The experience with percutaneous closure of atrial septal defect (ASD) in infants is limited. We sought to determine the feasibility, safety and efficacy of this procedure in children weighing < 20kg.
MethodsObservational study of a cohort of children weighing < 20kg undergoing percutaneous closure. Patients with right ventricular enlargement and evident symptoms were included. ANVISA approved devices were implanted under transesophageal echocardiography monitoring. Patients were evaluated 1, 3, 6 and 12 months after the procedure.
ResultsEighty patients were treated between October 1997 and May 2012. Median age and weight were 4 years (1-12) and 13.5kg (5-20), respectively, 20 patients had a genetic syndrome (25%) and 4 patients (5%) had an additional ASD. Only one patient required 2 devices. Two patients had associated defects that were treated in the same procedure (pulmonary valve stenosis and arteriovenous fistula). One patient developed total atrioventricular block during device implantation, solved spontaneously 36 hours after device removal, with no need for pacemaker implantation. This patient was successfully treated percutaneously 6 months later without complications. Seventy-nine patients were discharged within 24 hours after the procedure. A mild residual shunt (1-2mm) was observed in 5% of the cases before discharge. There was no residual shunt 6 months after the procedure. There were no complications in the late follow-up.
ConclusionsPercutaneous ASD closure in selected symptomatic infants is a feasible, safe and effective alternative and should be the first option therapy.
Factibilidade, Segurança e Eficácia doFechamento Percutâneo da Comunicação Interatrial em Crianças Pequenas
IntroduçãoA experiência com o fechamento percutâneo da comunicação interatrial (CIA) em crianças pequenas é limitada. Avaliamos a factibilidade, a segurança e a eficácia desse procedimento em crianças com peso < 20kg.
MétodosEstudo descritivo observacional de uma coorte de crianças < 20kg submetidas a tratamento percutâneo. Pacientes com dilatação ventricular direita e sintomas evidentes foram incluídos. Implantamos próteses aprovadas pela ANVISA, sob monitorização ecocardiográfica transesofágica. Os pacientes foram avaliados 1 mês, 3 meses, 6 meses e 12 meses após.
ResultadosEntre outubro de 1997 e maio de 2012, 80 pacientes foram tratados. As medianas de idade e peso foram de 4 anos (1-12) e 13,5kg (5-20), respectivamente, 20 pacientes apresentavam alguma síndrome genética (25%) e 4 pacientes (5%) apresentavam CIA adicional. Somente um paciente necessitou duas próteses. Dois pacientes tinham defeitos associados; os quais foram tratados no mesmo procedimento (estenose pulmonar valvar e fístula arteriovenosa). Um paciente desenvolveu bloqueio atrioventricular total durante o implante da prótese, resolvido espontaneamente 36 horas após a remoção da prótese, sem necessidade de implante de marca-passo. Esse paciente foi tratado percutaneamente 6 meses após com sucesso, sem complicações. Setenta e nove pacientes receberam alta hospitalar em até 24 horas após o procedimento. Fluxo residual discreto (1-2mm) foi observado em 5% dos casos antes da alta. Após 6 meses de seguimento, não foi detectado fluxo residual. Não houve complicações tardias no seguimento.
ConclusõesO fechamento percutâneo da CIA em crianças pequenas selecionadas e sintomáticas é uma alternativa terapêutica factível, segura e eficaz, devendo ser a primeira opção para seu tratamento.
The first description of interatrial septal defects dates back to 1875 and was performed by Rokitansky, but their physiopathology and clinical picture started to be revealed only after 1941.1Ostium secundum atrial septal defect (ASD) has the fourth or fifth highest incidence of congenital heart diseases, corresponding to approximately 5% to 10% of cases.2−4
With the gradual decrease in pulmonary vascular resistance, and improved hypertrophy and right ventricular compliance within the first year of life, ASD begins to have hemodynamic consequences with right chamber volume overload. However, most of these patients remain asymptomatic during the first years of life. The defect is usually electively corrected before the child reaches school age, at around 5 years old. Occasionally, patients younger than 5 years with ASD develop isolated symptoms generated by pulmonary hyperflow, including recurrent respiratory infections, bronchospasm, congestive heart failure, or failure to thrive.5,6 This scenario is aggravated when the ASD is associated with prematurity, chronic pulmonary diseases (asthma, bronchodysplasia), genetic syndromes, or other systemic diseases (renal and liver failure, among others). These patients require early treatment.
The surgical approach was considered the method of choice for ASD management for over four decades. Although it has very good results,7,8 it requires cardiopulmonary bypass, hemotherapy, and longer hospital stay, in addition to some postoperative morbidity, such as pain, infections, pericardial effusion, arrhythmias, and sternotomy scars.9−11 The first percutaneous ASD closure was described in 1976.12 With the technical advances and surgeons’ greater experience, percutaneous treatment has become the modality of choice for the management of most patients with ASD.13−19 Surgery is reserved for cases with unfavorable anatomy for the percutaneous approach, associated cardiac anomalies, or other types of ASD (e.g. ostium primum ASD, sinus venosus, and coronary sinus).19 Although the percutaneous treatment is well established for older children, adolescents, and adults, there are few reports on the use of this method in small children.20−24
This article describes an experience with the percutaneous treatment of ASD in children weighing < 20kg, assessing its feasibility, efficacy, and safety.
METHODSStudy designObservational, longitudinal descriptive study of a cohort of children weighing < 20kg submitted to percutaneous closure of ostium secundum ASD in two cardiology referral centers in Brazil. Data collection was performed retrospectively, through medical record analysis. Demographic, clinical, echocardiographic, and hemodynamic data were collected. Parents or guardians of patients were informed of the procedure and signed an informed consent approved by the ethics and research committee of both institutions.
Inclusion criteriaChildren weighing < 20kg with clinical and echocardiographic diagnosis of ostium secundum ASD with hemodynamic consequences, characterized by right ventricular volume overload associated with one or more of the following diagnoses:
- −
congestive heart failure;
- −
recurrent respiratory infections (six or more episodes in 12 months);
- −
chronic pulmonary disease (e.g. pulmonary bronchodysplasia, asthma, etc.).
