Previous studies suggest that inflammatory molecules play an important role in the pathophysiology of Bipolar Disorder (BD). The evidence suggests that BD may present a progressive course. Therefore there are theories that postulate the relationship between progression and stages of the disease with distinct peripheral biomarkers.
ObjectiveThe aim of this study was to carry out a systematic review of the literature of studies about the association between peripheral inflammatory markers and clinical variables related with staging in BD patients.
MethodsWe conducted a systematic review using electronic databases: PubMed, SciELO, LiLACS and PsycINFO. Keywords were divided into inflammatory markers and, BD and staging. Studies involving euthymic BD patients, studies evaluating peripheral biomarkers and studies correlating these with clinical variables related to neuroprogression or stage of BD were included.
ResultsWe present and discuss the methods and findings of ten articles. The inflammatory markers were measured with different techniques and show some contradictories results. The TNF superfamily and inflammatory cytokines may have a relationship with the neuroprogression of the disease.
ConclusionsThis study suggests that TNF and ILs could play a role in neuroprogression. However, longitudinal studies are needed to clarify the relationship between factors associated with neuroprogression.
Estudios previos indican que las moléculas inflamatorias tienen un papel importante en la fisiopatología del trastorno bipolar (TB). La evidencia apunta a que el TB puede presentar un curso progresivo. Por lo tanto, existen teorías que han postulado una relación entre la progresión y los estadios de la enfermedad con diferentes biomarcadores periféricos.
ObjetivoEl objetivo de este estudio es realizar una revisión sistemática de la literatura de los estudios sobre la asociación entre los marcadores inflamatorios periféricos y las variables clínicas relacionadas con la estadificación en los pacientes con TB.
MétodosSe llevo a cabo una revisión sistemática usando las bases de datos electrónicas PubMed, SciELO, LiLACS y PsycINFO. Las palabras clave se dividieron en marcadores inflamatorios y TB y estadificación. Se incluyeron estudios que evaluaron a pacientes con TB en fase de eutimia, estudios que evaluaron biomarcadores periféricos y estudios que correlacionaron dichos marcadores con las variables clínicas relacionadas con la neuroprogresión o estadificación del TB.
ResultadosSe presentan y se discuten los métodos y los hallazgos de 10 artículos. Los marcadores inflamatorios se determinaron con diferentes técnicas y mostraron resultados contradictorios. La superfamilia del factor de necrosis tumoral y las citocinas inflamatorias podrían tener una relación con la neuroprogresión de la enfermedad.
ConclusionesEl presente estudio indica que el factor de necrosis tumoral y las intereucinas pueden tener un papel en la neuroprogresión del TB. Sin embargo, se requieren estudios longitudinales con el fin de clarificar la relación entre los factores asociados con la neuroprogresión.
Bipolar disorder (BD) is a chronic, recurring and potentially progressive disease that affects between 1% and 3% of the population, and is one of the conditions that causes the most loss of life due to disability.1,2 It was previously thought that its clinical characteristics and nature were only associated with affective episodes, however in recent years cognitive impairment has been described, primarily in memory, attention span and executive function, including during the euthymia,3 as well as long-term progressive clinical deterioration in some patient subgroups.4 Of the neurobiological explanations it has been proposed that the disease exhibits neuroprogression, which would indicate a worse outlook, a rise in relapses and possible neuroanatomical, functional and biochemical changes.5,6 The allostatic load theory has been proposed to explain the neuroprogression and division in stages of the disease.7,8 In this theory a physiological wear and tear in a non adaptive way in response to multiple stressors in bipolar patients could be associated with oxidative stress, inflammation and neurotrophin expression.9,10 Also, various candidate biomarkers have been described for the stages of the disease, and there is consensus to search neuroimaging, peripheral inflammatory markers and genetic markers.11
Neuroprogression has led to the formulation of clinical staging models for BD I,12–16 which could be useful for diagnosis and specific interventions for each stage.14 The importance of staging classification systems lies in the possibility of a stage specific treatment regimen that could prevent the progression to late stages or remission to early stages.17,18 The International Society for Bipolar Disorders proposes dividing the staging into early and late, as well as the need for studies that validate and confirm the use of these models.14 The division of the proposed staging models is based on clinical variables such as the number of episodes, the length of illness, psychosocial functioning, neurocognition, quality of life and response to the treatment.14,18,19 But, it is essential include biological markers of progression that improved the physiopathological understanding of the disease, strengthen the clinical staging models and the search for other therapeutic targets.9,14 Studies which evaluated inflammatory markers have described the key role played by interleukins (IL), tumor necrosis factor (TNF) and other inflammatory factors in the physiopathology of BD.20 Many studies have evaluate the association between inflammatory biomarkers and BD.21,22 However, studies that sought the association of inflammatory biomarkers with staging are scarce.14
The goal of this study was to carry out a systematic review of the literature for all available studies that evaluated the serum or plasma levels for inflammatory markers in patients with BD, and its association with stages or clinical variables related to the staging of the disease.
