Surgical repair of some complex congenital heart diseases involves reconstruction of the right ventricular outflow tract using homografts, bioprostheses, bovine jugular grafts or other valved conduits between the right ventricle and the main pulmonary artery. Although these surgical procedures may be performed with low mortality rates, the life span of these implanted valves or conduits is usually short (< 10 years) due to either degeneration and/or calcification. Variable degrees of pulmonary stenosis, often associated with pulmonary insufficiency, are consequences of conduit degeneration. In 2000, Bonhoeffer et al. were the first to report the transcatheter pulmonary valve implantation (TPVI) of a bioprosthetic pulmonary valve later named Melody® valve (Medtronic, Minneapolis, USA). The technique was initially developed to limit the need for multiple surgical procedures, and, ultimately, to work as a surrogate of a new surgical valve replacement. Subsequent clinical studies in Europe and the United States confirmed the safety and efficacy of this technique in a larger number of patients. Since the National Sanitary Surveillance Agency (Agência Nacional de Vigilância Sanitária – Anvisa) granted approval for clinical use of the Melody® transcatheter pulmonary biological valve in February 2103, we deemed that a judicious. assessment of this new technology was timely and necessary before the widespread use in our country. The objective of this study was to perform a systematic literature review on the use of TPVI in patients with dysfunctional homografts, valved conduits and bioprostheses implanted surgically in the right ventricular outflow tract.
Implante Transcateter de Bioprótese Valvular Pulmonar: Revisão Sistemática da Literatura
A correção cirúrgica de algumas cardiopatias congênitas complexas envolve a reconstrução da via de saída do ventrículo direito com a interposição de homoenxertos, biopróteses, enxertos de jugular bovina ou outros condutos valvulados entre o ventrículo direito e o tronco da artéria pulmonar. Apesar de essas cirurgias poderem ser realizadas com baixa mortalidade, a vida útil das válvulas ou dos condutos implantados é normalmente pequena (< 10 anos), seja por degeneração e/ou calcificação. Graus variáveis de estenose pulmonar na maioria das vezes associada à insuficiência pulmonar são consequências da degeneração dos condutos. Em 2000, Bonhoeffer et al. foram os primeiros a relatar o implante transcateter de bioprótese valvular pulmonar (ITVP) com um dispositivo que posteriormente foi denominado de válvula Melody® (Medtronic – Minneapolis, Estados Unidos). A técnica foi inicialmente desenvolvida para limitar a necessidade de múltiplos procedimentos cirúrgicos, substituindo, em última análise, uma nova troca cirúrgica valvular. Estudos subsequentes na Europa e Estados Unidos atestaram para a segurança e eficácia dessa técnica em um número maior de pacientes. Como a Agência Nacional de Vigilância Sanitária (Anvisa) concedeu a aprovação para o uso clínico da válvula biológica pulmonar transcateter Melody® em fevereiro de 2013, consideramos necessária e oportuna a avaliação judiciosa da utilização dessa nova tecnologia antes que ela fosse aplicada em larga escala em nosso país. O objetivo deste estudo foi realizar uma revisão sistemática da literatura sobre o ITVP em pacientes com disfunções de homoenxertos, condutos valvulados e biopróteses implantados cirurgicamente na via de saída do ventrículo direito.
Surgical correction of some complex and less frequent congenital heart diseases involves reconstruction of the right ventricular (RV) outflow tract with the interposition of homografts, bovine jugular bioprostheses grafts, or other valved conduits between the right ventricle and the main pulmonary artery. Examples of diseases that may require such strategy include tetralogy of Fallot, pulmonary atresia with ventricular septal defect, double-outlet right ventricle with infundibular pulmonary stenosis, transposition of the great arteries with ventricular septal defect and infundibular pulmonary stenosis, and persistent truncus arteriosus. Ross procedure, used for reconstruction of the left ventricular outflow tract in case of double aortic lesion, also involves this strategy. Although these reconstructive surgeries of the right outflow tract ventricle can be performed with low mortality,1 the duration of the implanted valves or conduits is usually small (< ten years), due to degeneration and/or calcification of the materials used for their manufacture. Varying degrees of pulmonary stenosis, most often associated to pulmonary failure, are the result of conduit degeneration. The earlier the interposition is performed; the lower is the durability of the conduit. Such observation results in the need to perform several open-heartsurgeries,1 which have great impact on patients’ health and quality of life, especially in the case of children. In addition, a new pulmonary valve replacement surgical requires the use of cardiopulmonary bypass (CPB), which can worsen the RV function, usually already compromised.2,3
By mid-2000,4 the therapeutic alternatives to a new surgical valve replacement were limited. The percutaneous implantation of bare-metal stents in these stenotic conduits or bioprostheses was used both in Brazil and abroad,5,6 aiming to increase survival and minimize the need for repeated invasive procedures. Despite providing the possibility of postponing a new surgery, this strategy results in total pulmonary failure with currently well known deleterious effects, including arrhythmias, ventricular dysfunction, and decreased aerobic capacity. In 2000, Bonhoeffer et al.7 were the first to report the transcatheter pulmonary biological valve implantation, later named the Melody® valve (Medtronic, Minneapolis, USA). This valve is made with bovine jugular vein tissue and mounted on a stent.7,8 The technique was originally developed to limit the need for multiple surgical procedures, ultimately replacing a new surgical valve exchange.1 Subsequent studies confirmed the safety and efficacy of this technique,4,8 which has been used in over 1,000 patients in the world, especially in Europe.9
As it occur with the less prevalent diseases, to date there have been no prospective, randomized trials with a large number of patients to definitively guide the treatment of dysfunction in these conduits in the RV outflow tract. This is due to the difficulty in performing controlled and randomized clinical trials for a small population of patients with rare diseases. In addition, there are ethical issues that make it impossible to compare a less invasive procedure with more invasive surgical treatments. The Food and Drug Administration (FDA) has adapted to these particularities in treatment research for uncommon diseases. A review of the recent approvals demonstrates that some drugs were approved based on phase II studies and even in a historical series of cases. In the case of the transcatheter pulmonary valve Melody®, the FDA granted humanitarian approval to the device between late 2009 and early 2010, based on a study of a large case series performed in five americans centers of excellence. The Humanitarian Device Exemption (HDE) program was established in 1990, aiming to create an alternative path to accelerate the introduction in the market of technologies directed to the treatment of patients with rare diseases or conditions. According to FDA rules, when a device is intended to benefit patients with a disease or a condition that affects fewer than 4,000 individuals per year, there is an incentive from the U.S. government, which by a federal law offers the manufacturer exemption from the requirements of efficiency and effectiveness. However, it is necessary to demonstrate to the FDA that the device is safe for patients and that the benefits outweigh the risks. In addition, the applicant must demonstrate that there is no comparable device available for treatment or diagnosis of the same disease or condition. The goal of the humanitarian approval is to benefit a population that would not be treated if the requirements for approval of these devices for diseases and rare conditions were the same as for other devices. Moreover, this initiative encourages companies that manufacture medical devices to develop technologies that meet these populations’ needs.