- −
failure to thrive;
- −
genetic syndromes (e.g. Down syndrome, etc.);
- −
severe systemic disease (e.g. renal or liver failure, etc.).
Exclusion criteria included weight < 5kg, ASD classified as non-ostium secundum, fixed pulmonary artery hypertension, presence of associated intracardiac defects with surgical indication, and unfavorable anatomy for percutaneous closure of the defect.19−25 The absolute diameter of the ASD was not considered an exclusion factor for the procedure, nor was a deficient anteriorsuperior border, provided that the contralateral border was adequate.25 Patients with large ASDs that required devices that were too large for the size of the heart, with risk of interference with atrioventricular valve function and pulmonary venous or coronary sinus drainage were excluded. Patients with more than one deficient border (usually contralateral) around the defect, usually accompanied by thin and distensible atrial septum, were also excluded. Patients with two adjacent ASDs that could be occluded with only one prosthesis, with two large and distant ASDs, but with the possibility of occlusion with two devices; with two distant ASDs, but in which the smallest defect was < 3-4mm (therefore, with no clinical significance); with cribriform ASD with multiple small defects that could be occluded with only one prosthesis; and with simple additional heart defects that could be treated by catheterisation, such as patent ductus arteriosus (PDA), pulmonary valve stenosis, or vascular malformation were not excluded.
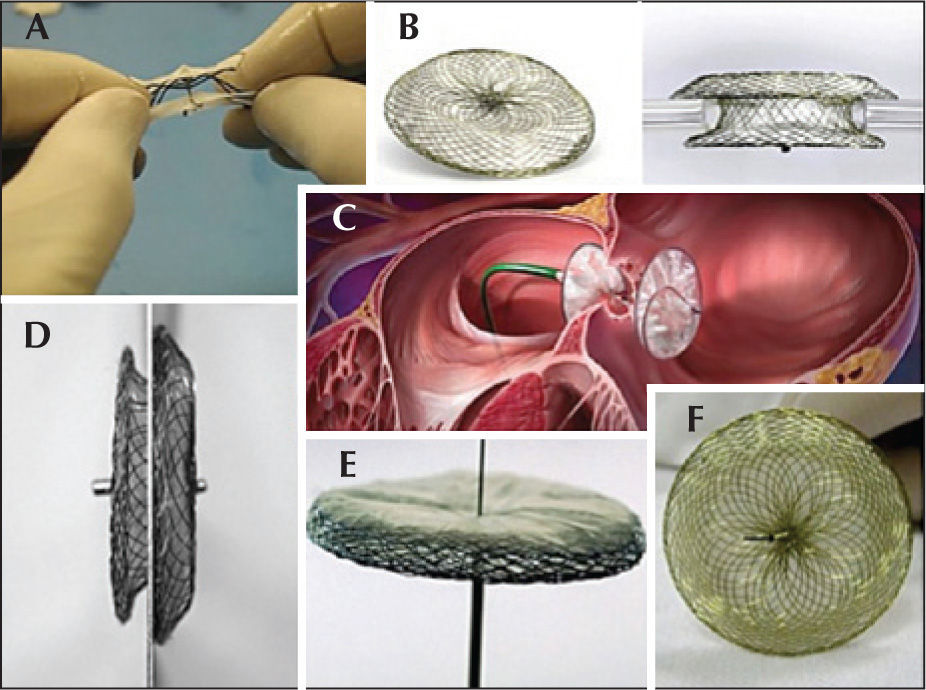
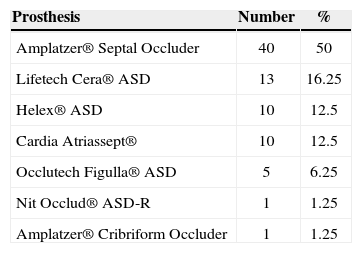
Prostheses used in the trialThe prostheses used in this trial were approved by the Brazilian Health Surveillance Agency (Agência Nacional de Vigilância Sanitária – ANVISA), with wellestablished safety and efficacy in previous experiments in Brazil, the United States, and Europe.17,18,26−28 These were: Amplatzer® Atrial Septal Occluder and Amplatzer® Cribriform Occluder (St. Jude Medical Inc. – St. Paul, United States), Gore Helex® Atrial Septal Occluder (W.L. Gore & Associate – Newark, United States), Lifetech Cera® ASD Occluder (Lifetech Scientific Corporation – Shenzhen, China), Occlutech Figulla® (Occlutech AB – Helsingborg, Sweden), Cardia® Atriassept (CardioLogic Ltd.), and Nit-Occlud ASD-R® (PFM Medical Ag – Koln, Germany) (Figure 1).
The spatial conformation of these prostheses consists of two discs of varying sizes with a central connecting waist.17,18,27,28 With the exception of the Helex® prosthesis, all others use nitinol – an alloy of nickel and titanium – as the main component of their self-expanding mesh. Occlusion is performed by the filling of the septal orifice by the self-centring waist, which exerts radial force on the ASD edges, as well as physical barrier to blood flow exerted by the discs positioned in each of the atria after implantation.
Nitinol is also present in the Helex® prosthesis, but in the form of a helical strut that provides support to a dense mesh of polytetrafluoroethylene (PTFE), which has occluding power after it is fully exposed and positioned. 26 In this prosthesis, the waist only connects the two disks and is not self-centring.