MethodsLiterature ResearchA systematic review of the literature was carried out following the PRISMA23 statement up to June of 2015, and in the main scientific databases: PubMed, SciELO, LiLACS and PsycINFO. There were no restrictions on year of publication or country, and the keywords were divided into 2 domains: a) Inflammatory markers using the MESH terms inflammation, cytokines and interleukins tumor necrosis factor, and b) Bipolar Disorders and staging using the MeSH terms staging OR bipolar disorder. Initially repeated articles were eliminated and the selection was made based on title and abstract in order to verify that they met the inclusion and exclusion criteria. Then the complete articles were evaluated. A review was carried out on the references from the original articles, as well as on some of the revisions that were found, with the aim of identifying other publications that could have been lost. The systematic search and selection of the references was carried out by two researchers (MCR and KD) and was overseen by a third researcher (CV).
Eligibility CriteriaSelection CriteriaThe papers retrieved from the research were screened first by title and abstract and then by the following criteria:
- 1.
The study population was 18 and over.
- 2.
The studies involved patients diagnosed with BD by a structured diagnostic interview based on criteria from the DSM or CID.
- 3.
The studies assessing circulating inflammatory markers with plasma or serum samples in vivo.
- 4.
The studies that used classification by stages or that included in the analysis clinical variables related to staging of BD: age of onset, the length of illness the number of episodes and cognitive functions.
- 1.
Studies evaluating genes related to inflammation and in vitro or morphological studies.
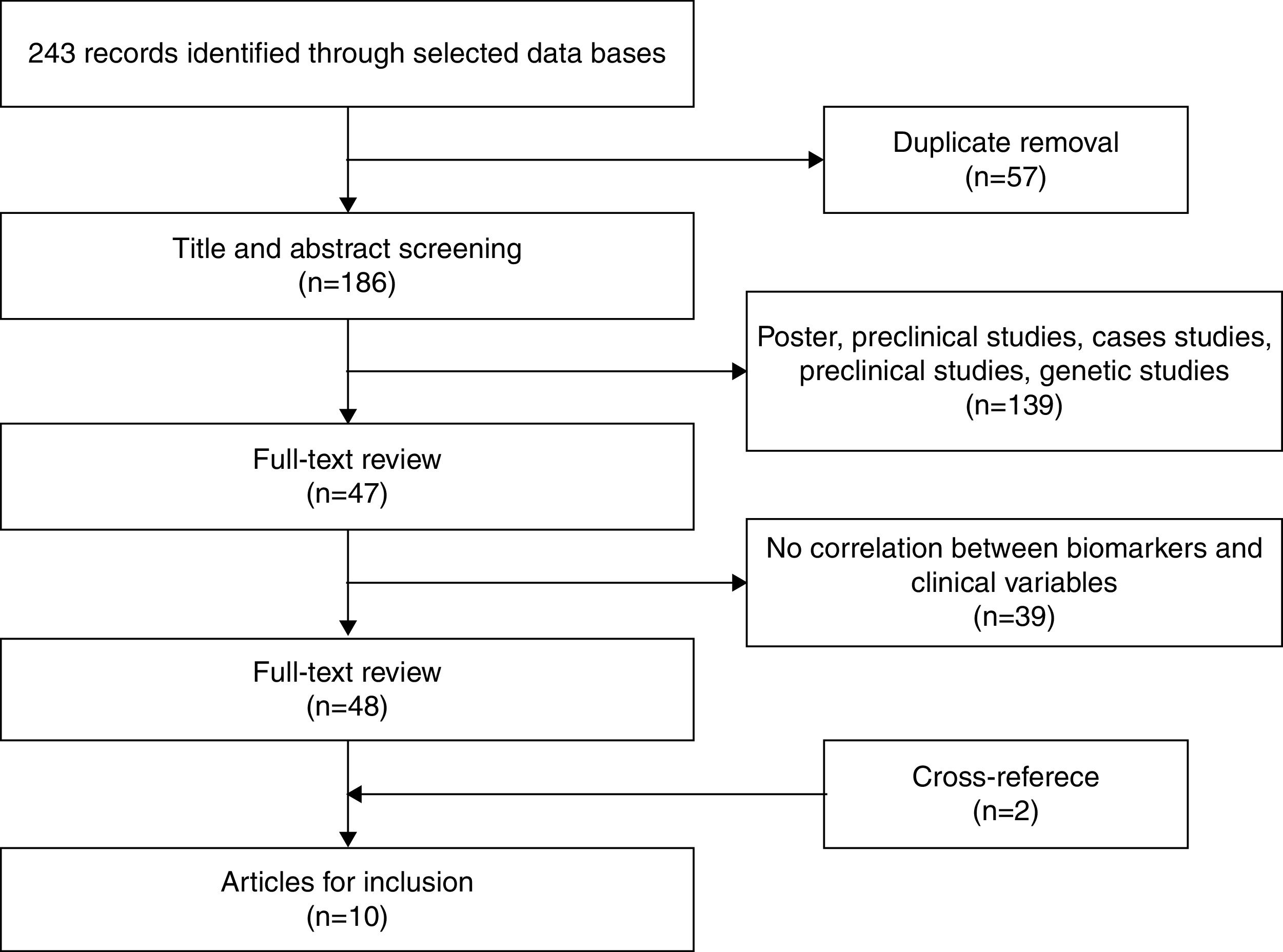
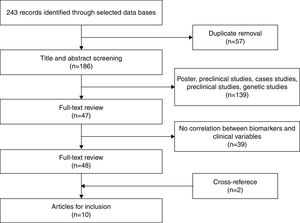
After eliminating duplicate references, 186 articles remained, which were examined by title and abstract. Of these, 139 were excluded as they were posters, preclinical or genetic studies, or they measured the effects of medication. Finally, 47 articles remained for more exhaustive review. After review, 39 articles were eliminated given that they did not correlate inflammatory biomarkers with variables related to staging. Two additional articles were obtained through cross-referencing. After finishing the selection process, 10 original articles were included in the systematic review that met the inclusion criteria. Figure 1 shows the selection process (Figure 1).