Evidence-based medicine uses techniques and tools that assist in the search and synthesis of the best available information in the literature. Currently, these techniques are being increasingly used for creating protocols and guidelines throughout the world, including Brazil. Since the Brazilian Health Surveillance Agency (Agência Nacional de Vigilância Sanitária – ANVISA) granted approval for clinical use of the Melody® transcatheter pulmonary biological valve in February 2103, a judicious assessment of this new technology was deemed timely and necessary before its widespread use in Brazil (Appendix).
OBJECTIVEThis study aimed to perform a systematic review the literature on the use of bioprosthesis transcatheter pulmonary valve implantation (TPVI) in patients with dysfunctional homografts, valved conduits, and surgicallyimplanted bioprostheses in the RV outflow tract.
METHODSLiterature review and an extensive search in the computerized databases EMBASE, LILACS, and MEDLINE were performed, using the words “transcatheter pulmonary valve” and “treatment”.
The combination of these terms provided the set of references considered for analysis. The search was restricted to articles in human subjects and of the following types: randomized controlled trials, systematic reviews or narratives, meta-analysis, guidelines, clinical studies, and case series. The search was not restricted regarding date or language.
Articles resulting from the analysis were reviewed, as well as the references of the current guidelines. No meta-analysis of the obtained data was performed.
In the Cochrane Library, the term “transcatheter pulmonary valve” was used to search for systematic reviews.
A search was also performed in annals of congresses of medical specialty societies, such as the Brazilian Society of Hemodynamics and Interventional Cardiology (Sociedade Brasileira de Hemodinâmica e Cardiologia Intervencionista – SBHCI) and the Brazilian Society of Cardiology (Sociedade Brasileira de Cardiologia – SBC).
The first two authors of this manuscript performed the literature review and initial drafting of the manuscript; both work for Evidências company. Subsequently, the article was critically reviewed by interventionists, specialized in congenital heart disease, who are familiar with the subject and will soon be involved in this type of procedure in Brazil. All interventionists are associated in some way to SBHCI, which supported this initiative.
Studied interventionThe intervention assessed in this review was the TPVI.
Description of the studied device for bioprosthesis transcatheter pulmonary valve implantationThere are two types of prosthesis used in TPVI. The oldest is called the Melody® valve, the only prosthesis approved in Brazil for use in the pulmonary position. Since almost all of the evidence found in the literature is based on the use of this device, this review will address mainly this type of prosthesis. The second prosthesis is the SAPIEN® valve (Edwards, Irvine, United States), approved in Brazil for use in the aortic position only, although the valve itself is practically the same.
