Post-traumatic stress disorder, or PTSD, is a condition that affects a subgroup of individuals that have suffered a previous traumatic event capable of generating changes at a psychological and behavioural level. These changes affect the personal, family, and social environment of those who suffer from this condition. Different genes have been identified as risk markers for development of this disorder. The population heterogeneity and individual differences (genetic and environmental) of each subject have made it difficult to identify valid markers in previous studies. For this reason, studies of Gene×Environment (G×E) have gathered importance in the last two decades, with the aim of identifying of the phenotypes of a particular disease. These studies have included genes such as SLC64A, FKBP5, and ADCYAP1R1, among others. Little is known about the interaction between the genes, pathways, and the molecular and neural circuitry that underlie PTSD. However their identification and association with stimuli and specific environments that stimulate the development of PTSD makes it focus of interest for identify genomic variations in this disorder. In turn, the epigenetic modifications that regulate the expression of genes involved in the hypothalamic–pituitary–adrenal (HPA) axis and the amygdala–hippocampal–medial prefrontal cortex circuits play a role in the identification of biomarkers and endophenotypes in PTSD. In this review, the advances in genetic and epigenetic that have occurred in the genomic era in PTSD are presented.
El trastorno por estrés postraumático (TEPT) es una condición que afecta a un subgrupo de individuos que han sufrido un evento traumático con la capacidad de generar cambios psicológicos y conductuales. Estos cambios interfieren el entorno personal, familiar y social de los individuos. Diferentes genes se han identificado como marcadores de riesgo de esta enfermedad; sin embargo, la heterogeneidad poblacional y las diferencias individuales (genéticas y ambientales) han hecho difícil la identificación de marcadores genéticos válidos en los estudios realizados. Por tal motivo, han cobrado gran importancia en las ultimas dos décadas los estudios de relación entre gen y ambiente con la intención de identificar fenotipos propios de la enfermedad. Se han estudiado ampliamente genes como SLC64A, FKBP5 y ADCYAP1R1, entre otros. Poco se conoce de su interacción con las vías y los circuitos moleculares y neuronales que subyacen al TEPT, pero su identificación y asociación con estímulos y ambientes específicos condicionantes de la aparición del TEPT los hacen centro de interés para el estudio de identificación de variables genómicas en TEPT. A su vez, las modificaciones epigenéticas que regulan la expresión de genes vinculados en el eje hipotálamo-pituitario-adrenal y en el circuito amígdala-corteza prefrontal media-hipocampo son de gran interés para la identificación de biomarcadores y endofenotipos en TEPT. En esta revisión se podrá elucidar los avances en genética y epigenética que han acontecido en la comprensión genómica del TEPT.
A traumatic event is an event which has the capacity to generate fear, helplessness or horror in response to the threat of injury or death.1 Approximately two thirds of the population may suffer a traumatic event at some point in their lives.2 Post-traumatic stress disorder (PTSD) is a condition that affects a subgroup of people who have suffered a traumatic event which results in psychological and behavioural changes, with maladaptive symptoms for the mental and physical health of the sufferer and limitations in the social and family environments.3–5 In the United States, the prevalence of PTSD over the course of people's lives is estimated to be 6.8%.6 In Colombia, according to the 2015 Mental Health Survey, 40.2% of adults aged 18–44 and 41.4% of those over 45 have suffered at least one traumatic event in their lives. The prevalence for measuring the positive risk indicator for PTSD is 3.29% for men and 3.84% for women.7
Little is known about the neurobiological bases involved in the development of PTSD. The systems that regulate the response to stress include endocrine components and neurotransmission pathways in brain areas, such as the amygdala, involved in the response to fear and stress.8 The hypothalamic–pituitary–adrenal (HPA) axis acts as the main organiser of the endocrine response to stress9–11 and several studies have identified dysfunctions in the HPA axis in the response to stress,12 anxiety13 and PTSD.14 Neurotransmitter alterations have been found involving catecholamines, serotonin, glutamate and GABA, which have been implicated in the neurocircuits that regulate the response to fear and stress.8 These alterations have been theorised about and subsequently investigated in the development of PTSD.
Advances in genome sequencing techniques have led to different genes being identified that confer risk of suffering from PTSD.15–18 However, many of the genes involved have lost statistical validity, as their association has been weak in sequencing studies and on some occasions they have not been identified in different study populations due to the heterogeneity of the population in PTSD (maltreated children, female victims of sexual abuse, male and female combatants in war, etc.).19 This has led over the last 20 years to a more translational approach to genetic studies of PTSD. Studies of genetic variations associated with an environmentally determined condition to identify a phenotype of interest have gained importance in population genetic studies in neuropsychiatric diseases.20 Similarly, Genome-wide Association Studies (GWAS) and exome sequencing have discovered new genetic markers linked to the risk of suffering from PTSD.19,21 One of the interesting aspects of this disorder as a study model is that it develops as a result of a previous traumatic experience. This has led research groups to consider questions relating to genetic variations that confer risk of becoming ill or determine the severity of the symptoms, or the inheritable epigenetic changes that modify the expression of genes linked to the development of the disorder without generating changes in the sequence of these genes.