ProcedureThe procedure was performed under general anaesthesia, controlled by fluoroscopy and transesophageal echocardiography (TEE) using a two-dimensional paediatric probe suitable for children weighing < 20kg with a Philips (Healthcare, DA – Best, the Netherlands) or General Electric (GE – United States) equipment. Femoral venous access was used in all patients, except in two, in whom the trans-hepatic route was necessary due to the absence of the hepatic portion of the inferior vena cava, caused by left atrial isomerism. Heparin at a dose of 100IU/kg was administered through the lateral route of the venous sheath, immediately after its insertion. The right cardiac chambers were catheterized and measures of pulmonary pressure were directly acquired. Calculations of pulmonary/systemic flow and resistance made using Fick’s method were restricted to cases with severe pulmonary hypertension.29
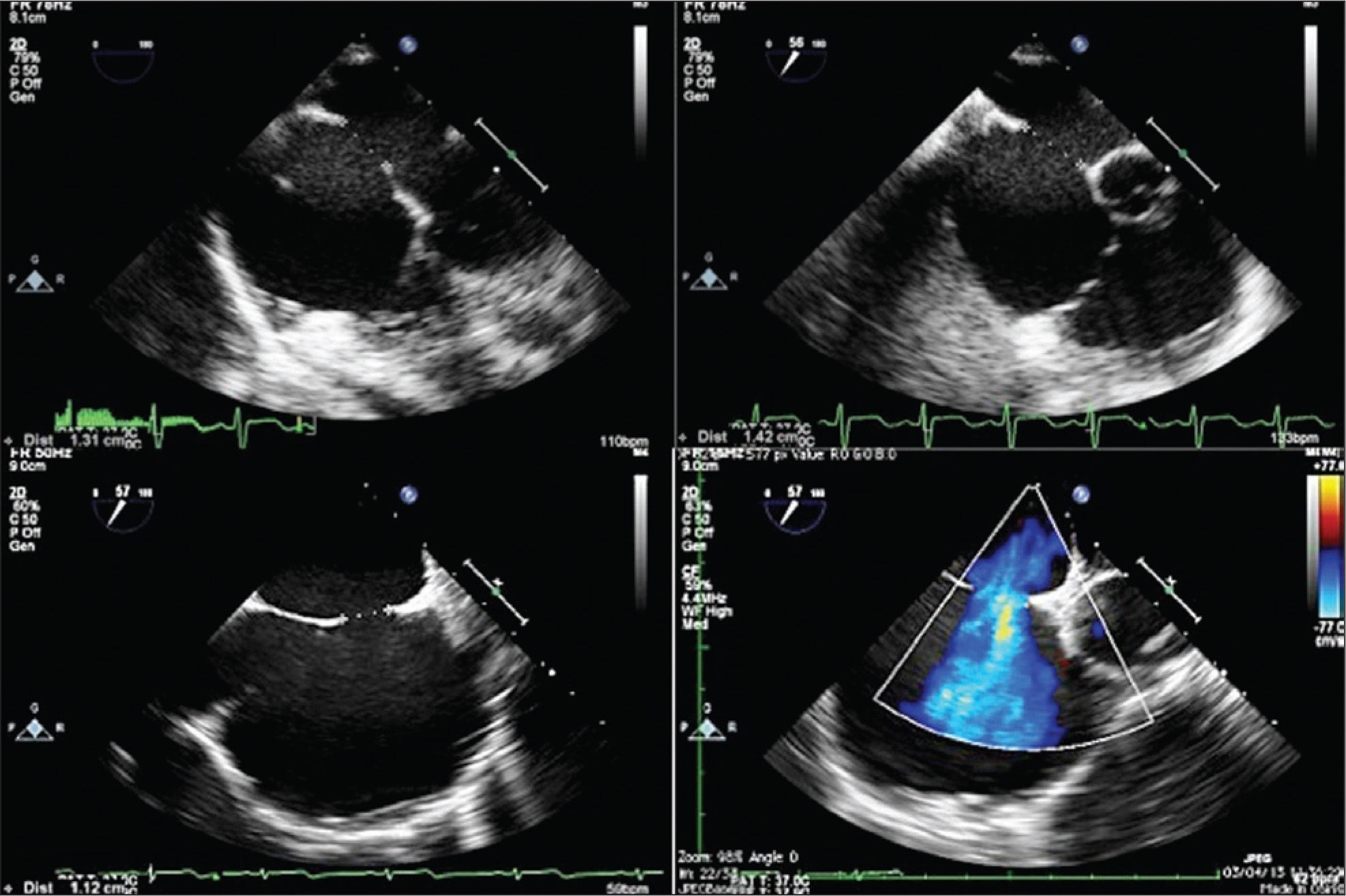
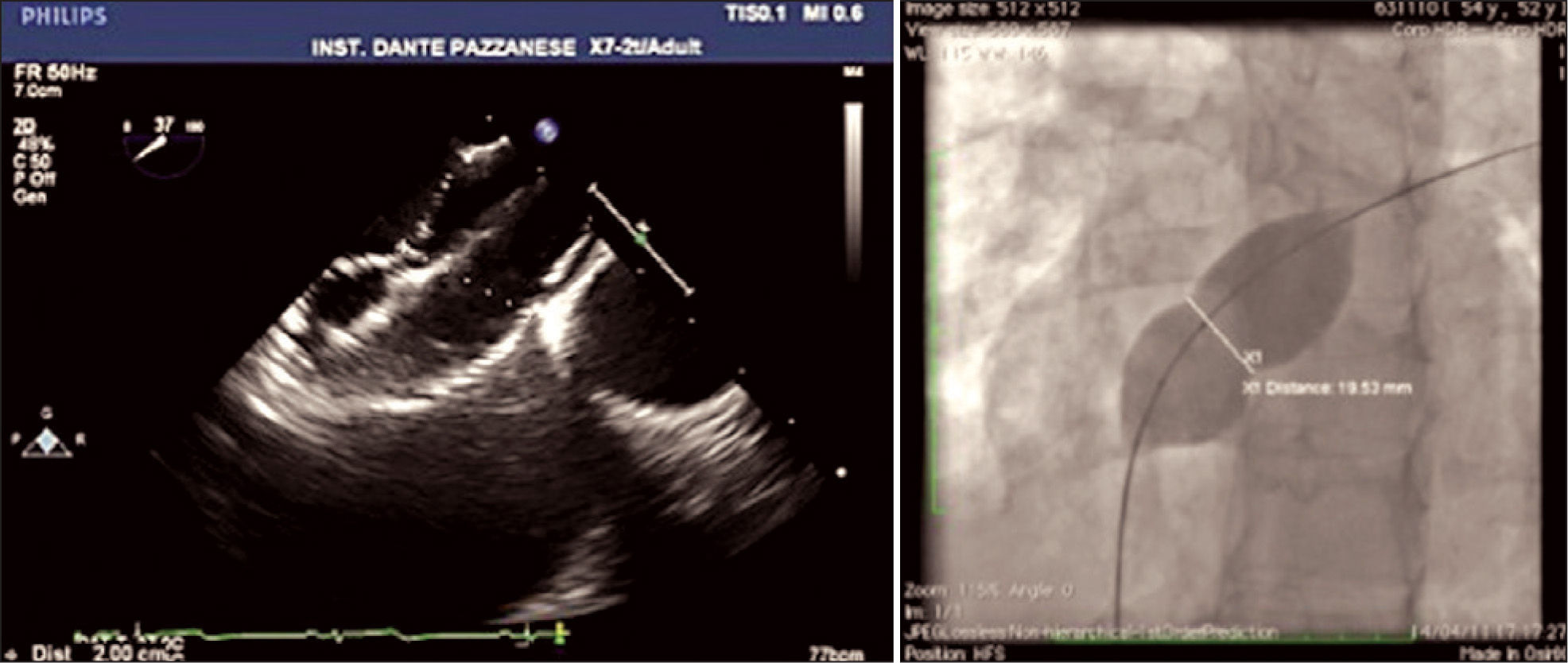
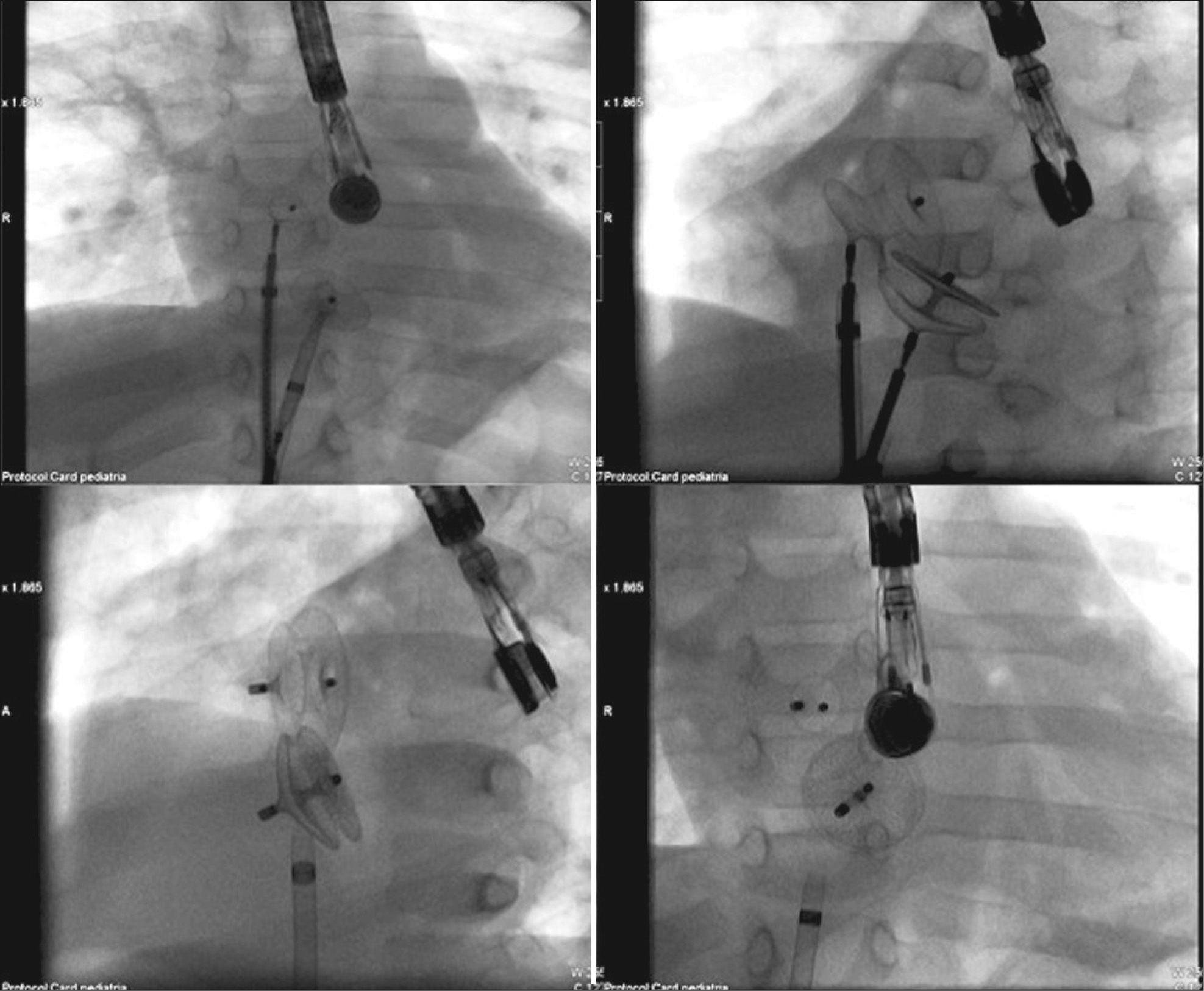
ASD measures assessed at baseline were evaluated, as well as the appearance of its borders, through TEE25 (Figure 2). The total atrial septum length in the anterior-posterior and superior-inferior views was determined using the four-chamber projection and the short axis at the level of the aortic valve, respectively. This measurement was performed to assess the adequacy of prosthesis size selected for the size of the patient’s heart. The stretched ASD diameter measurement using a low-pressure NuMed balloon (Hopkinton, United States) or