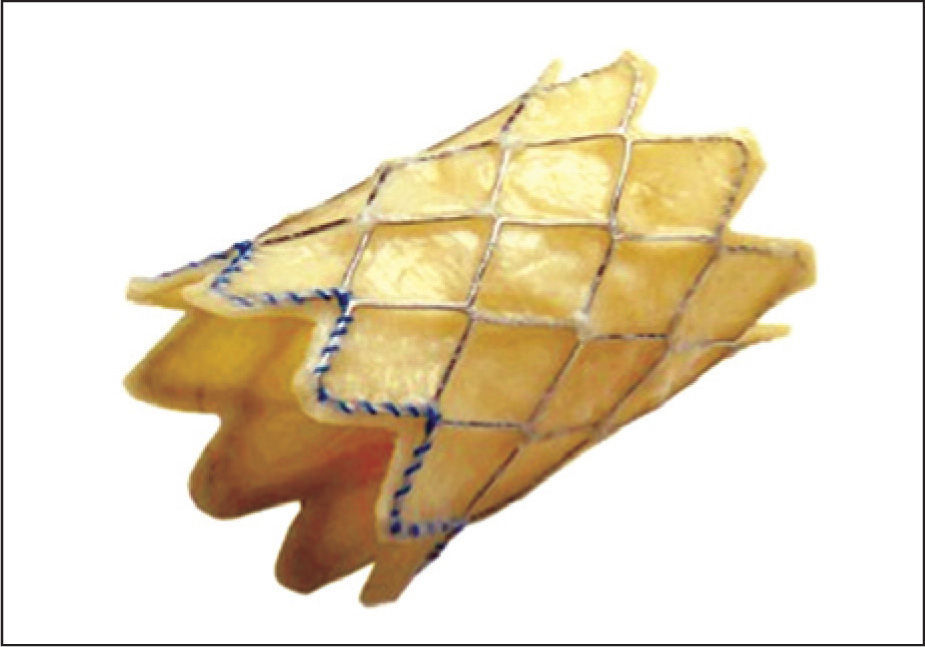
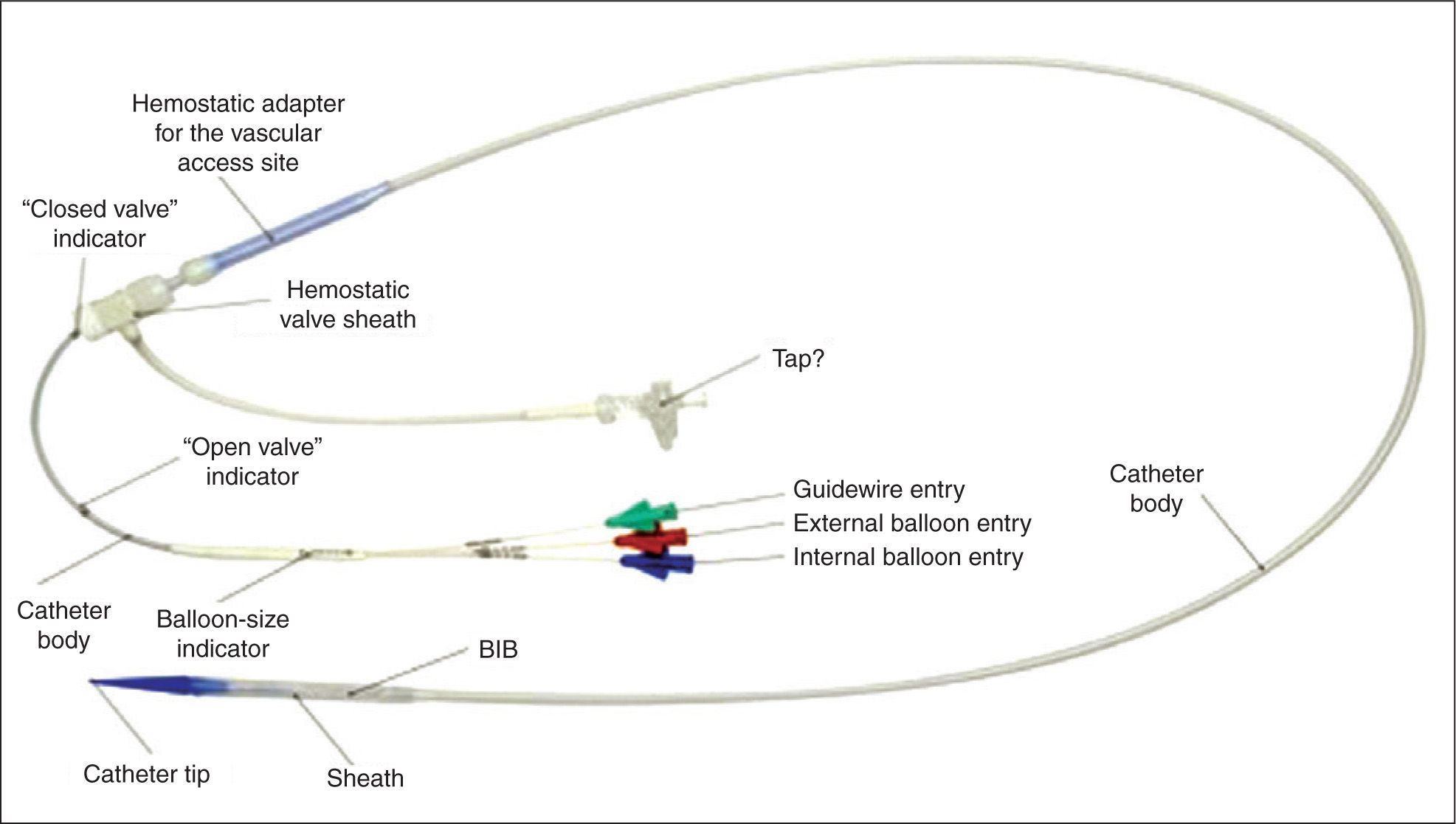
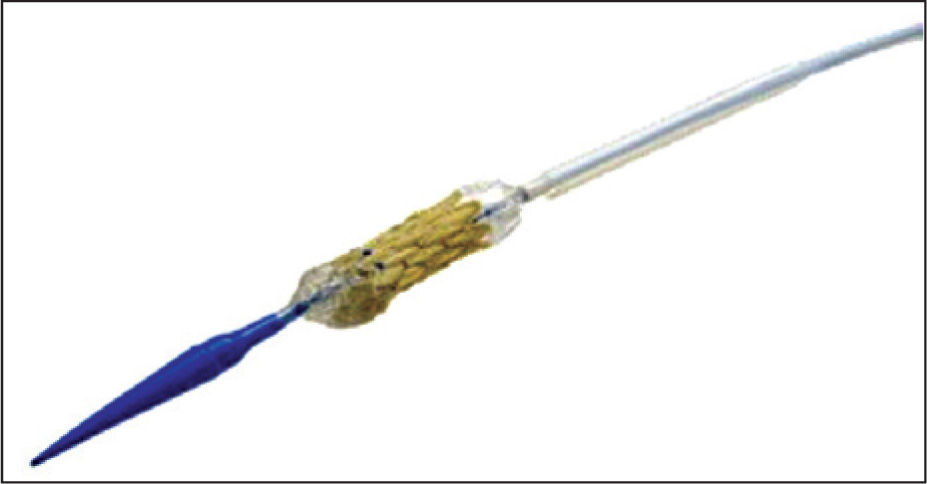
There are two components in the Melody® valve system: the Melody® transcatheter pulmonary valve itself, model PB10 (bovine jugular valve with stent) (Figure 1) and the Ensemble® transcatheter valve delivery system (Medtronic, Minneapolis, United States), NU10 model (Figures 2 and 3).
The Melody® transcatheter pulmonary valve is composed by a heterologous (bovine) jugular valve sutured within a platinum-iridium laser-welded stent, and the stent has gold-welded joints. The system is submitted to a final sterilisation procedure with an adequate sterilising agent containing 1% glutaraldehyde and 20% isopropyl alcohol, in which the valve and stent are preserved and packed until they are used.
The Ensemble® transcatheter valve delivery system consists of a balloon-in-balloon catheter with a polytetrafluoroethylene retractable sheath and a distal support that is large enough to frontally carry the valve, after the valve with the stent has been compressed and adapted to the balloon. The delivery system is available in sizes of 18mm, 20mm, and 22mm. The sheath of the catheter has a side port used to flush the system and a hemostatic sleeve on the sheath to minimize bleeding at insertion. The catheter has a conical distal obturator made of polyether-b-amide (Pebax®; Arkema – Colombes, France). The delivery system is compatible with a guide wire of 0.889mm.
Patients involvedPatients were both children and adults with objective dysfunctions (severe stenosis and/or severe insufficiencies) in homografts, valved conduits, and bioprostheses surgically implanted in the RV outflow tract.
Analysis of the results obtained in the study searchAll references retrieved through the search strategies had their title and abstracts read by two researchers, the first authors of this article. If there was any indication that a reference could meet the inclusion criteria of this study, it was included in a list of selected studies. The corresponding original article was obtained for every selected reference. Each of those articles was read by the researchers, who assessed whether they met the inclusion criteria.
All articles that met the inclusion criteria were separated for data extraction.
Data ExtractionA careful analysis and reading was performed for each article included, in order to extract data.
A specific form for data extraction was prepared. The data from each included study were extracted independently by two reviewers. The name of the first author and year of publication were used to identify the study. All data were extracted directly from the published articles.
Studied variablesThe following variables were assessed:
- •
characteristics of patients;
- •
characteristics of the procedure performed;
- •
hemodynamic results after TPVI; and
- •
safety data.