All of the studies were cross-sectional, analytic case-control studies, except one.24 There were 745 bipolar patients and 530 controls in total. In one of the studies the controls were relatives in the first degree of consanguinity,18 and in another they were patients with non-affective psychosis.25 All groups were matched based on sex and schooling, except one.25 One study used previous studies to define the percentiles for the C-reactive protein (CRP) levels and the cognitive functioning scores.24 One study included body mass index and cigarette consumption in the match.26
With regards to the clinical characteristics of the sample, 75% of the bipolar group was BD-I. Two of the studies did not define which were BD-I and BD-II,18,27 5 studies included only patients with BD-I,19,26,28–30 and the remaining studies included patients with BD-I and BD-II. The Hamilton Depression Rating Scale (HDRS) and the Young Mania Rating Scale (YMRS) were used for all studies to determine the status of euthymia, except for one that used the Montgomery-Asberg Depression Scale to measure depression with a cut-off point of <10.26 One study did not include scales that defined the severity of the status of euthymia with respect to affective symptoms.24
The inflammatory markers were measured in serum in 7 studies,18,19,24,26,27,30,31 and were measured in plasma in the studies remain. The concentration of cytokines was measured using enzyme-linked immunosorbent assay (ELISA)19,26,28–30; or determined the concentration by flow cytometry18,31 or using enzyme immunoassays (EIA).25,27,30
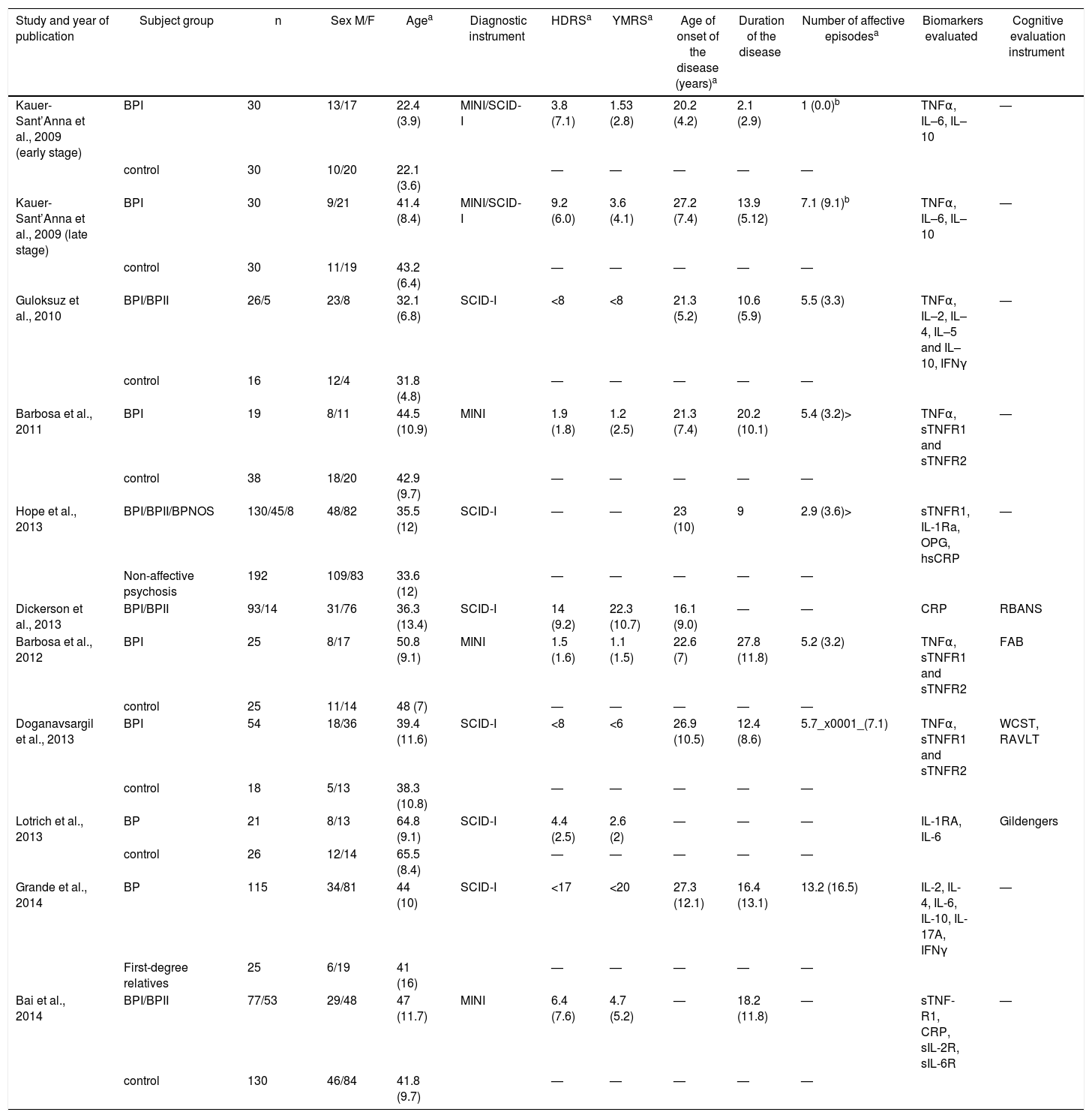
The most frequently evaluated clinical variables related to BD-I staging were the age of onset, the length of illness and the number of episodes. Four studies evaluated cognitive functions24,27,29,30 using, respectively, the Frontal Assessment Battery (FAB),32 the Repeatable Battery Assessment Neuropsychological Status (RBAS),33 the cognitive evaluation described by Gildengers,34 the Wisconsin Card Sorting Test and the Rey Auditory Verbal Learning Test. One included history of psychosis25 and 2 studies created a staging model which divided the patients into two groups: early and late stages of the disease.18,19 The Kauer-Sant’Anna et al. study definition of stage take in account the length of the disease, and the Grande et al. study determined early and late stages based on the functional assessment, age of onset, length of illness and number of episodes. Table 1 shows the characteristics of the studies included (Table 1).
