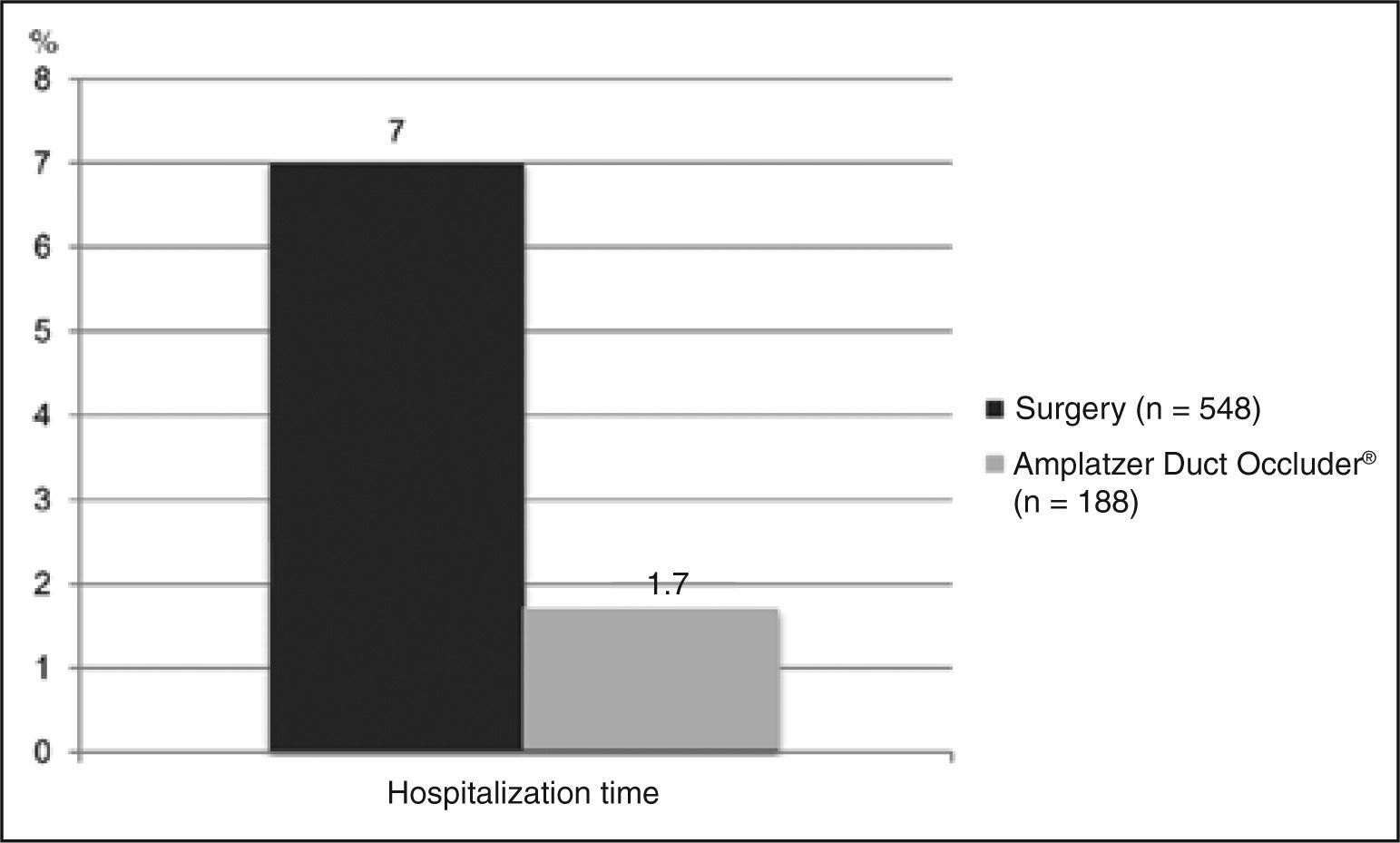
Average in-hospital length of stayOnly four studies reported mean hospitalization time, with a total of 548 patients. Three of them showed values between 8 and 9 days, and the last study had an average of 3.6 days. The weighted average of these studies was 7.01 days (Figure 6).