AGA balloon (Golden Valley, United States), with the stopped-flow technique30 was performed when there were thin, flexible, or aneurysmal borders or in the presence of additional ASD in addition to the main defect (Figure 3). Balloon measurement was also performed in all cases in which the Helex® prosthesis was chosen; the selected prosthesis was two-fold larger than the measured value. Self-centring prostheses from 0mm to 2mm larger than the central waist diameter of the balloon were chosen. In patients with single ASD with firm borders, the prosthesis of choice for implantation had a waist diameter that was 20% to 30% greater than the largest diameter of the defect at baseline. The self-centring prostheses with central waist (Amplatzer® ASO, Figulla® ASD, Nit Occlud® ASD and Cera®) were used in most cases (Figure 4). The Helex®, Figulla® PFO, or Amplatzer® Cribriform prostheses, not self-centring, were selected in the presence multifenestrated atrial septum, and were implanted through the most central orifice. The Helex® prosthesis was also occasionally employed for defects < 10–12mm, both single and central. When there were two ASDs, selfcentring prostheses were used, implanted in the larger defect (usually anterior-superior), with closure of the additional ASD (usually posterior-inferior) resulting from the coverage of the disks around the central waist. Two prostheses were necessary in only one case, one selfcentring and the other cribriform, in order to occlude a more anterior ASD and a cribriform posterior-inferior septum (Figure 5).