Features of the studies included in the systematic review.
| Study and year of publication | Subject group | n | Sex M/F | Agea | Diagnostic instrument | HDRSa | YMRSa | Age of onset of the disease (years)a | Duration of the disease | Number of affective episodesa | Biomarkers evaluated | Cognitive evaluation instrument |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Kauer-Sant’Anna et al., 2009 (early stage) | BPI | 30 | 13/17 | 22.4 (3.9) | MINI/SCID-I | 3.8 (7.1) | 1.53 (2.8) | 20.2 (4.2) | 2.1 (2.9) | 1 (0.0)b | TNFα, IL–6, IL–10 | — |
| control | 30 | 10/20 | 22.1 (3.6) | — | — | — | — | — | ||||
| Kauer-Sant’Anna et al., 2009 (late stage) | BPI | 30 | 9/21 | 41.4 (8.4) | MINI/SCID-I | 9.2 (6.0) | 3.6 (4.1) | 27.2 (7.4) | 13.9 (5.12) | 7.1 (9.1)b | TNFα, IL–6, IL–10 | — |
| control | 30 | 11/19 | 43.2 (6.4) | — | — | — | — | — | ||||
| Guloksuz et al., 2010 | BPI/BPII | 26/5 | 23/8 | 32.1 (6.8) | SCID-I | <8 | <8 | 21.3 (5.2) | 10.6 (5.9) | 5.5 (3.3) | TNFα, IL–2, IL–4, IL–5 and IL–10, IFNγ | — |
| control | 16 | 12/4 | 31.8 (4.8) | — | — | — | — | — | ||||
| Barbosa et al., 2011 | BPI | 19 | 8/11 | 44.5 (10.9) | MINI | 1.9 (1.8) | 1.2 (2.5) | 21.3 (7.4) | 20.2 (10.1) | 5.4 (3.2)> | TNFα, sTNFR1 and sTNFR2 | — |
| control | 38 | 18/20 | 42.9 (9.7) | — | — | — | — | — | ||||
| Hope et al., 2013 | BPI/BPII/BPNOS | 130/45/8 | 48/82 | 35.5 (12) | SCID-I | — | — | 23 (10) | 9 | 2.9 (3.6)> | sTNFR1, IL-1Ra, OPG, hsCRP | — |
| Non-affective psychosis | 192 | 109/83 | 33.6 (12) | — | — | — | — | — | ||||
| Dickerson et al., 2013 | BPI/BPII | 93/14 | 31/76 | 36.3 (13.4) | SCID-I | 14 (9.2) | 22.3 (10.7) | 16.1 (9.0) | — | — | CRP | RBANS |
| Barbosa et al., 2012 | BPI | 25 | 8/17 | 50.8 (9.1) | MINI | 1.5 (1.6) | 1.1 (1.5) | 22.6 (7) | 27.8 (11.8) | 5.2 (3.2) | TNFα, sTNFR1 and sTNFR2 | FAB |
| control | 25 | 11/14 | 48 (7) | — | — | — | — | — | ||||
| Doganavsargil et al., 2013 | BPI | 54 | 18/36 | 39.4 (11.6) | SCID-I | <8 | <6 | 26.9 (10.5) | 12.4 (8.6) | 5.7_x0001_(7.1) | TNFα, sTNFR1 and sTNFR2 | WCST, RAVLT |