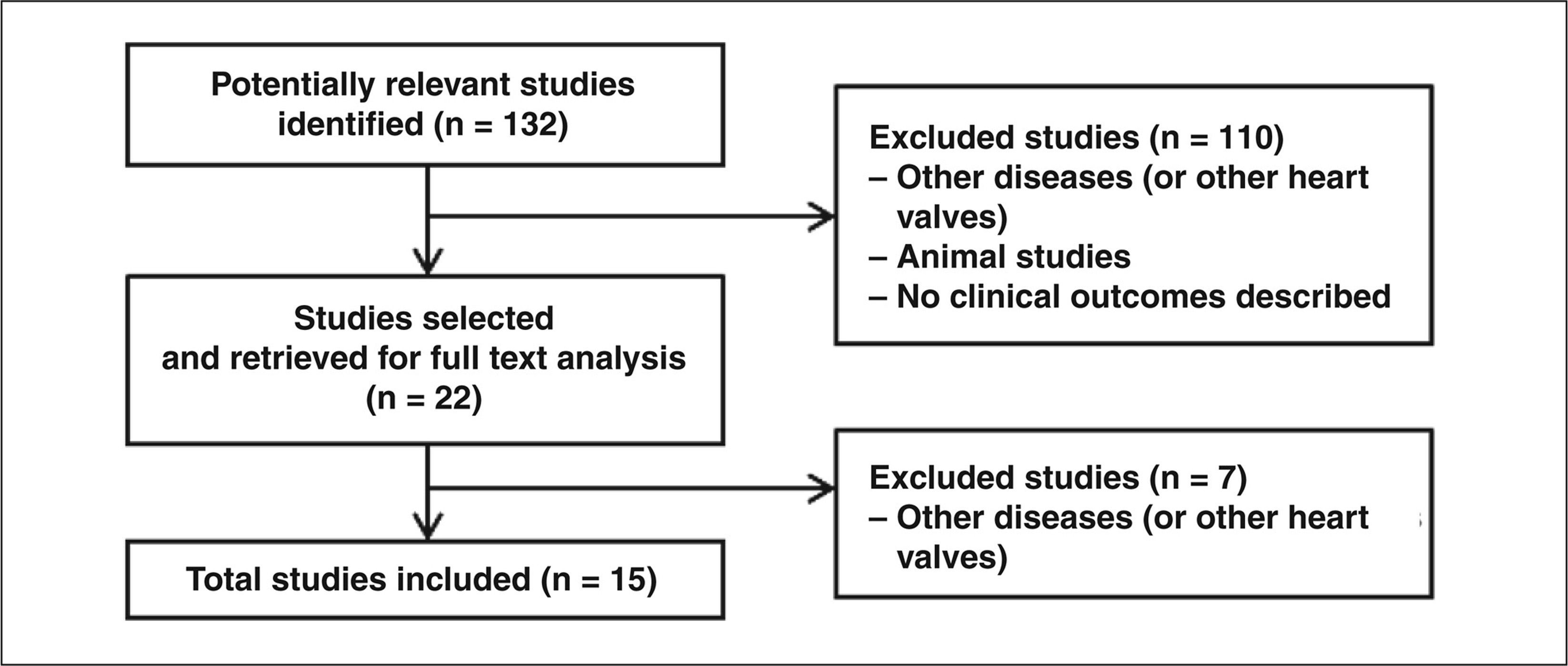
Figure 4 presents the flow chart used for identification of the included studies, as recommended by the Preferred Reporting Items for Systematic reviews and Meta-analysis (PRISMA).10 A total of 132 studies were obtained in the first search, whose articles on other (non-pulmonary) heart valves were not used for preparation of this systematic review. Additionally, animal studies and studies without a description of clinical or laboratory outcomes were excluded. Of this total, 15 studies met the inclusion criteria for this analysis.
Characteristics of patients and identified studiesMost studies included patients with RV outflow tract disorders (stenosis, pulmonary insufficiency, or mixed disorders), after one or more repairs for congenital heart disease with New York Heart Association (NYHA) functional class>II.
No randomized studies or systematic reviews were retrieved on the subject. The published studies are mostly case reports or retrospective studies.
In all studies, the procedure was performed under general anaesthesia, after the patient had been heparinized and received prophylactic antibiotics. The access route was reported in six studies;4,8,11-14 the femoral vein was most often chosen, followed by the jugular vein.
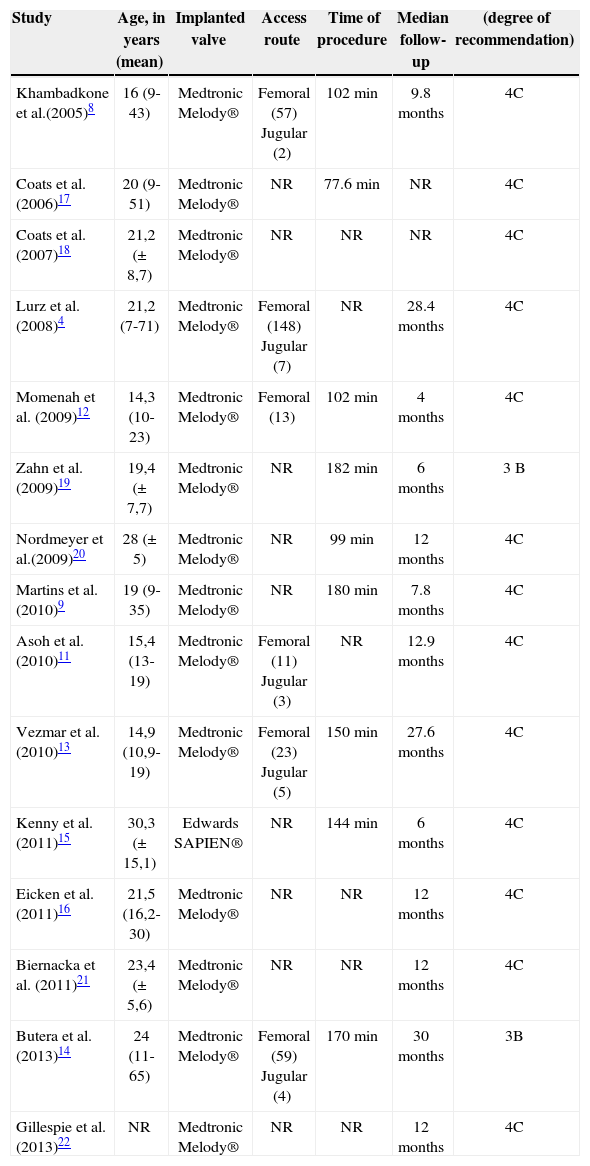
Age varied between studies, but most patients who underwent the implantation were young adults (Table 1). The Melody® valve was predominantly implanted in the published studies (94 %), with a success rate>90%. Only one study used the SAPIEN® valve.15 The mean time of procedure was 140 minutes (Table 1). Patients were discharged approximately two days after the procedure.11,16 The main indications for the procedure were:
- •
Significant pulmonary insufficiency and/or pulmonary stenosis;
- •
RV dilation;
- •
RV dysfunction;
- •
reduction in exercise tolerance; and
- •
diameter of the original conduit at the RV outflow tract>16mm and/or<22mm.