The purpose of this study is to publish the advances and new perspectives in the genetics and epigenetics of PTSD. We carried out a narrative review of the literature in the PubMed database in May 2016. We selected review articles, observational studies and analytical studies related to the purpose of our project. The following MeSH terms were included in the search: (“genetics” OR “gene” OR “epigenetic” OR “genetic advances”) AND (“post-traumatic stress disorder” OR “ptsd”). We selected articles from 2000 to date.
Genetic studies in families and identical twinsFamily studies have demonstrated the link of genetic variations in families of patients with PTSD. A study conducted by Yehuda et al. showed that the children of parents who survived the holocaust and had PTSD had a higher risk of PTSD than the children of parents who survived the holocaust and were not diagnosed with PTSD (p<0.0005).22 However, these studies faced the difficulty of not being able to differentiate between the environmental exposure that each member of the family may have been subject to and the genetic risks inherent to each of the families.
Genetic factors associated with the risk of exposure to traumatic events have been identified in studies of identical twins.15,16 Lyon et al. studied a sample of male twins from the Vietnam registry to examine genetic and environmental factors associated with exposure to traumatic events during combat. Inheritability explained 35–47% of the variance in exposure to the traumatic event during combat, but the family environment was not found to have a significant effect on the variable of interest.17,18 Other studies, such as the estimation of the inheritability of the symptoms of PTSD,23 the comorbidity24–29 and the physical condition,30–32 and gene–environment interaction studies33 have been carried out in monozygotic and dizygotic twins, with positive results for the association or linkage of genes. However, despite indicating that there is a risk of inheritability in the development of PTSD, these studies were unable to identify which gene is the direct link that confers the risk of PTSD, because of the multifactorial and polygenic component of this disorder and neuropsychiatric diseases in general.