| control | 18 | 5/13 | 38.3 (10.8) | — | — | — | — | — | ||||
| Lotrich et al., 2013 | BP | 21 | 8/13 | 64.8 (9.1) | SCID-I | 4.4 (2.5) | 2.6 (2) | — | — | — | IL-1RA, IL-6 | Gildengers |
| control | 26 | 12/14 | 65.5 (8.4) | — | — | — | — | — | ||||
| Grande et al., 2014 | BP | 115 | 34/81 | 44 (10) | SCID-I | <17 | <20 | 27.3 (12.1) | 16.4 (13.1) | 13.2 (16.5) | IL-2, IL-4, IL-6, IL-10, IL-17A, IFNγ | — |
| First-degree relatives | 25 | 6/19 | 41 (16) | — | — | — | — | — | ||||
| Bai et al., 2014 | BPI/BPII | 77/53 | 29/48 | 47 (11.7) | MINI | 6.4 (7.6) | 4.7 (5.2) | — | 18.2 (11.8) | — | sTNF-R1, CRP, sIL-2R, sIL-6R | — |
| control | 130 | 46/84 | 41.8 (9.7) | — | — | — | — | — |
BDNF: brain derived neurotrophic factor; BP: bipolar disorder; BPNOS: bipolar disorder no other specified; CRP: C reactive protein; FAB: Frontal Assessment Batery; HDRS: Hamilton Depression Rating Scale; hsCRP: high sensitivity reactive C protein; IFNγ: interferon gamma; IL: interleukin; IL–1Ra: IL–1 receptor antagonist; MINI: Mental International Neuropshychiatric Interview; OPG: osteoporgarina; RAVLT: Rey Auditory Verbal Learning Test; RBANS: Repeatable Battery for the Assessment of Neuropsychological Status; SCID-I: Structures Clinical Interview for DSM Disorders; sIL–2R: IL–2 soluble receptor; sTNFR: soluble receptor of tumoral necrosis factor; TNF: tumor necrosis factor; WCST: Wisconsin Sorting Card Test; YMRS: Young Manía Rating Scale.
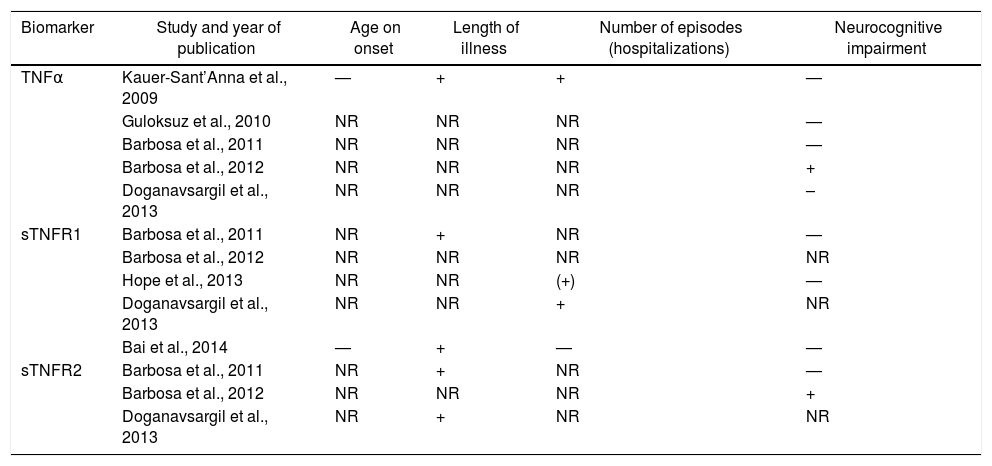
The TNF family was evaluated in 7 studies. These estudies find no association between TNF and age of onset of BD. Regarding the duration of illness, the only study which found a relationship of the TNFα with this and the number of depressive and manic episodes was the study of Kauer-Sant’Anna.19 In this, the TNFα levels were higher in patients in both stages when compared with controls, and a greater elevation was described in the late stage when compared with those found in early stage (F=12.1; d.f.=7,52; P=.001). The study of Barbosa et al. (2012) showed a positive correlation between TNFα plasma levels and the inhibitory control subtest of the FAB (ρ=.50; P=.02) and Doganavsargil-Baysal et al. (2013) showed a negative correlation with the RAVLT delayed recall (r=–.275; P=.04).
