Predominant polarity (PP) has been proposed as a specifier of bipolar disorder (BD) due to its relationship with clinical and prognostic variables. It is possible that this is due to a different underlying neurobiology, in such a way that the changes found by structural nuclear magnetic resonance imaging (sMRI) in BD are different and specific.
ObjectivesTo explore findings of structural neuroimaging in patients with BD type I (BD-I) according to PP.
MethodsCross-sectional study that evaluated 77 patients with BD-I using the DIGS interview. PP was established using the operative definition of two-thirds of all affective episodes throughout life to classify PP as manic (MPP), depressive (DPP) or indeterminate (IPP). MRI was performed during the euthymia phase to measure intracranial structures. The data obtained was analysed using a linear regression model adjusted for confounding variables (drug use, alcohol use, psychoactive substance use) and were compared between the three groups finding the standardised mean difference (SMD).
ResultsDifferences with adequate effect size were found in three brain structures after adjusting for confounding variables, specifically in the right fusiform gyrus and the left lingual gyrus, which were greater in the DPP group than in the MPP group (SMD = 0.92; 95% CI = 0.34–1.49 and SMD = 0.78; 95% CI = 0.21–1.35). Likewise, in the right thalamus, it was shown to be greater in the IPP group compared to MPP group (SMD 0.89, 95% CI = 0.31–1.46).
ConclusionsA reduction in the thickness of the right fusiform gyrus and the left lingual gyrus, as well as the right thalamic volume was observed in patients with BD-I with PPM, which supports the hypothesis that PP has a plausible neurobiological correlate and could have potential utility as a BD specifier.
La polaridad predominante (PP) se ha propuesto como un especificador del trastorno afectivo bipolar (TAB) por su relación con variables clínicas y pronósticas. Es posible que esto se deba a una neurobiología subyacente distinta, de tal manera que los cambios encontrados por resonancia magnética estructural (RMe) en el TAB sean diferentes y específicos.
ObjetivosExplorar hallazgos de neuroimagen estructural en pacientes con TAB I de acuerdo con la PP.
MétodosEstudio de corte transversal que evaluó a 77 pacientes con TAB I usando la entrevista DIGS. Se estableció la PP utilizando la definición operativa de los 2 tercios de todos los episodios afectivos a lo largo de la vida para clasificar la PP en maniaca (PPM), depresiva (PPD) o indeterminada (PPI). Se les realizó RMe durante la fase de eutimia para medir estructuras intracraneales. Los datos obtenidos se analizaron mediante un modelo de regresión lineal ajustado por variables de confusión (consumo de medicamentos, consumo de alcohol, consumo de sustancias psicoactivas) y se compararon entre los 3 grupos para hallar la diferencia de medias estandarizada (DME).
ResultadosSe encontraron diferencias con adecuado tamaño de efecto en 3 estructuras cerebrales tras ajustar por variables de confusión, específicamente en el giro fusiforme derecho y el giro lingual izquierdo, que fueron mayores en el grupo de PPD que en el de PPM (DME = 0,92; IC 95%, 0,34−1,49; DME = 0,78; IC 95%, 0,21−1,35). Asimismo en el tálamo derecho, que se mostró mayor en el grupo de PPI frente al de PPM (DME = 0,89; IC 95%, 0,31−1,46).
ConclusionesSe observó una reducción del espesor del giro fusiforme derecho y el giro lingual izquierdo, así como del volumen talámico derecho en pacientes con TAB I con PPM, lo que respalda la hipótesis de que la PP cuenta con un correlato neurobiológico plausible y podría tener potencial utilidad como especificador del TAB.