Multifactorial correlation in PTSD: studies of genes×environmentResearch into neuropsychiatric disorders and the multifactorial nature of mental illness have led us to understand that they cannot be explained by one single gene and, furthermore, that the gene–environment interaction goes beyond simple epistemological assumptions.34 Consequently, gene×environment (G×E) interaction studies have become increasingly important in recent years. The first studies in psychiatry to assess the interaction of genetic conditions and exposure to stimuli or environments and identify different disease phenotypes were in maltreated children who had a genotype conferring low concentrations of the enzyme monoamine oxidase A (MAOA), compared to maltreated children with high concentrations of MAOA, in relation to the appearance of behavioural disorders, antisocial behaviour and violence.35
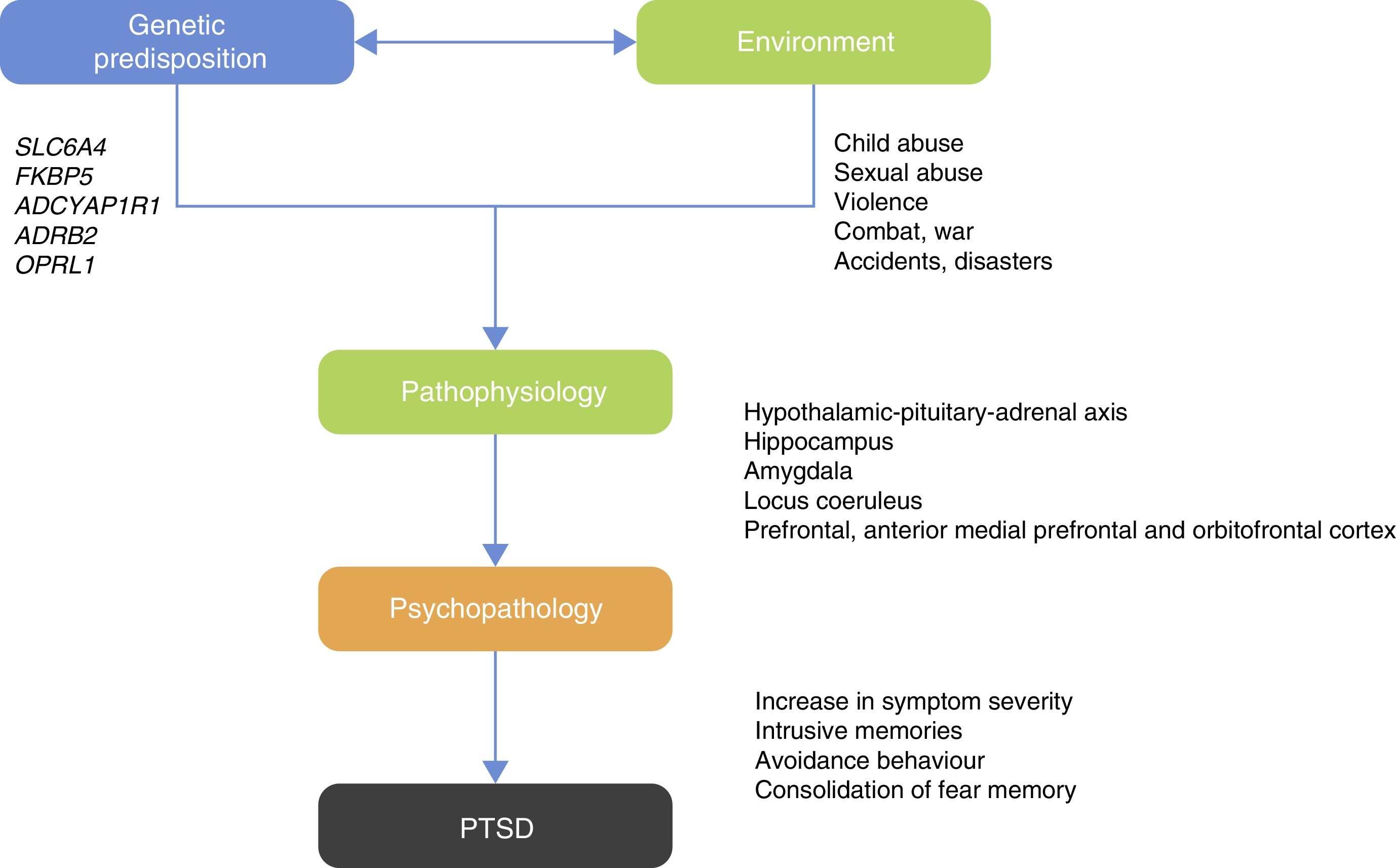
Those studies led researchers to ask which psychiatric disorders would be best to examine for G×E studies? PTSD can be considered an ideal candidate for G×E studies as one of the criteria for diagnosis is previous exposure to the traumatic event or exposure to a hostile environment that facilitates occurrence of the traumatic event20 (Fig. 1).
Of the G×E studies, the one conducted by Ressler et al. identifies a positive association for genetic risk between the single nucleotide polymorphism (SNP) rs2267735 in African-American women with a history of childhood and adult trauma and the risk of suffering PTSD.36 This study confers the association of risk of PTSD on the pituitary adenylate-cyclase 1 (PAC1) receptor, involved in the development of the nervous system, endocrine homeostasis, metabolism and the response to chronic stress.36,37 These findings suggest that the pituitary adenylate cyclase-activating polypeptide (PACAP)/PAC1 system is involved in the psychological and physiological response to stress resulting from trauma.
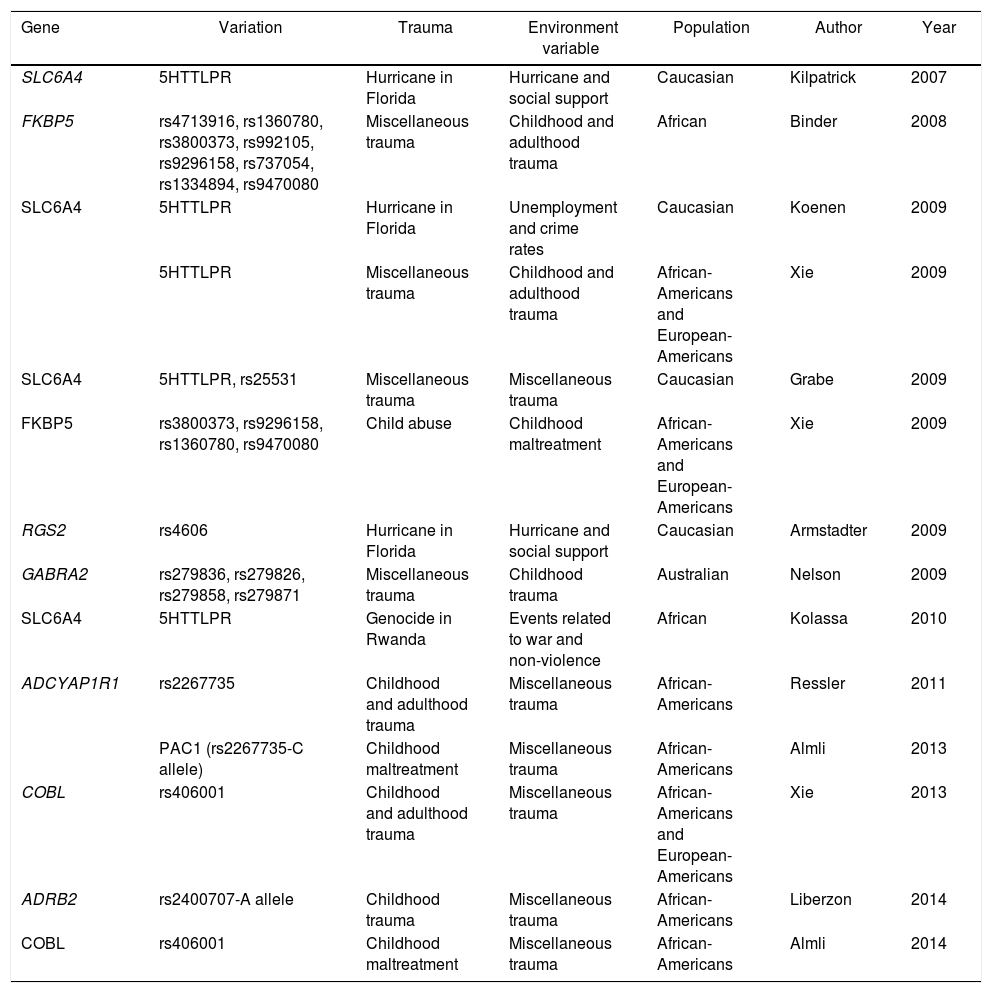
Another study to find a positive association between a gene and the environment contributing to traumatic events is that conducted by Kolassa et al. with patients who were refugees from the genocide in Rwanda. The study analysed 408 subjects (218 males and 190 females) living in a refugee camp in southwestern Uganda. All the subjects had experienced multiple traumatic situations. This study reported that the SLC6A4 gene that encodes the 5-hydroxytryptamine transporter gene, involved in previous studies of depression and response to medications,38 confers a risk from association of suffering PTSD after traumatic events.39 Other studies have associated different genes and polymorphisms linked to environmental variables (Table 1).