Characteristics of included studies
| Study | Age, in years (mean) | Implanted valve | Access route | Time of procedure | Median follow-up | (degree of recommendation) |
|---|---|---|---|---|---|---|
| Khambadkone et al.(2005)8 | 16 (9-43) | Medtronic Melody® | Femoral (57) Jugular (2) | 102min | 9.8months | 4C |
| Coats et al. (2006)17 | 20 (9-51) | Medtronic Melody® | NR | 77.6min | NR | 4C |
| Coats et al. (2007)18 | 21,2 (±8,7) | Medtronic Melody® | NR | NR | NR | 4C |
| Lurz et al. (2008)4 | 21,2 (7-71) | Medtronic Melody® | Femoral (148) Jugular (7) | NR | 28.4months | 4C |
| Momenah et al. (2009)12 | 14,3 (10-23) | Medtronic Melody® | Femoral (13) | 102min | 4months | 4C |
| Zahn et al. (2009)19 | 19,4 (±7,7) | Medtronic Melody® | NR | 182min | 6months | 3B |
| Nordmeyer et al.(2009)20 | 28 (±5) | Medtronic Melody® | NR | 99min | 12months | 4C |
| Martins et al. (2010)9 | 19 (9-35) | Medtronic Melody® | NR | 180min | 7.8months | 4C |
| Asoh et al. (2010)11 | 15,4 (13-19) | Medtronic Melody® | Femoral (11) Jugular (3) | NR | 12.9months | 4C |
| Vezmar et al. (2010)13 | 14,9 (10,9-19) | Medtronic Melody® | Femoral (23) Jugular (5) | 150min | 27.6months | 4C |
| Kenny et al. (2011)15 | 30,3 (±15,1) | Edwards SAPIEN® | NR | 144min | 6months | 4C |
| Eicken et al. (2011)16 | 21,5 (16,2-30) | Medtronic Melody® | NR | NR | 12months | 4C |
| Biernacka et al. (2011)21 | 23,4 (±5,6) | Medtronic Melody® | NR | NR | 12months | 4C |
| Butera et al. (2013)14 | 24 (11-65) | Medtronic Melody® | Femoral (59) Jugular (4) | 170min | 30months | 3B |
| Gillespie et al. (2013)22 | NR | Medtronic Melody® | NR | NR | 12months | 4C |
NR, Not Reported or not found
Patients with the following characteristics were excluded from the procedure:
- •
known heparin and acetylsalicylic acid allergies;
- •
pregnancy;
- •
active endocarditis;
- •
clinical or biological signs of infection; and
- •
obstruction of the central veins.
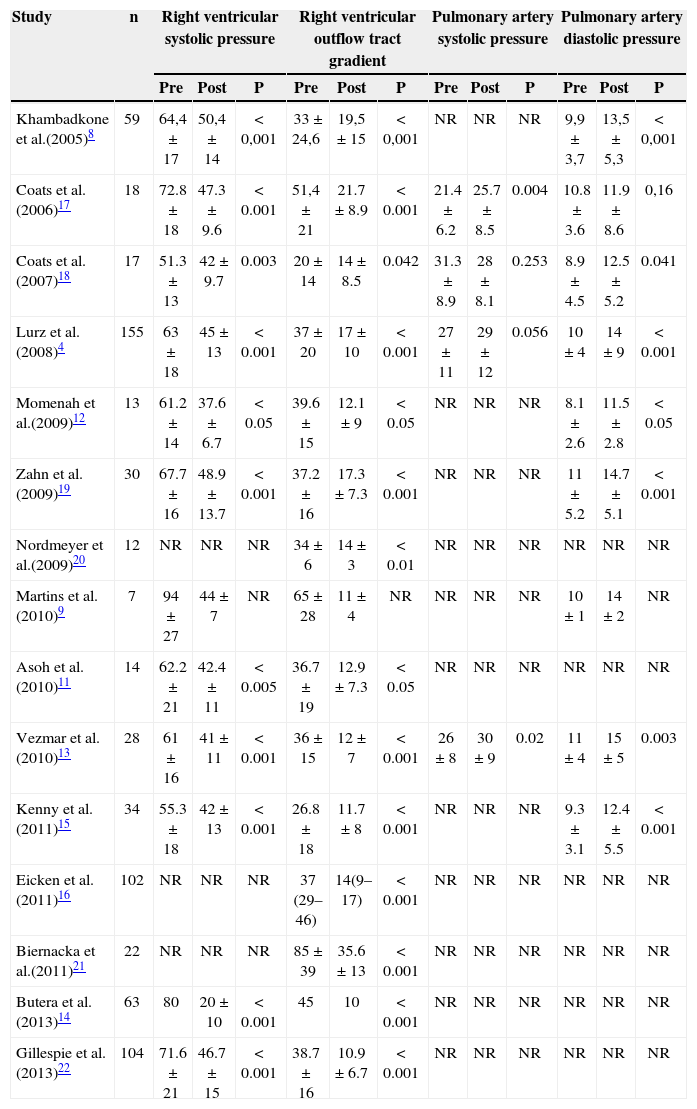
The first study retrieved was published in2005 by Khambadkone et al.8 This study reported the data of 59 patients previously submitted to surgery at the RV outflow tract due to congenital heart disease, with signs of dysfunction of outlet right ventricle and intervention indications such as RV hypertension (more than two-thirds of systemic blood pressure) with pulmonary stenosis or pulmonary insufficiency, RV dilation or failure. Most patients (58/59) were successfully submitted to TPVI via femoral artery (Table 2). Echocardiography performed 24 hours after the TPVI confirmed the immediate hemodynamic findings, showing gradient decrease in the RV outflow tract (63.4±23.4 to 40.5mmHg±18.2mmHg; P<0.001). The number of patients with pulmonary insufficiency>grade II also decreased significantly after the procedure (P<0.001).5 There was improvement in NYHA functional class I to II (P<0.001). Nuclear magnetic resonance imaging (NMRI) performed in 28 cases showed significant reduction in the pulmonary regurgitation fraction, from 21±13% to 3±4% (P<0.001) and in RV end-diastolic volume from 94±28mL to 82±24mL×beat−1 xm−2 (P<0.001).The rate of complications was small (Table 3).