In some patients, especially those whose weight was < 15kg and who had smaller dimensions of the left atrium (LA), in order to prevent extrinsic LA compression, the opening of the left disc was performed after the TEE probe was pulled. To avoid possible prolapse of left disc through the anterior portion (retroaortic) of the atrial septum, some technical manoeuvres were employed, including clockwise rotation of the sheath (which is almost in parallel with the column in anterior-posterior projection at the fluoroscopy) and opening of the left disk in the left or right superior pulmonary vein.
Prophylactic antibiotic therapy with second-generation cephalosporin was administered during prosthesis implantation and maintained for 24 hours. Acetylsalicylic acid was introduced five days before the procedure at a dose of 3mg/kg/day to 5mg/kg/day and maintained for six months. Patients were hospitalized for at least 24 hours for clinical observation, and were discharged from the hospital after undergoing a transthoracic echocardiography (TTE) and electrocardiography (ECG), and after ruling out acute complications of the treatment. The presence of residual shunt was determined through the analysis of the Doppler color flow through the atrial septum. Any immediate low-speed residual shunt through the prosthesis mesh was disregarded. Residual shunt was considered as adjacent to the prosthesis border and classified as minimum (< 1mm in diameter), small (1-2mm), medium (2-4mm), and large (> 4mm).31
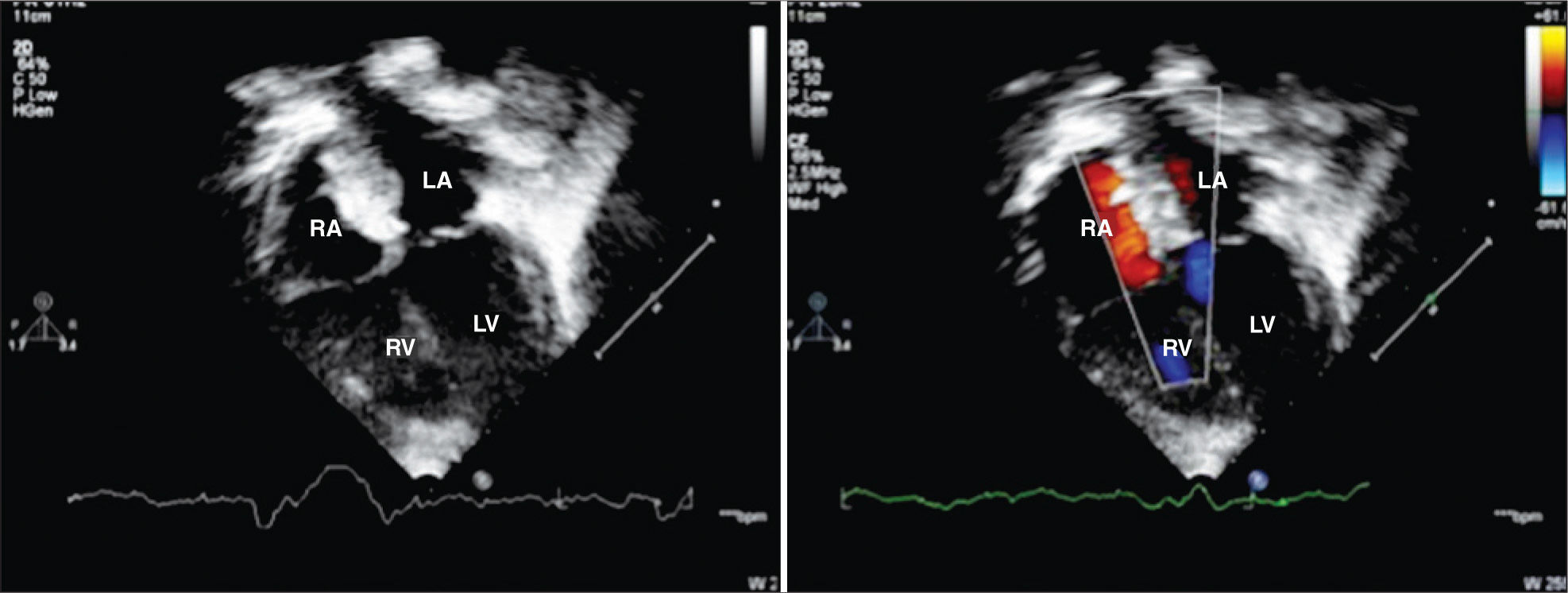
Follow-upOutpatient follow-up was performed at one month, three months, six months, and 12 months after the percutaneous treatment, accompanied by chest radiography, ECG, and TTE. After 12 months, follow-up was performed annually. Prophylaxis for infective endocarditis was recommended for up to six months after the procedure in cases with total defect occlusion (Figure 6), and indefinitely if any residual shunt was noticed through the atrial septum after six months.
– Transthoracic echocardiography showing good positioning of the two prostheses implanted in the interatrial septum, with no residual shunt six months after percutaneous treatment, as shown by the color flow mapping. RA, right atrium, LA, left atrium, RV, right ventricle; LV, left ventricle.