Published studies of gene–environment interaction in PTSD.
| Gene | Variation | Trauma | Environment variable | Population | Author | Year |
|---|---|---|---|---|---|---|
| SLC6A4 | 5HTTLPR | Hurricane in Florida | Hurricane and social support | Caucasian | Kilpatrick | 2007 |
| FKBP5 | rs4713916, rs1360780, rs3800373, rs992105, rs9296158, rs737054, rs1334894, rs9470080 | Miscellaneous trauma | Childhood and adulthood trauma | African | Binder | 2008 |
| SLC6A4 | 5HTTLPR | Hurricane in Florida | Unemployment and crime rates | Caucasian | Koenen | 2009 |
| 5HTTLPR | Miscellaneous trauma | Childhood and adulthood trauma | African-Americans and European-Americans | Xie | 2009 | |
| SLC6A4 | 5HTTLPR, rs25531 | Miscellaneous trauma | Miscellaneous trauma | Caucasian | Grabe | 2009 |
| FKBP5 | rs3800373, rs9296158, rs1360780, rs9470080 | Child abuse | Childhood maltreatment | African-Americans and European-Americans | Xie | 2009 |
| RGS2 | rs4606 | Hurricane in Florida | Hurricane and social support | Caucasian | Armstadter | 2009 |
| GABRA2 | rs279836, rs279826, rs279858, rs279871 | Miscellaneous trauma | Childhood trauma | Australian | Nelson | 2009 |
| SLC6A4 | 5HTTLPR | Genocide in Rwanda | Events related to war and non-violence | African | Kolassa | 2010 |
| ADCYAP1R1 | rs2267735 | Childhood and adulthood trauma | Miscellaneous trauma | African-Americans | Ressler | 2011 |
| PAC1 (rs2267735-C allele) | Childhood maltreatment | Miscellaneous trauma | African-Americans | Almli | 2013 | |
| COBL | rs406001 | Childhood and adulthood trauma | Miscellaneous trauma | African-Americans and European-Americans | Xie | 2013 |
| ADRB2 | rs2400707-A allele | Childhood trauma | Miscellaneous trauma | African-Americans | Liberzon | 2014 |
| COBL | rs406001 | Childhood maltreatment | Miscellaneous trauma | African-Americans | Almli | 2014 |
5HTTLPR: serotonin-transporter-linked polymorphic region; ADCYAP1R1: adenylate cyclase-activating polypeptide 1 (pituitary) receptor type I; ADRB2: beta-2-adrenergic receptor, surface; COBL: Cordon-bleu WH2 repeat protein; FKBP5: FK506 Binding Protein 5; GABRA 2: gamma-aminobutyric acid (GABA) A receptor, alpha 2; PAC1: pituitary adenylate cyclase-activating polypeptide type I receptor; RGS2: regulator of G-protein signalling 2; SLC6A4: solute carrier family 6 (neurotransmitter transporter) member 4.