Bipolar disorder (BD) is a serious psychiatric illness that covers a group of clinical mood disorders with multifactorial aetiology. It is considered a significant cause of disability because of the related cognitive and functional deterioration and is associated with high mortality rates, including deaths from suicide.1,2 BD is a relatively common disorder, with a worldwide lifetime prevalence of the entire bipolar spectrum of 2.4% and of BD type I (bipolar I disorder; BD-I) of 0.6%.3,4 It is a chronic, episodic and recurrent disorder, with a heterogeneous clinical presentation, defined by at least one manic episode and a variable number of affective episodes, whether other manias, hypomanias, major depressive episodes (MDE) or mixed episodes, usually with symptom-free or euthymic periods in between.5,6 It has been suggested that affected people may have different longitudinal trajectories. Jules Angst (1978)7 described three “clinical types” in a bipolar population: an “MD” type, to describe those hospitalised for depression and mania in equal measure, a manic type, "Md", and a depressive type, “Dm”, where hospital admission was mainly due to mania or depression respectively. These observations led to the concept of predominant polarity (PP), for which two operational definitions are proposed. The first, by Colom et al8 in Barcelona, is the most frequently used and defines as PP where one polarity occurs during at least two thirds of the total number of previous episodes. The second is the Harvard index, which comes from a ratio between the number of manic (and hypomanic) episodes and the total number of depressive episodes; if the score is >1, the patient is considered “predominantly manic” and if it is <1, “predominantly depressive”.9,10
Different studies have described an association between PP and clinical and prognostic variables, which is why PP has been proposed as a specifier of the course and prognosis of BD-I, despite not being included as such in the DSM-5.11 For example, it has been found that the type of first affective episode is a predictor of the subsequent PP, with a positive predictive value of 75–80%.12 Manic PP (MPP) is more common in males with a higher educational level and a family history of mood disorders, an earlier onset of the disease, hospitalisation at an earlier age, greater use of psychoactive substances and a greater likelihood of psychosis. Depressive PP (DPP) has been associated with being female, with a greater likelihood of finding a partner and getting married, a longer delay in diagnosis and initiation of appropriate treatment, with a seasonal pattern of presentation, comorbidity with personality disorders, alcohol use and an increased risk of suicidal behaviour, and need for electroconvulsive therapy.12–16 There are also differences in treatment. It has been found that patients with MPP are more likely to take mood stabilisers (mainly lithium, valproic acid and lamotrigine) and antipsychotics, alone or in combination with mood stabilisers, while patients with DPP take more antidepressants and benzodiazepines.17 DPP has also been found to be a predictor of a good response to lamotrigine18,19, while in MPP there is a good response to lithium.20
Taking into account the specific characteristics of each PP and how they may affect the disease progression in each patient, it would be helpful to assess whether these characteristics have some neurobiological correlate that might explain clinical aspects of the disorder in which we can intervene and prevent future negative outcomes. A useful instrument for neurobiology study is structural magnetic resonance imaging (MRI), with which it is possible to identify changes in brain morphology. However, although such changes could help explain the variability of the disorder, and in particular of PP, they have been shown to often have inconclusive associations and be influenced by factors that are difficult to control, rendering the findings invalid. It is also difficult to determine how much heterogeneity of the disorder and the PP is due to different underlying neurobiology in each type of PP, or whether the results are simply difficult to interpret because of methodological problems with studies involving neuroimaging analysis.
Many studies in the scientific literature evaluate neuroanatomical aspects in samples of patients with BD, both with structural MRI and functional MRI (fMRI), with different results. For example, there are findings which show changes or abnormalities in different structures, or modifications in activation and connectivity patterns in different brain regions, depending on the type of affective episode. This points to the possibility that these neuroanatomical findings are specific to the episode of mania or bipolar depression, suggesting the hypothesis that there may also be specific findings depending on the type of PP. The aim of our study was to identify the differences in structural MRI images among patients with BD-I according to their type of PP in a sample of patients from Antioquia in Colombia.