Immediate hemodynamics results after transcatheter pulmonary valve bioprosthesis implantation
| Study | n | Right ventricular systolic pressure | Right ventricular outflow tract gradient | Pulmonary artery systolic pressure | Pulmonary artery diastolic pressure | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pre | Post | P | Pre | Post | P | Pre | Post | P | Pre | Post | P | ||
| Khambadkone et al.(2005)8 | 59 | 64,4±17 | 50,4±14 | <0,001 | 33±24,6 | 19,5±15 | <0,001 | NR | NR | NR | 9,9±3,7 | 13,5±5,3 | <0,001 |
| Coats et al.(2006)17 | 18 | 72.8±18 | 47.3±9.6 | <0.001 | 51,4±21 | 21.7±8.9 | <0.001 | 21.4±6.2 | 25.7±8.5 | 0.004 | 10.8±3.6 | 11.9±8.6 | 0,16 |
| Coats et al.(2007)18 | 17 | 51.3±13 | 42±9.7 | 0.003 | 20±14 | 14±8.5 | 0.042 | 31.3±8.9 | 28±8.1 | 0.253 | 8.9±4.5 | 12.5±5.2 | 0.041 |
| Lurz et al.(2008)4 | 155 | 63±18 | 45±13 | <0.001 | 37±20 | 17±10 | <0.001 | 27±11 | 29±12 | 0.056 | 10±4 | 14±9 | <0.001 |
| Momenah et al.(2009)12 | 13 | 61.2±14 | 37.6±6.7 | <0.05 | 39.6±15 | 12.1±9 | <0.05 | NR | NR | NR | 8.1±2.6 | 11.5±2.8 | <0.05 |
| Zahn et al.(2009)19 | 30 | 67.7±16 | 48.9±13.7 | <0.001 | 37.2±16 | 17.3±7.3 | <0.001 | NR | NR | NR | 11±5.2 | 14.7±5.1 | <0.001 |
| Nordmeyer et al.(2009)20 | 12 | NR | NR | NR | 34±6 | 14±3 | <0.01 | NR | NR | NR | NR | NR | NR |
| Martins et al.(2010)9 | 7 | 94±27 | 44±7 | NR | 65±28 | 11±4 | NR | NR | NR | NR | 10±1 | 14±2 | NR |
| Asoh et al.(2010)11 | 14 | 62.2±21 | 42.4±11 | <0.005 | 36.7±19 | 12.9±7.3 | <0.05 | NR | NR | NR | NR | NR | NR |
| Vezmar et al.(2010)13 | 28 | 61±16 | 41±11 | <0.001 | 36±15 | 12±7 | <0.001 | 26±8 | 30±9 | 0.02 | 11±4 | 15±5 | 0.003 |
| Kenny et al.(2011)15 | 34 | 55.3±18 | 42±13 | <0.001 | 26.8±18 | 11.7±8 | <0.001 | NR | NR | NR | 9.3±3.1 | 12.4±5.5 | <0.001 |
| Eicken et al.(2011)16 | 102 | NR | NR | NR | 37 (29–46) | 14(9–17) | <0.001 | NR | NR | NR | NR | NR | NR |
| Biernacka et al.(2011)21 | 22 | NR | NR | NR | 85±39 | 35.6±13 | <0.001 | NR | NR | NR | NR | NR | NR |
| Butera et al.(2013)14 | 63 | 80 | 20±10 | <0.001 | 45 | 10 | <0.001 | NR | NR | NR | NR | NR | NR |
| Gillespie et al.(2013)22 | 104 | 71.6±21 | 46.7±15 | <0.001 | 38.7±16 | 10.9±6.7 | <0.001 | NR | NR | NR | NR | NR | NR |
NR, Not Reported or not found
Rate of procedure-related complications during follow-up
| Study | n | Stent fracture | Arrhythmias | Endocarditis | Mortality |
|---|---|---|---|---|---|
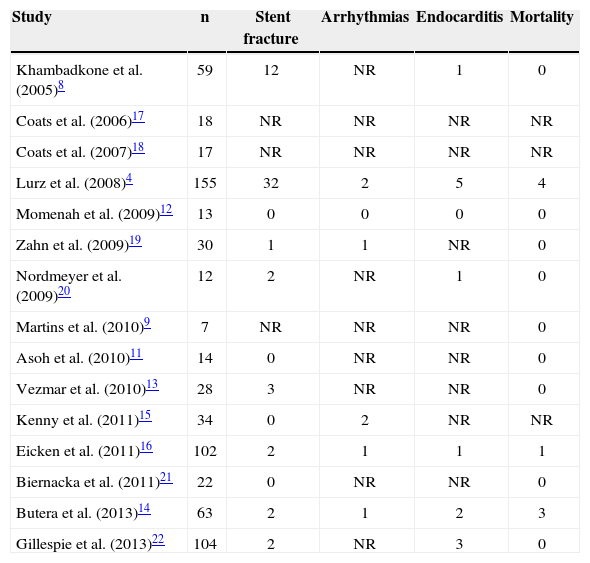
| Khambadkone et al. (2005)8 | 59 | 12 | NR | 1 | 0 |
| Coats et al. (2006)17 | 18 | NR | NR | NR | NR |
| Coats et al. (2007)18 | 17 | NR | NR | NR | NR |
| Lurz et al. (2008)4 | 155 | 32 | 2 | 5 | 4 |
| Momenah et al. (2009)12 | 13 | 0 | 0 | 0 | 0 |
| Zahn et al. (2009)19 | 30 | 1 | 1 | NR | 0 |
| Nordmeyer et al. (2009)20 | 12 | 2 | NR | 1 | 0 |
| Martins et al. (2010)9 | 7 | NR | NR | NR | 0 |
| Asoh et al. (2010)11 | 14 | 0 | NR | NR | 0 |
| Vezmar et al. (2010)13 | 28 | 3 | NR | NR | 0 |
| Kenny et al. (2011)15 | 34 | 0 | 2 | NR | NR |
| Eicken et al. (2011)16 | 102 | 2 | 1 | 1 | 1 |
| Biernacka et al. (2011)21 | 22 | 0 | NR | NR | 0 |
| Butera et al. (2013)14 | 63 | 2 | 1 | 2 | 3 |
| Gillespie et al. (2013)22 | 104 | 2 | NR | 3 | 0 |
NR, Not Reported or not found
In 2006, Coats et al.17 reported the results of 18 patients with pure isolated pulmonary stenosis in 93 patients undergoing TPVI. The results also were favorable to the procedure. There was a significant improvement in NYHA functional class I to II (P<0.001) one month after the procedure. The echocardiography revealed a significant decrease in RV systolic pressure (from 84.9±17.5mmHg to 50.7±14.4mmHg; P<0.001) and in the RV outflow tract gradient (85.2±19mmHg to 41.1±12.3mmHg; P<0.001). In the following year, the same authors published data on 17 patients with pure isolated pulmonary insufficiency undergoingTPVI.18 The results were also favorable to the procedure, with significant improvement in NYHA functional class from II to I (P<0.001). The improvement in hemodynamics was more significant in patients with pure pulmonary insufficiency, when compared to those with pulmonary insufficiency, and is described in Table 2.
Lurz 2008 (level of evidence 4C)The largest case series was published in 2008 by Lurz et al.,4 with 155 patients, and included patients who underwent the procedure at the initial phase of the learning curve. Most patients had NYHA functional class>II. Among the patients, 61 had predominantly pulmonary stenosis, 46 had pulmonary insufficiency, and 44 had combined dysfunctions. Hemodynamic benefits are described in Table 2. The absence of reintervention in70 months was 70%. In this group, most reinterventions were performed due to valved stent fractures, which is why the preparation of the conduit with previous implantation of a conventional stent (non-valved) in conduit (pre-stenting) was later included in the technique. Another risk factor for reintervention was the finding of immediate residual gradients>25mmHg, indicating the need to eliminate gradients through the technique of preparing the conduit with bare-metal stents. The learning curve also played an important role in reducing the need for new procedures, with significant improvement in latest two-thirds of the treated cohort. In 2009, three more studies12,19,20 were published, with positive hemodynamic results for TPVI.