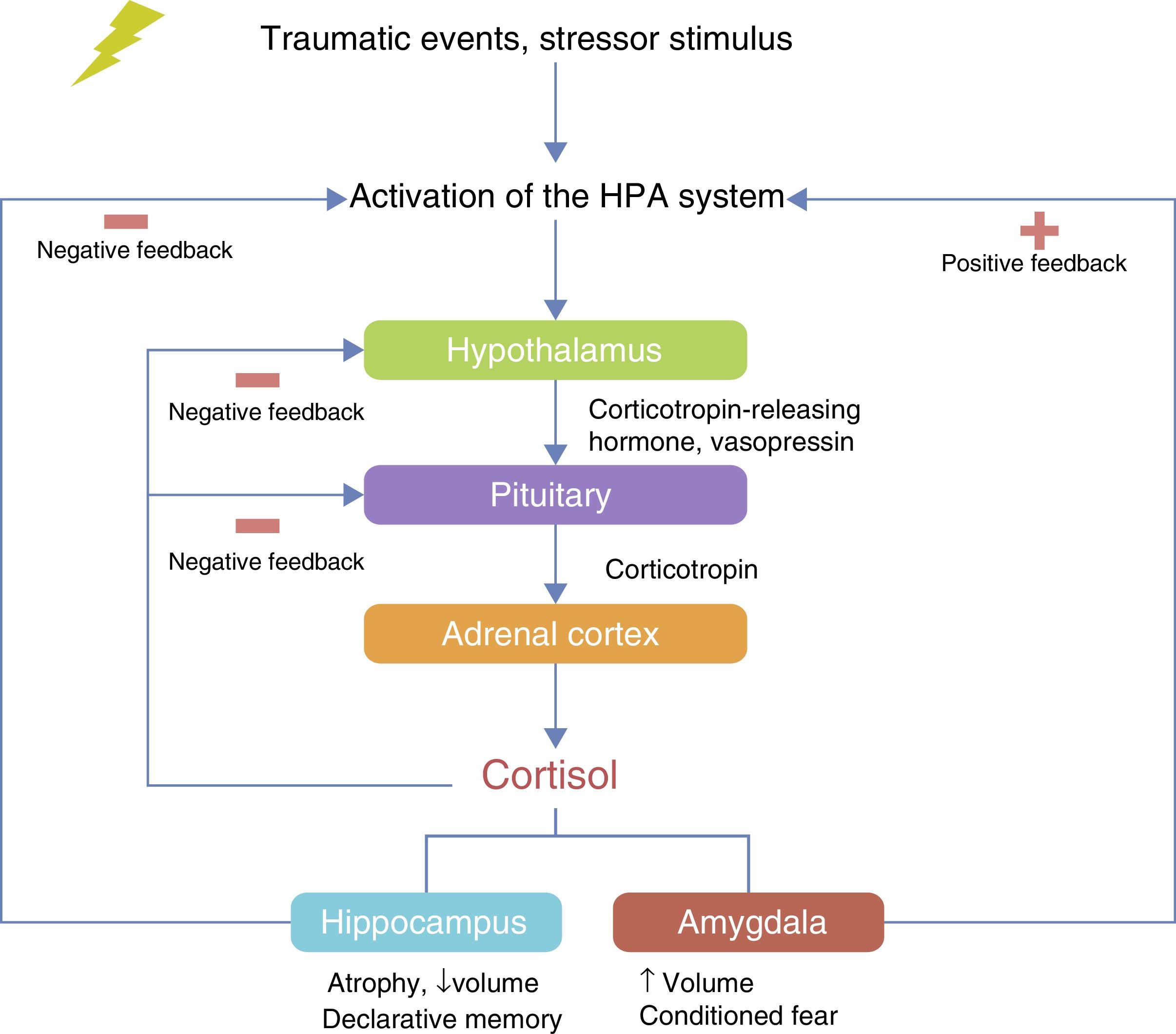
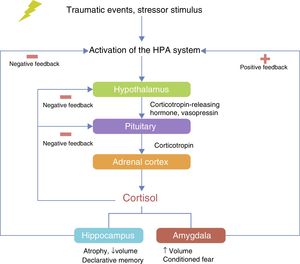
The HPA axis is involved in the neuroendocrine response to stress. The hypothalamus controls the secretion of corticotropin (adrenocorticotropic hormone; ACTH) from the anterior pituitary gland and stimulates the consequent secretion of cortisol by the adrenal gland.40 Under conditions of acute stress, the pulsatile release of corticotropin-releasing hormone (CRH) and vasopressin (arginine vasopressin, AVP) are increased, thereby increasing the release of ACTH and cortisol and other factors, such as angiotensin II, various cytokines and inflammatory mediators40–42 (Fig. 2). The neurobiological interaction between genes that act on the HPA axis has been studied extensively in different neuropsychiatric disorders such as depression,43 bipolar disorder,44 substance abuse,45 alcoholism46 and PTSD.20
Immunophilins (cytosolic proteins) were discovered because of their ability to bind and mediate the immunosuppressive effect of drugs such as ciclosporin and rapamycin.47 The FKBP5 gene (FK506 Binding Protein 5) encodes one of the immunophilins that binds to the immunosuppressant FK506 and rapamycin48 and plays a role as a regulatory gene for the HPA axis.49 Several studies have identified the FKBP5 gene in the development of PTSD50–52; one of the first groups to associate this gene was Koenen et al. in 2005; their study included 46 children who had already suffered car accidents, gunshot or stab wounds and other types of trauma. The polymorphisms rs3800373 and rs1360780 were strongly associated with the development of peri-traumatic dissociation in this group of patients.52 In a study of 900 predominantly Caucasian subjects, 4 SNPs of FKBP5 interacted with the severity of the sexual abuse of children as predictors of PTSD symptoms in adulthood.53
A recent study evaluated the FKBP5 polymorphisms (rs9296158, rs3800373, rs1360780, rs947008) in two populations of European-American US military veterans. The first population was of 1585 veterans and the second, of 577. The results of this study showed that the four polymorphisms were associated with the PTSD symptom severity in both samples.50 This genetic variation is a risk factor for later PTSD in adulthood, a link which had already been made in children who were victims of sexual abuse.53,54 The results indicate that child abuse and the presence of these polymorphisms in a population exposed to a traumatic event in life (such as the populations in this study) confer risk of suffering severe PTSD symptoms in adulthood.
Furthermore, FKBP5 as a co-chaperone in the regulation of the glucocorticoid receptor (GCCR/NR3C1) plays a fundamental role in the consolidation of intrusive memories. Memories of this type are repetitive and unwanted and generate an involuntary sensory/perceptual experience which is usually associated with negative memories in patients with PTSD.55 Various studies have reported that cortisol enhances the consolidation of emotional memory.56,57 The SNPs rs3800373, rs9296158, rs1360780 and rs9470080 present in FKBP5 were analysed in a study of 46 subjects included for genetic analysis, and it was concluded that the participants homozygous for the risk alleles of the studied polymorphisms were more likely to consolidate memories of negative images because of the modulation by FKBP5 of the GCCR/NR3C1 receptor in the response to stress.58