The realisation of the procedure was defined as successful implantation with prosthesis release and appropriate positioning in the atrial septum. Safety was assessed by complication rates.31 Higher incidence of death, brain embolism, cardiac perforation with tamponade, endocarditis, need for reintervention (surgical or percutaneous), arrhythmias requiring permanent cardiac pacemaker or antiarrhythmic drugs for prolonged periods, pleural or pericardial effusion requiring surgical drainage, and need for surgery due to device embolisation were considered complications.
Minor complications included prosthesis embolisation with percutaneous removal, cardiac arrhythmias with no need for prolonged treatment, hematoma at the venous access site, retroperitoneal hematoma with no need for intervention, anaemia requiring blood products, non-cardiac infection, and several respiratory conditions (pulmonary edema, atelectasis, laryngitis). Efficacy was assessed by the total occlusion rates or by the presence of residual shunt with no hemodynamics consequences (with normal right ventricular dimensions) during follow-up.
Statistical AnalysisStatistical analysis was performed using the Sigmastat software, version 2011. The data are shown as absolute values and frequencies, means and standard deviations, or medians and ranges, according to sample distribution.
RESULTSBetween October of 1997 and May of 2012, 80 patients (49 females) with a median age of 4 years (1-12years) and median weight of 13.6kg (5-20kg) were submitted to percutaneous ASD closure in two Brazilian cardiology referral centers. Of these 80 patients, 18 (22.5%) were younger than 2 years and 9 (11%) weighed < 10kg. Twenty patients (25%) had genetic syndromes (Down syndrome, Holt-Oram syndrome, and other unknown syndromes) or multiple malformations (gastrointestinal, renal, neurological, bone). Ten (12.5%) had a history of prematurity. During routine catheterisation, none of the patients showed severe pulmonary artery hypertension, and all patients had mean pressure < 30mmHg in the pulmonary artery. Seventy-six patients (95%) had a single ASD, with a mean diameter of 12±4mm at the TEE. Four patients had ASD with one or more additional orifices (5%). The balloon-stretched diameter assessment was performed in 40 patients (50%), with a mean value of 16±5mm. The mean diameter of the chosen prosthesis was 16.9±5mm and the mean profile of the long delivery sheath was 8±1.5F. The mean duration of procedures was 80±15 minutes. The distribution of prostheses used in the procedures is shown in Table 1.
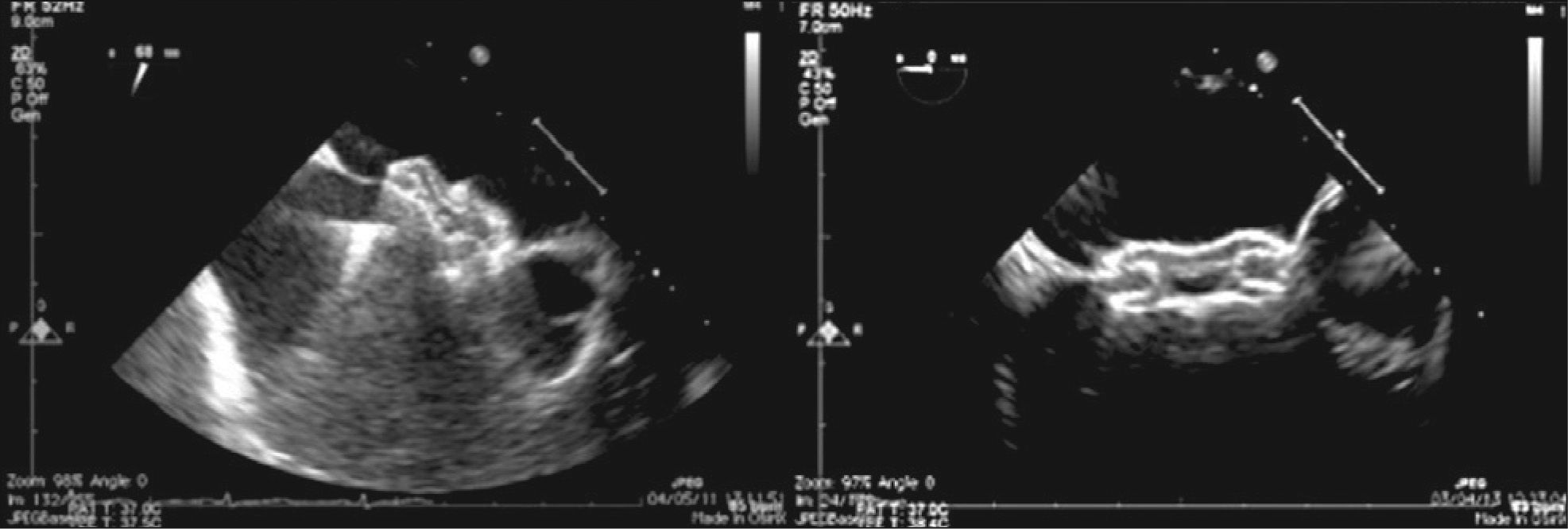
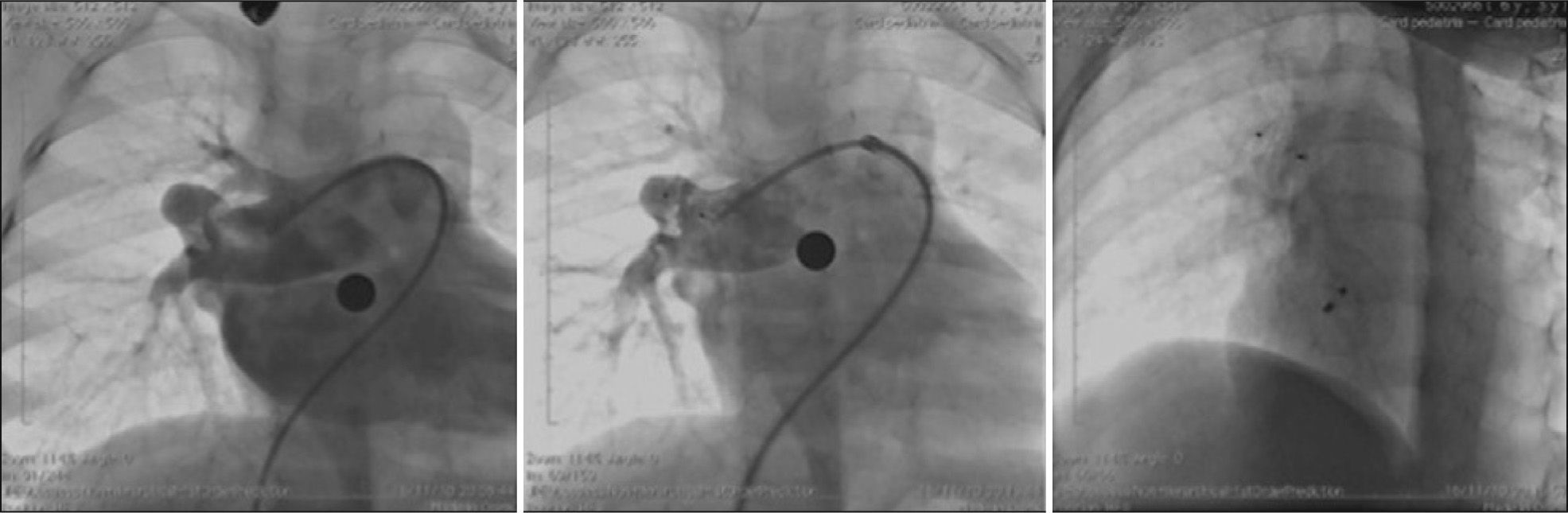
Two patients had associated heart defects, treated percutaneously in the same procedure. One had moderate pulmonary valve stenosis and was submitted to balloon pulmonary valvuloplasty prior to ASD occlusion. The other patient had pulmonary arteriovenous fistula presented in right lung base that resulted in significant cyanosis, which was successfully occluded with the Amplatzer® Duct Occluder II (Figure 7).
– To the left, coronary angiography demonstrating the presence of large pulmonary arteriovenous fistula originating from the middle lobar branch of the right pulmonary artery. In the center, control coronary angiography showing good prosthesis positioning, with fistula occlusion. To the right, chest fluoroscopy in the left oblique projection with cranial angulation showing the spatial association between the implanted prosthesis in the fistula and in the atrial septal defect (Amplatzer® ADO II and Amplatzer® ASD).
Technical success was achieved in all 80 patients (100%). However, a patient submitted to Helex® prosthesis implantation had total atrioventricular block (TAVB) during prosthesis positioning, with no improvement after its full opening and locking into the final position in the atrial septum. The prosthesis was removed according to the usual technique. Since the patient had adequate junctional escape rhythm with no signs of low output, he was taken to the intensive care unit for monitoring, receiving intravenous corticosteroids, with no need for pacemaker stimulation during evolution.