Zahn, 2009 (level of evidence 3B)Zahn et al.19 reported data on 30 patients with a success rate of the procedure in 29 Melody® valve implantations. During the study follow-up, 100% of the patients were free of new procedures, and 79% of the 24 patients with NYHA functional class≥II showed functional class improvement. This study was continued, and culminated in another study that resulted in the approval of the Melody® prosthesis by the FDA under the afore mentioned HDE provision.
Nordmeyer, 2009 (level of evidence 4C)Nordmeyer et al.20 published the results of12 patients undergoing TPVI, of whom 50% had predominantly pulmonary stenosis, 33% had pulmonary insufficiency, and 17% had mixed dysfunctions. The success rate of the procedure was 100 %, and no acute complications were related to the procedure.
Momenah, 2009 (level of evidence 4C)In the study by Momenah et al.,12 the procedure was performed in 13 patients with no acute or late procedurerelated complications. This study had a follow-up of only four months. The echocardiography performed 24 hours after the TPVI procedure showed reduction in RV systolic pressure and in the RV outflow tract gradient. Three other studies were published in 2010.9,11,13
Martins, 2010 (level of evidence 4C)Martins et al.9 reported data on 13 patients submitted to TPVI. The predominant dysfunction in the RV outflow tract was the mixed lesion, and all patients underwent pre-stenting of the conduit. Angiographic results showed resolution of stenosis and/or insufficiency in all patients. There were no procedure-related complications.
Vezmar, 2010 (level of evidence 4C)In the study by Vezmar et al.,13 17 patients (61%)had mixed lesions, nine (32%) had pure pulmonary stenosis, and two (7%) had isolated pulmonary insufficiency. The parameters of the echocardiography performed within the first 24 hours showed decreased RV pressure and RV outflow tract gradient. One month after the TPVI, 80%of the patients had no detectable pulmonary insufficiency, 68% of whom had pulmonary insufficiency≥grade 3 before the procedure (P<0.001).The time free from reintervention was 83% at 36months of follow-up.
Asoh, 2010 (level of evidence 4C)Asoh et al.11 published a retrospective study of 14 patients with dysfunctional conduits in the RV outflow tract, of whom ten had mixed lesions, two had pulmonary stenosis, and two had pulmonary insufficiency. The parameters of the echocardiography performed within the first 24 hours also showed decrease in RV pressure (82.2±15.6mmHg to 61±10mmHg; P<0.01) and in the RV outflow tract gradient (59.6±26.8mmHg to 41±19.1mmHg; P<0.05). The hemodynamic improvement of these three studies is described in Table 2.
Three studies published in 2011 were retrieved.15,16,21
Biernacka, 2011 (level of evidence 4C)Biernacka et al.21 presented data on 22 patients submitted to TPVI at the European Congress. There were nine patients with pure pulmonary stenosis, 11 with mixed dysfunction, and two with pure pulmonary insufficiency. The procedural success rate was 96%. They observed improvement in NYHA functional class six months after the procedure, which remained stable at 12 months and24 months (P<0.005). There was a significant improvement in the mean pulmonary insufficiency fraction one month after the TPVI (15.7±11.1%to 2.6±2.9%; P=0.0005).
Eicken, 2011 (level of evidence 4C)Eicken et al.16 reported data on 102 patients, of which 36 had pulmonary stenosis, 18 had pulmonary insufficiency and 48 had mixed lesion. Pulmonary insufficiency, assessed by nuclear magnetic resonance (NMR), was significantly reduced (P<0.001). RV-end diastolic volume was also evaluated by NMR and decreased from 106mL/m2 (93–133mL/m2) to90 mL/m2 (71–108mL/m2, P<0.001).
Kenny, 2011 (level of evidence 4C)Kenny et al.15 published the only study using a valve different than Melody®, the SAPIEN® Pulmonic THV (Edwards, Irvine, California, United States), which included 34 patients. The implantation success rate was 97.1%. There was improvement in hemodynamics, as observed with the Melody® valve (Table 2). After the procedure, pulmonary insufficiency was classified as minimal in 31 of 33 implantation procedures.
Two studies were published in 2013.14,22
Gillespie, 2013 (level of evidence 4C)Gillespie et al.22 presented a series of 104 cases from eight centers in the United States submitted to TPVI with Melody® valve. There were hemodynamic benefits, described in Table 2, with no morbidities or deaths related to the procedure. After 12 months of follow-up, no patient had mild pulmonary insufficiency and only four patients had a gradient>30mmHg in the RV outflow tract.
Butera, 2013 (level of evidence 3B)A prospective, multicenter study published by Butera et al.14 reported an experience in 63 patients undergoing TPVI. The success rate of the procedure was 97%. In 51 patients with pulmonary stenosis (21with pure pulmonary stenosis and 30 with associated pulmonary insufficiency), RV pressure and RV outflow tract gradient decreased significantly (Table 2). In the 42 patients with severe pulmonary insufficiency (12 with pure pulmonary insufficiency and 30 with associated pulmonary stenosis), the degree of pulmonary insufficiency also decreased (pre-TPVI: 42 patients with grade≥2; post-TPVI: four patients).
Immediate hemodynamic resultsAccording to the NYHA criteria, there was statistically significant improvement in functional class in most studies (class II to I). The same occurred with hemodynamics parameters. A significant decrease in RV systolic pressure and reduction in the RV outflow tract gradient were observed in 100% of the analysed studies (Table 2).
Few studies4,13,17,18 reported data on systolic pulmonary artery pressure. For this parameter, only two studies13,17 showed a significant increase of 26mmHg to 30mmHg.
Regarding diastolic blood pressure in the pulmonary artery,sevenstudies4,8,12,13,19 reported this outcome; there was significant pressure increase in all of them (Table 2).
SafetyThe main complications are described in Table 3. In general, the immediate mortality rate related to TPVI was<1%. The rate of endocarditis was small and often associated with dental procedure without prophylaxis at the late follow-up.4,8,16 In many studies,4,20 when stent fracture occurred during follow-up, TPVI was performed again without complications.
Recommendations from medical specialties and regulatory entitiesANVISA approved the use of the transcatheter pulmonary valve (Melody®) for the treatment of patients with the following clinical conditions:
- •
Patients with prosthetic conduits in the RV out-flow tract, with pulmonary insufficiency and clinical indication for invasive or surgical intervention;
- •
Patients with prosthetic conduits in the RV outflow tract with pulmonary stenosis and in whom the risk of regurgitation worsening is a relative contraindication to balloon dilation or stent implantation;
- •
existence of a complete conduit (circumferential) in the RV outflow tract, whose original diameter was≥16mm and<22mm when implanted.