Amygdala, hippocampus and prefrontal cortex in patients with PTSDThe relationship between the amygdala, the hippocampus and the prefrontal cortex has been the subject of neurobiological research in PTSD. These brain regions are rich in glucocorticoid receptors and very sensitive to stress, which makes them a very important circuit for the conditioning of fear.59–62
Neuroimaging studies have investigated the activation of these areas during exposure to stimuli related to traumatic experience. It was found that the medial prefrontal cortex (mPFC), which includes the anterior cingulate cortex, the subcallosal cortex and the middle frontal gyrus, is involved in the processes of extinction of conditioned fear.63,64 Increases in cerebral blood flow in the amygdala and reduced flow in the middle frontal gyrus have been detected by positron-emission tomography in combat veterans.65 This would indicate hypersensitivity in the amygdala's response to stimuli related to a traumatic event66. Kirby et al.67 demonstrated that emotions affect the formation of memories. In situations of fear, in the hippocampus, which is involved in the formation of declarative memories, the basolateral amygdala induces the generation of new neurons from a single population of neural stem cells in the dentate gyrus of the adult hippocampus. The formation of these new neurons has been associated with the fact that emotional events are remembered more easily and for longer than everyday experiences, with this having important implications in the pathophysiology of PTSD.68 In a recent study involving maltreated young people with PTSD, a decrease in the volume of the ventromedial prefrontal cortex (vmPFC) was found on functional magnetic resonance imaging.62
Studies of the expression of genes identified in areas involved in the neural circuitry of PTSD have produced different genetic markers which are of interest in the genetic and molecular study of this condition, both in animal models and in humans. One of these is the gene OPRL1 (opioid receptor-like 1), which encodes the nociceptin/orphanin FQ receptor. This receptor–ligand system regulates a variety of biological functions, such as the response to stress,69 anxiety,70 learning and memory,71 locomotor activity72 and immune and inflammatory responses.73 In the murine animal model, expression of OPRL1 was found in the central amygdala. Using the microarray technique, regulation of OPRL1 mRNA was greater in the group of animals with fear expression than in the control group.74 In that same study, Andero et al. used the peptide SR-8993, a new selective nociceptin receptor agonist, to potentiate impairment in fear learning (conditioning). The findings from that study indicate that intraperitoneal injection in the animal model of SR-8993 prevents fear memory consolidation.74 Andero et al. also sought to find association between polymorphisms present in the OPRL1 gene and the development of PTSD. Using 1847 subjects who had already suffered traumatic events, they found a positive association between the SNP rs6010719 and the increased symptoms in individuals with PTSD with a history of child abuse.