The TAVB was solved in approximately 36 hours and the patient was discharged within 72 hours after confirmation of sustained sinus rhythm in Holter monitoring. This patient returned after – six months, was successfully submitted to ASD occlusion with Amplatzer® without complications. Only one patient with multiple distant ASDs required a second prosthesis, both successfully implanted. In the other patients with multiple ASDs, additional defects were occluded after device implantation in the main ASD. Except for one patient who had TAVB, and another with a congenital syndrome that awaited discharge to return to home care, all others were discharged within 24 hours after the procedure. There were no hemodynamic, vascular, or pulmonary complications.
Slight/mild residual shunt (1-2mm) was observed in four patients (5%) at the TTE prior to hospital discharge. The outpatient follow-up comprised 95% of the patients, lasting 4±3 years. The rate of ASD occlusion in the first clinical evaluation at one month and six months after the procedure was 100%. The echocardiography showed no signs of interference with atrioventricular valve function or blood flow in the right superior pulmonary vein or coronary sinus. There were no clinical complications detected during follow-up.
DISCUSSIONThis prospective observational longitudinal study demonstrated that in children weighing < 20kg with ASD with hemodynamic consequences and significant clinical impact, percutaneous closure is feasible, safe, and effective.
Clinical indications for early percutaneous closureThe decision to treat an ASD in children weighing < 20kg was reserved for those patients in whom pulmonary blood flow resulting from the defect brought significant clinical consequences, characterized by congestive heart failure, recurrent respiratory conditions (six or more episodes in 12 months) and low weight gain. In addition, patients who benefit the most from early percutaneous approach are those with chronic pulmonary disease, several genetic syndromes, and severe systemic diseases.
In this clinical practice, when these conditions are not present, percutaneous treatment of ASD with hemodynamic consequences (with enlarged right ventricle) is usually scheduled to occur when the child is aged between 4 and 5 years.
Procedure feasibility in young childrenUntil recently, there were some dogmas and uncertainties regarding the feasibility of performing percutaneous closure in small children. The catheter profile, the size of the devices, and the possibility of intracardiac trauma, among others, were reasons to justify the impossibility of percutaneous treatment. In this study, although such problems were not observed, considerable care was taken regarding the procedure. The femoral vein, although of a smaller calibre in young children, was not a limiting factor to the procedure in this series. The sheaths were advanced without technical difficulties and without vascular complications in all patients. The mean profile of 8F sheaths was suitable for the median weight of the sample (13kg). The procedure was feasible even in a patient weighing 5kg, with a genetic syndrome, in whom a long 7F sheath was used to implant a 9-mm Amplatzer® prosthesis. Even in patients who do not have normal femoral venous access, the trans-hepatic route, used in two patients in this sample, allows the procedure to be performed. In these two cases, long 9F or 10F sheaths (the largest sheaths used in the present study) were employed uneventfully.
The smaller size of the left atrium can cause some difficulty to open the left disk of the commercially available prostheses, and thus should be performed more carefully and slowly. Pulling the TEE probe helped to prevent a possible extrinsic compression, which would further reduce the size of the left atrium. In patients with an appropriate window (common in young children), another possibility to properly guide the procedure would be the use of TTE.32 It was chosen not to use intracardiac echocardiography in this population due to the need for a second venous access. In this experience, the manoeuvres used to avoid disc prolapse on the left through the anterior portion (retroaortic) of the septum were effective. However, greater care when performing these manoeuvres was required due to the increased fragility of intracardiac structures. Despite the small number of patients with multiple ASDs in this study, the ASDs were occluded in all five patients with one or even two prostheses.