MethodsDescriptive cross-sectional study. The study was approved by the Ethics Committee of the Faculty of Medicine at Universidad de Antioquia. The participants signed an informed consent form. This research project involved minimal risk for the participants and complied with the standards for research in humans, according to the provisions of Resolution No. 008430 of 1993 of the Ministry of Health of Colombia and in the Declaration of Helsinki of 2013.
PopulationWe included patients aged from 18 to 60 from a cohort of the PRISMA21 project with a diagnosis of BD-I, according to DSM-IV-TR diagnostic criteria, established by the diagnostic interview for genetic studies (DIGS), who would have had structural MRI during euthymia, defined by a Young Mania Rating Scale <6 points and a Hamilton Depression Rating Scale <7 points (initially 82 patients). We excluded participants with neurological disease, moderate or severe intellectual disability, autism, personality disorders, other mood disorders, schizophrenia, history of head injury, history of electroconvulsive therapy or contraindications to structural MRI (pacemaker, implantable cardioverter-defibrillator, cochlear implant, metallic vascular clips, vascular prosthesis, vascular stent, intrauterine device, orthopaedic prostheses, or metal fragments in the body, such as firearm projectiles) and obtained a definitive sample of 77 patients.
Instruments and proceduresDiagnostic Interview for Genetic Studies (DIGS) version 3.0Developed by the National Institute of Mental Health (NIMH) in 1994 for research into the genetics of mental illness, it is validated for application to the Colombian population. It is designed for the comprehensive evaluation and diagnosis of individuals with severe mental disorders, such as BD-I.22,23 For this study, the DIGS was applied by trained psychiatrists and psychiatry residents. Sociodemographic and clinical information was obtained from this interview (age of onset, time since onset, number and type of affective episodes, psychopharmacological treatment received, history of psychotic symptoms, use of psychoactive substances and suicidal behaviour).
Predominant polarityParticipants were classified according to their PP, using the Barcelona group criteria,8 where at least two thirds of a patient’s episodes in the longitudinal course of the disorder must be MDE or mania (or hypomania) to be considered DPP or MPP respectively. Participants in whom neither of the above two PP were identified were classified as having indeterminate PP (IPP).
Structural brain MRI imagesStructural brain MRI were performed with a Nova Dual Philips Achieva 3 Tesla resonator and took approximately 1 h. T1-weighted sequences were obtained, and the Statistical Parametric Mapping program version 2005 (SPM5) (Department of Cognitive Neurology, University College, London) was used for data preprocessing and analysis. All images were realigned to correct for any patient movement during the scan by performing a six-parameter rigid body transformation and creating an average image of all sessions for the data obtained. Sessions with realignment parameters >4 mm in any of the planes of motion (x, y, z axes) were excluded from the statistical analysis, as were sessions with motion >0.05 radians in the planes of rotation. The average image was then spatially normalised to the Montreal Neurology Institute (MNI) human brain template using a 12-parameter transformation with 12 non-linear replicates and 7 × 8 × 7 basis functions. After normalisation, all volumetric images were sampled with 2 × 2 × 2 voxels with trilinear spatial interpolation. All images were smoothed with an amplitude of 8 mm at half maximum isotropism from a Gaussian filter to compensate for between-subject variability and allow Gaussian random field theory to render correct statistical inferences. We analysed cortical and subcortical structures bilaterally. For the analysis of cortical structures, we used the Desikan and Killiany atlas of brain regions of interest. A database was generated taking into account the measurements of thickness (mm), area (mm2) and volume (mm3) obtained from the Freesurfer software (http://surfer.nmr.mgh.harvard.edu). The volume was calculated by multiplying the thickness by the area. To normalise the volume of brain structures and enable comparison, we used the equation v′ = v/ICV, where v′ is the normalised volume, v is the non-normalised volume, and ICV, the total intracranial volume.