Percutaneous intervention with the Amplatzer Duct Occluder®Seventeen articles studying the ADO prosthesis were included, with a total of 1,705 patients. The final year of inclusion of patients in the articles ranged between 1999 and 2009. The mean age of these patients was 4.8 years. The mean size of PDA ranged between 2.0 and 6.5 mm. The follow-up period ranged from a few days up to five years.
DeathThirteen studies (n = 1,465) reported mortality data, with only one perioperative death. Thus, the proportion of perioperative and overall death was equal, equivalent to 0.06% (Figure 2).
Need for a second procedureThirteen studies (n = 1,368) reported data on need for a surgical procedure due to incomplete closure of the defect, or by embolization. The weighted average for occurrence of this event was 1.46% (Figure 3). Conversely, 12 studies reported the necessity of a new hemodynamic procedure (n = 1,216). On average, this outcome occurred in 0.82% of patients.
Residual flow and other complicationsEight studies (n = 553) were included in the evaluation of the residual shunt outcome, with a mean incidence of 0.18% (Figure 4). With regard to other complications, seven studies reported outcomes appropriately (n = 514), with a mean estimated incidence of 2.52% (Figure 5).
Mean in-hospital lengthof stayOnly three studies reported data on mean in-hospital length of stay, with a total of 188 patients. The weighted average obtained was 1.73 day (Figure 6).
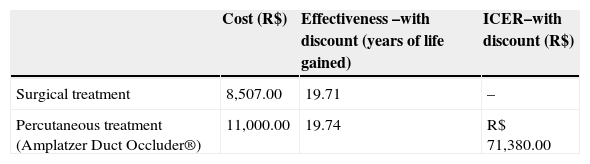
Economic analysisBase-case: model using estimates of the systematic literature reviewConsidering the costs incurred in the sequence of events and all possible outcomes (closure, complications, and re-interventions), an incremental cost-effectiveness ratio (ICER) of R$ 71,380.00 was found per year of life gained for the percutaneous closure of PDA with the ADO prosthesis. This value was obtained in the main analysis, with life expectancies discounted at 5% per year, a value higher than the limit value for an intervention to be considered cost-effective - R$ 57,000.00 per year of life gained, corresponding to three times the value of Brazilian GDP per capita in 2010 (R$ 19,016.00). Data relative to ICER are shown in Table 2.
Cost-effectiveness and incremental cost-effectiveness ratio (ICER) of surgical and percutaneous treatment strategies for patent ductus arteriosus