The duration of illness showed positive correlation with the soluble Tumor Necrosis Factor Receptor-1 (sTNFR1) in 2 studies (P=.36; P=.01; r=.278; P=.0001)26,28 and with the sTNFR2 in 1 more studies (P=.42; P=.05; r=.32; P=.01).28,30 Furthermore, a positive correlation was found between the sTNFR1, both for the number of episodes (r=.428; P=.001)30 and for the length of hospitalization (P=.23; P=.04).25 The other studies found no relationship between sTNFR and the studied variables. The relationship between neurocognitive impairment and TNF family were contradictory.
Table 2 shows the studies that included TNF superfamily and its correlation with clinical variables.
Studies that included the TNF superfamily and its correlation with clinical variables.
| Biomarker | Study and year of publication | Age on onset | Length of illness | Number of episodes (hospitalizations) | Neurocognitive impairment |
|---|---|---|---|---|---|
| TNFα | Kauer-Sant’Anna et al., 2009 | — | + | + | — |
| Guloksuz et al., 2010 | NR | NR | NR | — | |
| Barbosa et al., 2011 | NR | NR | NR | — | |
| Barbosa et al., 2012 | NR | NR | NR | + | |
| Doganavsargil et al., 2013 | NR | NR | NR | – | |
| sTNFR1 | Barbosa et al., 2011 | NR | + | NR | — |
| Barbosa et al., 2012 | NR | NR | NR | NR | |
| Hope et al., 2013 | NR | NR | (+) | — | |
| Doganavsargil et al., 2013 | NR | NR | + | NR | |
| Bai et al., 2014 | — | + | — | — | |
| sTNFR2 | Barbosa et al., 2011 | NR | + | NR | — |
| Barbosa et al., 2012 | NR | NR | NR | + | |
| Doganavsargil et al., 2013 | NR | + | NR | NR |
–: inverse correlation; —: not evaluated;+ positive correlation; NR: no relationship; sTNFR: soluble receptor of the tumor necrosis factor; TNF: tumor necrosis factor.
Serum osteoprotegerin levels (OPG) in patients with BD-I showed no correlation with the clinical variables associated with neuroprogression.25
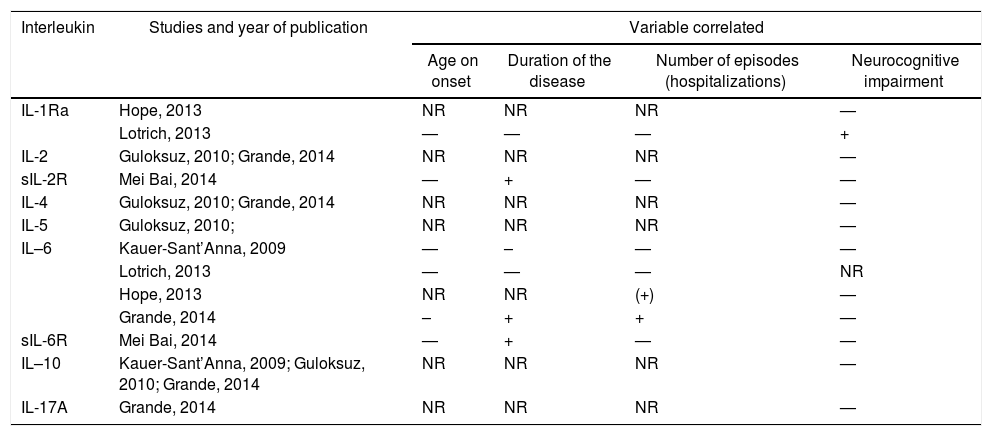
InterleukinsInterleukins (ILs) were evaluated in 6 studies, however not all measured the same families of ILs.18,19,25,27,31 The studies that investigated correlation between ILs and clinical variables can be found on Table 3.