The OPRL1 gene is worthy of more attention and further research in biomedical and in G×E studies, as it has previously been linked to alcoholism,75 a condition frequently shared by individuals who have PTSD.76–78 OPRL1 also plays an important role in the HPA axis circuit in the stress response. There is evidence implicating the nociceptin system in the stress response through a control of regulation on the HPA axis.79–81 Devine et al. demonstrated that the administration of nociceptin/orphanin FQ increases plasma cortisol in experimental rats.79
As already mentioned, the FKBP5 gene modulates glucocorticoid receptor activity and has high expression levels in the hippocampus. A positive association has been found between the “risk” T allele of the SNP rs1360780 and an increase in the transcription of FKBP5, with a consequent increase in the inhibition of glucocorticoid signalling.82 Low concentrations of glucocorticoids have been associated with risk of PTSD.65 This allele has also been related to structural differences in the left posterior cingulate cortex characterised by a decrease in fibre density, axonal diameter and myelination in this region.83 The polymorphism rs1360780 (TT/TC) has been associated with alterations in the structure of the left hippocampus in the CA1 region and increased attention to threat in attention tasks.84
Other genes of interest related to differences in brain function in PTSD are SLC6A4 (serotonin transporter gene) and ADCYAP1R1. The SNP alleles rs16965628 G and 5-HTTLPR S are associated with decreased expression of SLC6A4; the first affects the activity of the ventrolateral prefrontal cortex (vlPFC), while the second affects the activity of the amygdala.65 The PAC1 receptor is encoded by the ADCYAP1R1 gene. This receptor is widely expressed in the amygdala, the hypothalamus and the hippocampus, and is associated with an increased risk of PTSD, especially in women.85 The ADCYAP1R1 SNP rs2267735 C risk allele has been associated with a decrease in the expression of ADCYAP1R1 and less of its mRNA; this allele modulates the activity of the amygdala and the hippocampus, which leads to greater activation of these structures in response to threatening stimuli.86
There are other genetic variations associated with differences in brain structure in PTSD which involve the COMT and BDNF genes. The genetic variations in the COMT SNP rs4680 are associated with decreased grey matter volume in the anterior cingulate cortex (ACC)87 and those in the BDNF SNP rs6265 are associated with an alteration in fear extinction in humans and murine animal models.88 BDNF has been found to interact with glucocorticoid signalling (important for brain plasticity), and higher levels of BDNF are associated with an improvement in glucocorticoid signalling and a lower level of vulnerability to stress.89
These data suggest that the linkage of genes involved in the amygdala–mPFC–hippocampus circuit could be worthy of study for the identification of biological markers of PTSD in individuals with previous exposure to traumatic events.
Noradrenergic systemNoradrenaline is an important element in the pathophysiology of PTSD. This catecholamine acts as a neurotransmitter synthesised in the locus coeruleus in the brainstem,90,91 and as a hormone when released by the adrenal medulla.92 Noradrenaline carries out its biological function in the groups of alpha and beta adrenergic receptors,93 and as the main neurotransmitter in the sympathetic nervous system.94 Some studies have linked the noradrenergic system with the response to stress and fear generated in patients with PTSD.95 One group of researchers managed to identify tonic concentrations of noradrenaline in the cerebrospinal fluid (CSF) of American war veterans with PTSD compared to the controls.96,97 This confirms the hypothesis that the noradrenergic system becomes hyperactive in subjects with PTSD, in addition to over-consolidation of fear memory and associated intrusive memories.16
Catechol-O-methyltransferase is an enzyme involved in the catalysis and inactivation of catecholamines such as dopamine, noradrenaline and adrenaline. The COMT Val158Met polymorphism has been associated with the development of PTSD and the abolition of the conditioned fear response.98–100 This polymorphism has been the object of experimentation in previous studies which linked it to anxiety disorders, especially in homozygous Met/Met carriers.101 A recent study associated Val158Met with a reduction in the capacity of human beings to recover from traumatic events or painful situations, known as resilience.102 It was also found that methylation of CpG islands in COMT regions, including the promoter region, was influenced by the presence of the Val158Met polymorphism; Val158Met regulated gene expression and the function of the enzyme103,104 and was associated with impaired inhibition of fear previously generated in a conditioning paradigm model.104
Epigenetics in PTSDEpigenetic studies have been attracting increasing amounts of interest in recent years. Epigenetics is the “study of changes in gene function which are mitotically inheritable and do not involve a change in the DNA sequence”.105 Identifying epigenetic modifications in experimental studies, they have been standardised as: (a) DNA methylation; (b) histone post-translational modification; (c) chromatin remodelling; (d) histone variants, and (e) non-coding RNA.106 Epigenetic studies in PTSD could explain individual differences in subjects exposed to trauma who are vulnerable to PTSD. Such studies allow a molecular approach considering family vulnerability, predisposition to certain environments and prolonged exposure to certain trauma effectors.107