| Cost (R$) | Effectiveness –with discount (years of life gained) | ICER–with discount (R$) | |
|---|---|---|---|
| Surgical treatment | 8,507.00 | 19.71 | – |
| Percutaneous treatment (Amplatzer Duct Occluder®) | 11,000.00 | 19.74 | R$ 71,380.00 |
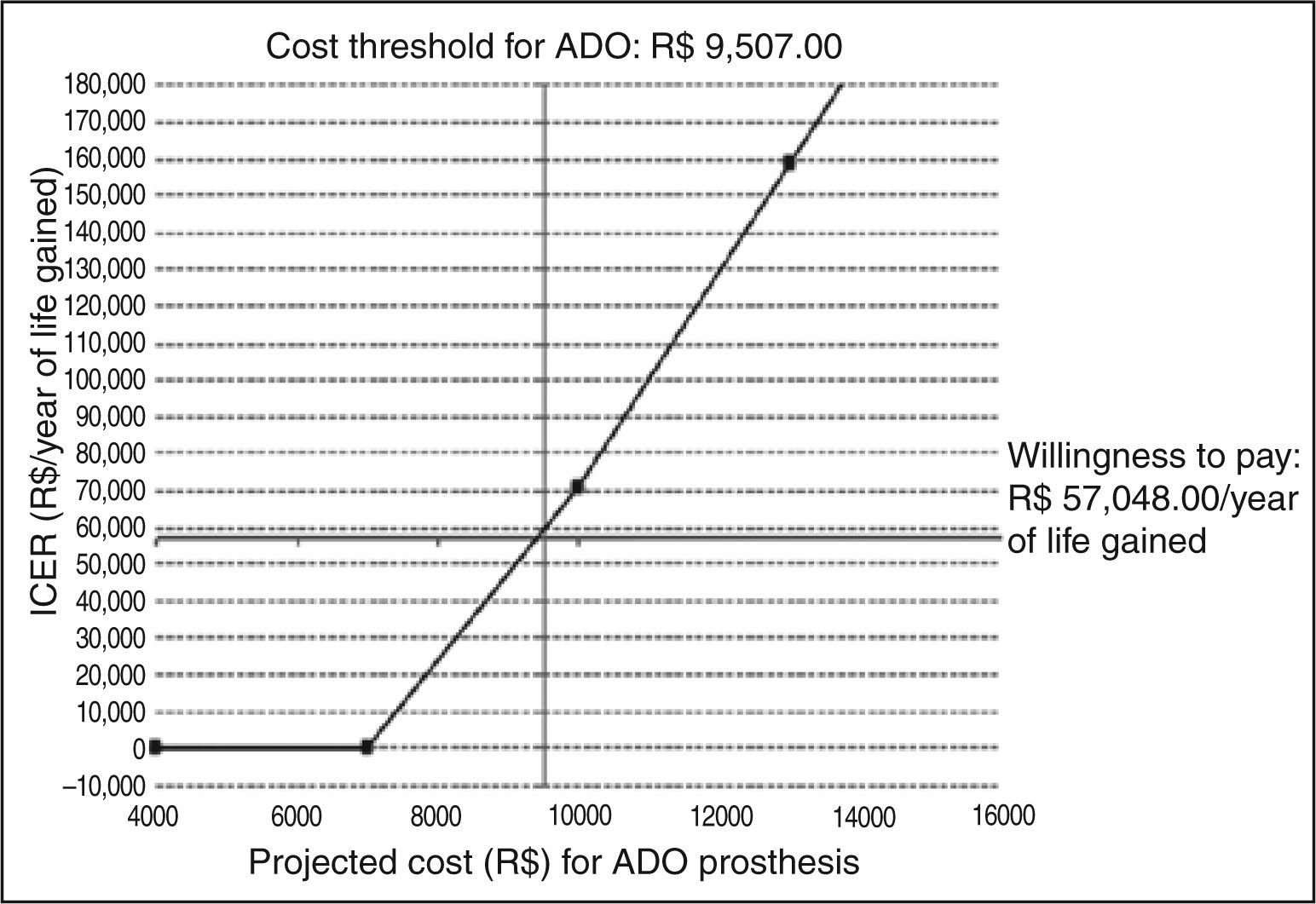
From the base-case derived of literature data and including a discount rate of 5% in the evaluation of effectiveness, a threshold analysis was conducted to determine the maximum limit-cost, so the use of the ADO prosthesis achieved an ICER of up to R$ 57,048.00/ year of life. In these terms, the ADO prosthesis would present an ICER considered acceptable for reimbursement at a maximum cost of R$ 9,507.00, including the cost of the delivery system. On the basis of this value, from an economic standpoint the surgical treatment would be the most attractive option. Figure 7 summarizes the threshold analysis and shows the expected variation of ICER for variations in the cost of the ADO prosthesis between R$ 4,000.00 and R$ 16,000.00.
DISCUSSIONPDA is one of the most common congenital heart diseases, and its timely treatment leads to patient's healing and to a life expectancy equal to that of the general population. From 1990 onwards, there has been a marked progress in the techniques of percutaneous closure of this lesion. Today, this procedure is one of the most frequently performed in the catheterization laboratories of large referral centers. However, there are still few the studies comparing the results of this method with the surgical approach.
This study sought to identify the evidence on the safety and efficacy of both treatment options, and to perform an analysis of incremental cost-effectiveness as a basis for possible incorporation of the technology (PDA closure with plug-type prosthesis) by the SUS. The extensive literature review conducted here showed that the efficacy data of both treatment strategies are based on observational studies and uncontrolled experiments, with no direct comparison in randomized trials. Considering that the percutaneous treatment has already been adopted as procedure of choice in children weighing > 6 kg in major world centers, it is unlikely that both strategies will be compared in future randomized clinical trials. Thus, the available evidence for an evaluation will not increase in quality in the future.