Studies that included interleukins and their correlation with clinical variables.
| Interleukin | Studies and year of publication | Variable correlated | |||
|---|---|---|---|---|---|
| Age on onset | Duration of the disease | Number of episodes (hospitalizations) | Neurocognitive impairment | ||
| IL-1Ra | Hope, 2013 | NR | NR | NR | — |
| Lotrich, 2013 | — | — | — | + | |
| IL-2 | Guloksuz, 2010; Grande, 2014 | NR | NR | NR | — |
| sIL-2R | Mei Bai, 2014 | — | + | — | — |
| IL-4 | Guloksuz, 2010; Grande, 2014 | NR | NR | NR | — |
| IL-5 | Guloksuz, 2010; | NR | NR | NR | — |
| IL–6 | Kauer-Sant’Anna, 2009 | — | – | — | — |
| Lotrich, 2013 | — | — | — | NR | |
| Hope, 2013 | NR | NR | (+) | — | |
| Grande, 2014 | – | + | + | — | |
| sIL-6R | Mei Bai, 2014 | — | + | — | — |
| IL–10 | Kauer-Sant’Anna, 2009; Guloksuz, 2010; Grande, 2014 | NR | NR | NR | — |
| IL-17A | Grande, 2014 | NR | NR | NR | — |
–: inverse correlation; —: not evaluated;+ positive correlation; IL: interleukin; IL-Ra: antagonist receptor for IL-1; NR: no relationship; sIL-2R: soluble receptor for interleukin 2; sIL-6R: soluble receptor for interleukin 6.
Hope et al.25 found a positive correlation between the IL-1 receptor antagonist (IL–1Ra) with a history of psychosis. In the study of Lotrich et al.,27 the IL–1Ra was inversely correlated with three cognitive function factors and global cognition (r=–.37; P=.01). The patients with high levels of IL-1Ra had worse performance in visual cognition, memory cognition, speed/executive cognition and global cognitive function. No relationship between age of onset, illness duration and number of episodes with IL-1 was found.
Three studies evaluated the IL-2 family.18,26,31 Only Bai et al.26 found a positive correlation between the soluble receptor of the IL-2 (sIL-2R) and the length of the disease (r=.133; P=.03).
The IL-6 family was evaluated in 5 studies.18,19,26,27 Two studies that divided the patients into early and late stages found a relationship between the IL-6 and the stages. The Kauer-Sant’Anna et al. study found a significant decrease from the early stage (within the first 3 years of a first manic episode) to late stage (at least 10 years after the diagnosis of BD) (F=6.0; d.f.=7,52; P=.018) and negative correlation with duration of illness (r=x0.41; P=.001). The Grande et al. study suggest the distinction of two stages: one, referred to as early stage, with better functioning, fewer episodes, late age of onset and low levels of IL-6; and the other, referred to as late stage, with a greater compromise of functioning, more episodes, earlier age of onset and raised levels of IL-6 (OR=–2.03; P=.02). Hope et al.25 found a positive correlation between the IL-6 with a history of psychosis and the length of hospitalization (P<.05), but no correlation with age of onset and the duration of hospitalization were found. Lotrich et al. (2014) looked a negative result for the relationship of IL-6 with cognitive function. The Soluble Interleukin-6 receptor (sIL-6R) exhibited positive correlation with the length of illness, and the levels found in patients with the disease independent of the affective status were higher than in the controls (r=.227; P=.0001).26
There was no correlation between the variables related to staging and the IL-4,18,31 the IL-5,31 the IL-10,14,19,31 nor with IL-17A.18
Other MarkersThe interferon gamma (IFN-y) was evaluated in two studies and no correlation was found with the staging variables.18,31
Raised levels of reactive C protein (CRP) were associated with low cognitive function, greater change in global cognitive function, short-term memory, attention span, language and TMT (with t statistic; P<.05).24 Furthermore, there was a positive correlation with the length of illness (r=.14; P=.01).26
DiscussionSummary of EvidenceThe results of this review suggest that TNF activity measured by TNFα, sTNFR1 and sTNFR2 could be related to the length of illness, the number of episodes and changes to the cognitive function in adult patients with BD during euthymia. Raised levels of TNFα, sTNFR1 and sTNFR2 indicate that there may be a proinflammatory status in more advanced stages of BD. However, elevated levels have been reported when patients are suffering maniac or depressive episodes.22 A possible explanation for these findings could be that the patients remain in a chronic inflammatory state, or that the raised levels in periods of euthymia owe to the cumulative effect of the affective episodes.7
Elevated levels of TNFα were only found in one of the studies19 that measured it. This could be because of the effect of the treatment, as suggested by the comparison of patients who are free of medication with patients under treatment with lithium31; it could also be explained by the fact that TNFα is a more unstable molecule, and for this reason more consistent associations were found with the soluble receptors sTNFR1 and sTNFR2, which are easier to detect in peripheral fluids.35,36
The results suggest that there is a relationship between the IL-1Ra and sIL-2R and specific symptoms of the disease: the IL-1Ra with the severity of cognitive function and the number of episodes, and the sIL-2R with the length of illness. Studies have found elevated levels of IL-1Ra and sIL-2R in mania episodes, but not in states of euthymia,22,37 which would indicate a relationship between the IL-1 and IL-2 system and the deterioration of specific symptoms, and express a state of chronic inflammation. Further cohort studies are required that analyze the levels of these markers in mania episodes and euthymia in early and late stages of the disease, so as to clarify whether or not they are related to the stage or to specific symptoms.