There is evidence that DNA methylation is involved in all stages of consolidation, reconsolidation and maintenance of memory formation in both the hippocampus and the cerebral cortex.108 The studies in murine animal models clarified that the acetylation of histone H3 in the CA1 area of the hippocampus occurred in the conditioned fear model, and, with the use of inhibitors of the histone deacetylase enzyme, it was possible to enhance the formation of the memory.108,109
A group of researchers led by Weaver110 discovered that changes in the DNA methylation of the glucocorticoid receptor gene in the hippocampus caused by variations in the maternal behaviour of rats towards their offspring generated a regulation in the HPA axis. The breeding model, in which the mother rats were removed early after having given birth to their offspring to prevent natural interaction (licking and grooming their offspring), generated inheritable changes in the expression of the glucocorticoid receptor gene in the hippocampus. These changes were generated by hypermethylation of the gene promoter. DNA methylation has also been demonstrated in immune response cells in peripheral blood of subjects with PTSD.111
DiscussionGenetic studies identifying polymorphisms and genes associated with illness in PTSD have linked the genes SLC6A4,38,39,65,86FKBP5,49–53,58,82–84,100ADCYAP1R1,85,86ADRB2112 and OPRL1,74 among others, as candidates in the risk of PTSD. The difficulty posed by genetic association studies is the lack of reproducibility in different population groups.19 This may be due to differences in the environment to which the study subjects are exposed and sometimes also to the size of the samples used by researchers. However, studies of the G×E relationship are of great interest, particularly in psychiatric conditions such as PTSD, where it is essential to meet the diagnostic criterion of having experienced a traumatic event before the onset of symptoms.20 It is important to note that most of the studies of G×E have been conducted with the diagnostic considerations pre-established in the DSM-IV for the diagnosis of PTSD. However, since the publication of the DSM-V in 2013 with the diagnostic modifications and the inclusion of new criteria for the diagnosis of PTSD, further studies could be conducted not only to examine those who have suffered directly from the traumatic event, but also people who have indirectly shown symptoms associated with PTSD. Another aspect to look at would be whether the genetic variations most associated with risk of disease in those who directly witnessed the traumatic event might be the same for people who have the symptoms of PTSD but through indirect contact with the trauma.
The first molecular studies on PTSD focused on the HPA axis.20,49 However, whole-genome sequencing studies (genome-wide association studies [GWAS]) in large population samples have identified new candidate genes which are of interest when analysing the molecular mechanisms in which these genes are involved.19,21 The genetic variations present in neuropsychiatric diseases involve magnitudes of risk of suffering from a certain disorder. However, they are polygenic and multifactorial mental illnesses15,20 and more precise study of the interaction is therefore required: genetic variation due to intracellular and extracellular signalling pathways; genetic variation through gene expression; genetic variation due to structural changes; genetic variation resulting from epigenetic modifications. All of this can be transferred to the experimental field by way of cellular and molecular studies and with experimentation in animal models.
The amygdala–mPFC–hippocampal system requires closer examination in genetics and molecular studies, particularly in relation to the genes involved in the synaptic and neurotransmission mechanisms in the processes of fear, anxiety, learning and memory. Different neurotransmitters and their respective receptors have been linked to cognitive processes such as memory, learning and executive functions.8 These cognitive components are related to the symptoms of patients with PTSD, especially the intrusive memories and memories of the event.62–66
ConclusionsPTSD is a serious psychiatric condition which becomes disabling. Although susceptibility to suffering a traumatic event in life is >50%, depending on the population studied, the prevalence of PTSD is 7–12%, suggesting that genetic and environmental predisposition leads to differences between subjects who suffer from the disorder.6,113 Studies of G×E interaction seek to associate genetic variations and environmental characteristics as factors linked to the development of the disorder. For these polygenic and complex mental disorders, epidemiological research through G×E studies is an interesting and useful tool for addressing multifactorial relationships in PTSD.
The HPA axis, the noradrenergic system and the amygdala–mPFC–hippocampal circuit are considered pivotal in the neurobiological circuit involved in the development of PTSD. However, different genes related to synaptic transmission and neurotransmitters such as serotonin, glutamate, GABA and dopamine have also been involved in animal experimentation and population studies.
Epigenetic mechanisms, such as methylation of CpG islands in promoter regions of genes involved in the development of PTSD, indicate inheritable mechanisms that are worth examining, as they could help identify risk biomarkers and/or explain disease progression. Continuity in the studies of genetic association with large population samples and their reproducibility in cell and animal models will help us to better understand the cellular mechanisms that underlie this condition.
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: Guillén-Burgos HF, Gutiérrez-Ruiz K. Avances genéticos en el trastorno por estrés postraumático. Rev Colomb Psiquiat. 2018;47:108–118.