Percutaneous treatment of associated heart diseases using the same anaesthesia time of the ASD occlusion added no technical difficulty, nor did it influence the success of the procedure. The only additional care that was planned for the catheterisation was treating the associated disease first, thus preventing additional intracardiac manipulations after the prosthesis implantation and release in the atrial septum, which could theoretically cause prosthesis displacement or embolisation. Thus, both the balloon pulmonary valvuloplasty and the occlusion of the pulmonary atrioventricular fistula were performed before the ASD occlusion.
Taking into consideration all the aforementioned aspects, technical success was achieved in 100% of cases. The only unsuccessful case was caused by an electrical complication requiring device removal. This high success rate reflects the group’s experience with the techniques and prostheses used and the management of young children with severe congenital heart defects.
Although weight < 5kg was considered an exclusion criterion in this study, the percutaneous closure of ASD in preterm neonates weighing < 2–3kg with bronchodysplasia and on mechanical ventilation has been performed in some centers, resulting in early extubation and optimal outcomes (Dr. John Cheatham, personal communication). This will probably be the next challenge in future experiments.
Safety in this populationIn this series, percutaneous ASD closure in young children was shown to be very safe. The only severe complication was the development of TAVB in one patient. This TAVB probably resulted from the lack of the self-centring mechanism in the device used (Helex®) causing the device to fall into the coronary sinus, temporarily injuring the region of the atrioventricular node, which required device removal and a subsequent new procedure. The implantation of a self-centring prosthesis a few months later minimized this lower migration and prevented the occurrence of TAVB. The small size of the atrial cavities, typical of a small child, may also have influenced the emergence of this complication during the attempted implantation. The transitory nature of TAVB and the successful implantation of a new prosthesis – the Amplatzer® – confirm the hypothesis that there was no permanent injury to atrioventricular node or electrical conduction bundles. In one study, percutaneous closure of ASD in young children resulted in rates of approximately 4% of TAVB.33 However, large prostheses were used in that experiment.
This observation, together with those obtained from this and other trials, show the importance of proper patient selection for the percutaneous procedure, especially regarding the stringent selection of the size and type of device for the underlying anatomy.34
The use of balloons to assess the stretched ASD diameter did not involve complications resulting from the technique in this selected group of young children, demonstrating safety when well indicated. As for the cases in which it was decided not to use them (50%), there were no complications secondary to prosthesis under – or overestimation. The use of the balloon was restricted to the ASDs with features that complicated the choice of prosthesis (e.g. thin and redundant borders, etc.) or when the option was the use of prosthesis without self-centring mechanism (e.g., Helex®). Previously published works corroborate the non-mandatory use of this resource when choosing the prosthesis, with no impairment regarding the safety or the efficacy of the procedure in selected cases.30
One of the authors’ concerns was to avoid, as much as possible, the overestimation of the prosthesis chosen for implantation, which could interfere with the atrioventricular valve function or the blood flow in the left superior pulmonary vein or coronary sinus. None of the patients had such complications. The strategy of measuring the total length of the interatrial septum at the echocardiography (TEE and TTE) and avoiding prosthetic discs greater than those measures was probably effective in preventing these complications. Therefore, some authors recommend performing the angiography with the catheter placed in the left superior pulmonary vein in hepatoclavicular projection to obtain the interatrial septum length measurement (Dr. Ziyad M. Hijazi, personal communication).
In this study, no vascular complications were observed due to the adequacy of the profile of the prostheses used (mean 8F) to the size of patients (median weight of 13kg). In this sense, the use of the Amplatzer® in this population has been preferred, due to the lower profile of their implant sheaths when compared to similar prostheses, such as the Cera® or Figulla®. The launch of the Figulla Flex® II is expected, which will reduce the calibre of the sheath by 2F to 3F, allowing for a safer use of this device in this population.
EfficacyThe occlusion rate of 100% observed from one month to six months in all followed patients (95%) is consistent with previous experiences reported in the literature, and demonstrates the high efficacy of this procedure. The progressive endothelialisation of the prostheses explains the spontaneous occlusion of immediate residual shunt.35 Although this aspect was not assessed in this study, the concept of clinical efficacy must not be forgotten. Previous studies have demonstrated that young patients with ASD and who are highly symptomatic greatly benefit from the percutaneous procedure, with fast weight gain and significant improvement of recurrent pulmonary conditions.23
Study limitationsThis study has obvious limitations, due to its descriptive design and lack of a control group. The lack of data for evaluation of clinical efficacy for this type of procedure (weight gain, improved symptoms) is also an important limitation.
CONCLUSIONSPercutaneous closure of ASD in very symptomatic selected young children is a feasible, safe, and effective therapeutic alternative. As it is less invasive than surgery, especially considering that this subgroup of patients is at high-surgical risk, this therapeutic modality should be regarded as the option of choice in the management of these patients, and should be offered early.
CONFLICTS OF INTERESTCarlos Pedra is a consultant for the following representation and manufacturer companies: Bioassist and St. Jude (Amplatzer®), Boynton and Lifetech (Cera®), CMS (PFM), and TecMedic (Figulla®). The other authors declare to have no conflicts of interest.
CLARIFICATIONSAfter completion of data collection and drafting of this manuscript, the percutaneous closure of ASD was performed in seven more patients weighing < 20kg between May of 2012 and May of 2013. Although similar results were obtained, a severe complication was observed in a patient undergoing occlusion by transhepatic route, due to the impossibility of peripheral venous access due to multiple previous hospitalisations. This patient with genetic syndrome, weighing 7kg, with severe gastroesophageal reflux and chronic pulmonary disease, developed right hemothorax secondary to liver puncture. After drainage, there was satisfactory progress without additional complications. This complication, which was not related to the occlusion procedure itself, but rather to the process of obtaining an access route, indicates the frailty of some of these patients and does not invalidate the aforementioned observations.