To the authors’ knowledge, in the Brazilian literature there are no published studies comparing the costs of both treatment methods (surgical vs. percutaneous) of PDA, whether from the perspective of public health or within the realm of supplementary health. This is not only a pioneering study in this direction, but also in its economic analysis. The authors evaluated the incremental cost-effectiveness (during life) of both treatment strategies, which is crucial for the incorporation of a new technology in the public health system. The Brazilian Ministry of Health itself mandates the conduction of this type of economic analysis.13
This study is based on a systematic literature review with analysis of the main possible outcomes in both treatment strategies, for creating a decision-making model similar to that observed in daily clinical practice. The model generated showed an adjusted cumulative effectiveness estimate (lifelong survival) very similar between the two therapies, with slightly higher benefit for percutaneous therapy with the ADO prosthesis. However, the incremental cost-effectiveness ratio with the use of ADO was rather high, at about R$ 71,000.00 per year of life saved. In the threshold analysis performed, it was perceived that a small reduction in the cost of the device (approximately R$ 500.00) would bring this ratio to an acceptable value, R$ 57,000.00 per year of life saved, corresponding to three times the value of Brazilian GDP per capita in 2010 (R$ 19,016.00).
Even with a slightly elevated incremental cost- effectiveness ratio with the values stipulated in this study, there are several reasons for a possible incorporation of this technology by the SUS. First, it is important to understand the context in which the ADO prosthesis would be used in the context of the SUS, so that its indication would be individualized, and with consequent impact on cost reduction. This study considered that all patients with PDA would be treated with the ADO prosthesis, and this is not really necessary in clinical practice. According to a study from Toronto, with respect to the ductus arteriosus, about one-third measure < 2.5 mm at the angiography, and almost 90% of these defects exhibit an A-, D-, or E-type constriction on the pulmonary side.6 In patients exhibiting channelswith these types and sizes, the use of Gianturco coils, which are devices already covered by SUS, is very safe and results in high rates of occlusion, as seen in experiments in this scenario.10 These patients may still be treated with the use of springs, with an excellent incremental cost-effectiveness ratio, considering that, today, the SUS pays R$ 1,953.00 for this device, including its release system. Such individualization of strategies, according to the basic anatomy of the patient, would optimize the overall cost-effectiveness ratio of percutaneous closure of PDA, and would bring this result to an acceptable threshold for incorporating the ADO prosthesis in the SUS.
It is acknowledged that the type and size distribution of arterial channels observed in Toronto may not be the same as in Brazil. However, most Brazilian centers performing percutaneous closure of PDA are situated in areas with similar geographical characteristics to those of Ontario, Canada, where the mentioned study was conducted. Considering that Brazil does not have a significant portion of the population living at high altitudes, is unlikely to find a high prevalence of no- constriction, large-diameter channels, as observed in Mexico City and La Paz, Bolivia.16
Additionally, it is noteworthy to that the present cost analysis took into account only the direct costs related to the procedures, such as using the hemo-dynamics laboratory, surgical center, materials and supplies, and medical and support team expenses. These items are the easiest to measure. Conversely, indirect costs, which are more difficult to estimate, were not accounted for in this study. While acknowledging the speculative nature of this observation, it is believed that if such values had been considered, the incremental cost-effectiveness ratio would be much higher for the surgical treatment. As it is known, the surgery for patent ductus arteriosus requires greater use of blood products,5 with a consequent increase in costs related to the use of the blood bank (collection and distribution of bags, serology and blood typing, among others).