The FDA and specialty societies in cardiology in Europe (European Society of Cardiology [ESC], European Association for Cardio-Thoracic Surgery [EACTS],and National Institute for Clinical Excellence [NICE]) also approve the performance of TPVI for patients with pulmonary insufficiency and pulmonary stenosis.23-26 It is important to remember that the study that culminated with the approval of Melody® valve by the FDA through HDE was a continuation of the first trial published by Zahn et al.19 In this second study with over150 patients, recently published by McElhinney et al.,27 the risks of procedures requiring immediate surgical intervention were very low (<1%) and included coronary artery compression by the valve, conduit rupture, or stent migration. Initially observed hemodynamics benefits were maintained during follow-up, with most patients in functional class I or II, non-significant gradients in the RV outflow tract, and adequate valve function during a three-year period. Stent fractures were observed in 25% of cases, in a mean follow-up of 12 months, and 38% of these patients needed to have a second device implanted.
The American Heart Association (AHA) recently published a document to define guidelines for percutaneous procedures in congenital heart defects, in which they recommend TPVI as class II A – level of evidence B.28
Recommendations for percutaneous implantation of pulmonary valve, adapted from the American Heart Association recommendationsClass IIaPercutaneous implantation of pulmonary valve is indicated in patients weighing>20-30kg in the postoperative period of surgeries during which conduits were used to restore the continuity of the right ventricle to the pulmonary artery, and that have objective evidence of conduit dysfunction (significant stenosis and/or regurgitation) and meet the pre-established inclusion and exclusion criteria (level of evidence: B).
The SBHCI is at the final stages of preparing its guidelines for percutaneous treatment of congenital heart diseases, which is in agreement with the position adopted by the AHA (Carlos A. C. Stone, personal communication).
FINAL CONSIDERATIONSDysfunctions in the RV outflow tract, especially pulmonary insufficiency when associated with pulmonary stenosis, is associated with undesirable hemodynamic effects in the long term, such as RV dilation and dysfunction, tricuspid valve regurgitation, arrhythmias, and death.1-3 The conduits used in the surgical correction of RV outflow tract dysfunctions frequently develop regurgitation and/or progressive stenosis requiring multiple surgeries, which result in significant mobility and mortality in these patients.1-3 The Melody® valve prosthesis, developed by Bonhoeffer et al.7 to be percutaneously implanted, brought considerable benefits to patients with dysfunctions in the RV outflow tract, as it simultaneously corrects pulmonary insufficiency and stenosis. A significant decrease in RV systolic pressure can be observed when using this valved device, due to stenosis relief in its outflow tract, also determining are duction or abolition of pulmonary reflow in those cases with predominant regurgitation. The hemodynamic improvement observed after TPVI and during the late follow-up of these patients was also confirmed by echocardiography assessment and magnetic resonance imaging, directly related to reduction in the RV intracavitary pressure and of the systolic gradient in its outflow tract and in the pulmonary regurgitation rate.
From a clinical standpoint, TPVI is also associated with improved functional capacity of patients. The analysed studies demonstrated that, after the implantation, most of the patients developed mild symptoms or even no symptoms, little or no limitation of routine activities, and comfortat rest. This reflects a significant improvement in quality of life of this population. TPVI also showed immediate mortality<1%, significant periprocedural morbidity<4%, and medium-term survival of 96.6%. Thus, the procedure was shown to be safe, providing an increase in years of survival for the patients.
It is important to recall that TPVI is a high-complexity procedure and should be performed by surgeons acquainted with the percutaneous treatment of congenital heart disease, particularly the implant of stents in conduits and pulmonary arteries. It requires a variety of materials for its performance and possible treatment of complications (e.g. coated stent implantation in the conduit in case of ruptured conduit). Specific training to allow the surgeon to perform this type of procedure is necessary before its large-scale use. In this sense, SBHCI has led this initiative: the training guidelines have already been established and consolidated for percutaneous aortic valve implantation in the elderly.
Finally, the aim of this article was to address more specifically the use of the Melody® valve, according to its on-label use in conduits between the right ventricle and the pulmonary artery. There are numerous cases of off-label use for treatment of native outflow tracts, where there is a clear anchor point for the device (John Cheatham, personal communication).The Melody® valve has been used in the tricuspid, mitral, and aortic positions with optimal initial published results.29,30 Such specific uses must be considered in patients and institutions in an individualized and personalized manner.
CONCLUSIONSTPVI is a safe and effective procedure in the treatment of dysfunctions (pulmonary stenosis, pulmonary regurgitation, or both) of homografts, bioprosthesis, and other valved conduits surgically implanted in the RV outflow. Such functional recovery is achieved without the need for CPB, and is associated with great immediate and medium-term outcomes. Although there have been no studies comparing the percutaneous and the surgical techniques, current evidence in the literature suggests that TPVI should be the first-choice procedure or, at least, an excellent therapeutic alternative for patients with dysfunctions in the conduits of RV outflow tract.
CONFLICTS OF INTERESTEvidências company was hired and provided services to Medtronic to perform this systematic review. Carlos A. C. Pedra is a lecturer at Medtronic. The remaining authors declare to have no conflicts of interests.
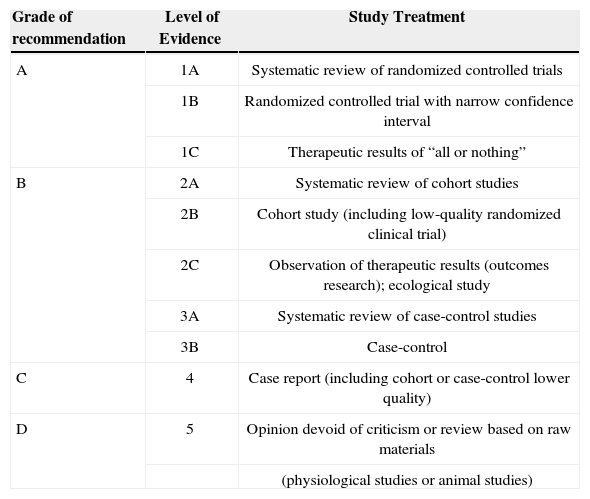
Levels of scientific evidence according to the classification of the Oxford Centre for Evidence-Based Medicine
| Grade of recommendation | Level of Evidence | Study Treatment |
|---|---|---|
| A | 1A | Systematic review of randomized controlled trials |
| 1B | Randomized controlled trial with narrow confidence interval | |
| 1C | Therapeutic results of “all or nothing” | |
| B | 2A | Systematic review of cohort studies |
| 2B | Cohort study (including low-quality randomized clinical trial) | |
| 2C | Observation of therapeutic results (outcomes research); ecological study | |
| 3A | Systematic review of case-control studies | |
| 3B | Case-control | |
| C | 4 | Case report (including cohort or case-control lower quality) |
| D | 5 | Opinion devoid of criticism or review based on raw materials |
| (physiological studies or animal studies) |