The levels of IL-6 show contradictory results in the studies that were under review.18,19 These differences can be explained by the lack of uniformity in the definition of early and late stages. Where Kauer-Sant’Anna define early stage those patients within first three years of a first manic episode and patients in late stage those with minimum ten years after diagnosis of BD. Furthermore, Grande considered the functioning assessment, number of episodes, age of onset and time elapsed since first episode to delimit early and late stages. In the Kauer-Sant’Anna study the IL-6 levels were found to be lower in the late stage, which could be because of the effect of the modulators on IL-6 levels, which decrease after 6 weeks of treatment according to a previous study.38 Conversely the Grande study included patients with subsyndromatic symptoms, which could be related to elevated IL-6 levels. This was also the case for the Bai YM study, which found raised levels of sIL-6R in patients who have has the disease for longer. There is a correlation if one takes into account that patients with more advanced ages have greater difficulties reaching complete clinical remission and continue to suffer subsyndromatic symptoms.39
CRP is a protein generated in the liver and secreted in the blood, and plays a key role in the systematic response to the inflammation.40 Elevated levels of CRP in patients with advanced age and changes to cognitive function suggest that this might be a neuroprogression marker. Nonetheless studies have shown that these levels may also be elevated in cardiovascular diseases,41 for which the probability of suffering them increases with age in patients with BD.42
OPG is a soluble member from the superfamily of receptors of the tumor necrosis factor, and has a central effect on the immune system.43 It is activated by proinflammatory cytokines and has been linked with the risk of suffering cardiovascular disease and diabetes.44,45 However, the Hope study found no association with the severity of BD-I, which may be explained by two factors: either by the anti-inflammatory effect of IL-1Ra, which was found to be elevated and could offset OPG activation, or because the prevalence of arterial hypertension and diabetes was only 5% in the study sample, which would indicate greater specificity of OPG for these diseases and lesser for BD.
The results suggest that the levels of the IL-1, IL-2 and IL-6 families could play a key role in neuroprogression and may be potential markers for staging diagnosis and therapeutic targets for BD-I.
Limitations of the StudiesThe studies that were reviewed have various limitations. One important limitation is that these studies are cross-sectional, meaning that researchers are unable to use causality as a variable. Additional limitations are related to small study samples, entailing a Type I error for multiple comparisons; the inclusion of patients with BD-II, which could be a possible source of error through the possibility of having neuroprogression different to BD-I; as well as the inclusion of some studies of patients with affective episodes who had previously demonstrated association with changes to the values of the inflammatory markers.26,28
Moreover there may be further sources of error that remain unmeasured. One such source could be the effect of the treatment on the inflammatory biomarker levels. These were only measured in one study, which suggests that lithium may have a confounding effect.31 Likewise, the levels of inflammatory biomarker could be affect for some diseases like obesity, hypertension and diabetes that the studies did not take in count.
Limitations of the Systematic ReviewThe most significant limitation of the review is that the results that relate biomarkers with variables involved in staging are the product of secondary analyses, except in 2 studies. For the Kauer-Sant’Anna and Grande studies, the main goal was to establish the relationship between inflammatory markers and TB I stages, but there was no homogeneity of criteria that split patients into stages.
Although the selection criteria were homogenous, the samples were different in some clinical variables. The mean patient age, mean length of illness and mean number of episodes were different in the studies, which makes it difficult to generalize the results.
The levels of cytokines can be affected by some conditions like the use of novel technologies, sampling, use of anticoagulants and storage.46 The sample collection protocols and biochemical assays in the studies were different, which may affect the comparisons between the results.
Another limitation is the inclusion of studies with different analytical methods given the scarcity of workable studies, making the generalization of results difficult.
ConclusionsTen studies with original data, which compared inflammatory marker levels to variables related to staging, were chosen for this study. The most significant preliminary findings, although inconsistent across all studies, are that the TNF superfamily and inflammatory cytokines may have a relationship with the neuroprogression of the disease, and support the hypothesis that they may be considered stage markers based on the staging model of BD. These results strengthened the inflammatory theory of BD, and suggest that the relationships between the inflammatory factors and clinical features of neuroprogression are complex.
Longitudinal studies are needed, with larger and multisite samples and greater uniformity of criteria, which allow the research to establish causality, clarify the relationship between factors associated with neuroprogression, measure the effect of the treatment and facilitate the creation of stage diagnostic criteria for BD. Also, further investigations evaluating the relationship between inflammatory biomarkers and clinical variables related with early and late stages of bipolar disorders, genetic markers, imagenologic or neurocognitive variables should be performed.
Conflicts of interestThe authors have no conflicts of interest to declare.