Although this study was not designed to perform a comparison of clinical outcomes in relation to the safety and efficacy of each therapeutic method studied, the systematic review of the literature performed here corroborated observations previously found in the literature, showing that the percutaneous closure of PDA has similar efficacy, in addition of lower complication rates and shorter hospitalization time, when compared to the surgical procedure. These observations are consistent with findings from the clinical study performed at this institution and funded by the Brazilian Ministry of Heal thin order to compare the safety and efficacy of both treatment methods for PDA.5 It is probable that the greater morbidity (mainly by infectious and respiratory complications) and the hospitalization time of the surgical treatment of PDA would result in greater indirect costs.
In addition, the longer hospitalization time hinders the rapid return of patients to their routine activities, with possible work losses for parents or guardians. Indirect costs of these potential losses are difficult to quantify. It is also noteworthy that the longer hospitalization time required to treat this relatively simple congenital heart disease hinders the rapid turnover of beds in centers performing surgeries for more complex heart diseases, reducing the capacity and effectiveness of the hospital system, especially if it is considered that there is a repressed demand for the surgical treatment of congenital heart diseases.17
Even though a willingness-to-pay threshold of R$ 57,000.00 was used (three times the per capita Brazilian GDP in 2010) per QALY in this study, other methods for determining thresholds are used in other (usually developed) countries, which take into account a fixed value of US$ 50,000.00.18 If this criterion had been used, the closure of PDA with the ADO prosthesis would have been cost-effective from the incremental point of view, and amenable to incorporation.
This study has some limitations. The absence of a threshold analysis related to quality of life and loss of workdays by parents or guardians is one limitation. Although the effect of severe complications in mortality rates and the chances of re-intervention were contemplated in this analysis, the impact on quality of life and on school- and employment-absences was not evaluated. The procedure of thoracotomy and the pain due to the incision probably reduced the quality of life of patients in the first month after surgery, further delaying the return to routine activities. In view of this, parents or guardians reduce their workload to care for the patient, indirectly incurring in a burden on society. In this study, the SUS table currently available for reimbursement of cardiac catheterization for closure of PDA was used. The authors recognize the need to update this table, both for reimbursement in order to cover hospital expenses such as doctors' fees, as well as for medical fees. Such an update would increase the incremental cost-effectiveness of percutaneous closure. The outcome analysis was performed considering the use of the ADO prosthesis, which has its safety and effectiveness widely documented in the literature and in clinical practice. Although other plug-type nitinol prostheses from other brands and manufacturers are available in the Brazilian market, the literature relevant to these devices is still scarce. Currently, it cannot be stated with certainty that the use of other devices would result in clinical outcomes similar to those observed with the ADO prosthesis.
This study can be considered as a first step in a series of actions for the possible incorporation of this technology by the SUS. A study of incremental cost- effectiveness similar to the present, but more accurate and appropriate to the contemporaneous reality, should take into account the following aspects: about one-third of patients with PDA can be treated with Gianturco coils; the reimbursement values of cardiac catheterization (including medical fees) must be updated; and the indirect costs should be accounted for. The authors believe that such a study is feasible, for subsequent calculation of the impact on the Brazilian budget, and for a definitive incorporation of this technology. Considering that the prevalence of cases of patent ductus arteriosus in the general population is very low, it can be speculated that such impact would be limited.
CONCLUSIONSThe percutaneous treatment of patent ductus arteriosus with the ADO prosthesis presented lower morbidity and shorter in-hospital length of stay, and an effectiveness similar to that of surgical treatment. With the direct values stipulated in this study and assuming that the entire population of patients with patent ductus arteriosus would be treated with the ADO, the procedure of percutaneous closure had lower incremental cost-effectiveness. However, with a small reduction in values for the kit of materials, the percutaneous procedure would be within an acceptable threshold of incremental cost-effectiveness ratio for possible incorporation by the SUS.
CONFLICTS OF INTERESTCarlos Augusto Cardoso Stone is a consultant for Lifetech (China) and Occlutech (Germany), and is a proctor for St. Jude (United States) and PFM (Germany), manufacturers of prostheses for percutaneous closure of ductus arteriosus.
FUNDING SOURCEThis work received financial support from the Brazilian Ministry of Health, in partnership with the Hospital do Coração, Associação do Sanatòrio Sírio, São Paulo (SP), as part of one of its philanthropic projects.