Statistical analysisThe demographic and clinical characteristics of each PP group are described with the median [interquartile range] if continuous, and with frequencies and percentages if categorical. The structural neuroanatomy data is described with the mean ± standard deviation, and the comparison between the groups of each PP was carried out using a linear regression model adjusted for the consumption of benzodiazepines, alcohol and psychoactive substances, lithium, antipsychotics, antidepressants and valproic acid, and assuming the predominantly manic group as the reference group because it is the most common. The results are presented as standardised mean difference (SMD) with their respective 95% confidence interval (95% CI). SMD values above 0.80, 0.50 or 0.20 were interpreted as large, moderate or small differences respectively.24 Given the limitations of the p value, and following the suggestions of Greenland25, we calculated the s value (Shannon information or surprise value) as s = –log2(p), where p is the value of the statistical test used.26 The s value should be interpreted as a continuous measure of the amount of information or bits provided by the statistical test against the contrasted hypothesis (in our case, the hypothesis of no differences between the different study groups). All analyses were performed using R/R-studio.27,28
ResultsParticipant characteristicsSeventy-seven individuals aged from 20 to 58 were included, the majority males (63.6%). The PP was identified in 58 patients (75.3%), while in 19 (24.7%) the PP was indeterminate. The sociodemographic and clinical characterisation is shown in Table 1.
Sociodemographic and clinical characteristics of a group of patients with BD-I according to the predominant polarity.
| Characteristic | MPP | DPP | PP | Total |
|---|---|---|---|---|
| Patients | 39 (50.6) | 19 (24.7) | 19 (24.7) | 77 (100) |
| Male | 24 (61.5) | 13 (68.4) | 12 (63.2) | 49 (63.6) |
| Age (years) | 47 [38.5−52.5] | 33 [25–41] | 46 [31–51] | 43 [32–52] |
| Marital status | ||||
| Married/cohabiting | 9 (23.1) | 4 (21.1) | 5 (26.3) | 18 (23.4) |
| Divorced/separated | 4 (10.3) | 3 (15.8) | 1 (5.3) | 8 (10.4) |
| Widowed | 0 | 1 (5.3) | 1 (5.3) | 2 (2.6) |
| Single | 26 (66.7) | 11 (57.9) | 12 (63.2) | 49 (63.6) |
| Occupation | ||||
| Students | 5 (12.8) | 5 (26.3) | 1 (5.3) | 11 (14.3) |
| Unemployed | 8 (20.5) | 6 (31.6) | 4 (21.1) | 18 (23.4) |
| Stay-at-home mum | 8 (20.5) | 4 (21.1) | 7 (36.8) | 19 (24.7) |
| Trade/sales | 8 (20.5) | 2 (10.5) | 0 | 10 (13) |
| Other | 10 (25.6) | 2 (10.5) | 7 (36.8) | 19 (24.7) |
| Education level achieved | ||||
| Primary | 15 (38.5) | 2 (10.5) | 5 (26.5) | 22 (28.6) |
| Secondary | 9 (23.1) | 3 (15.8) | 6 (31.6) | 18 (23.4) |
| University/Technical | 14 (35.9) | 14 (73.7) | 7 (36.8) | 35 (45.5) |
| Postgraduate/Masters | 1 (2.6) | 0 | 1 (5.3) | 2 (2.6) |
| Children | 1 [0.0−1.5] | 0 [0.0−2.0] | 1 [0.0−1.0] | 1 [0.0−2.0] |
| School years passed | 11 [8.0−14.0] | 13 [11.5−15.0] | 11 [6.5−13.5] | 12 [9.0−14.0] |
| Number of affective episodes | ||||
| Total | 5 [3–7] | 6 [4.5−12] | 4 [2−8.5] | 5 [3–8] |
| Depressive | 1 [0−1] | 5 [3.5−7] | 1 [1–3] | 1 [1–3] |
| Mania/Hypomania | 4 [2−5.5] | 1 [1−2.5] | 1 [1−2.5] | 2 [1–5] |
| Mixed | 0 | 0 [0−0.5] | 0 [0−1] | 0 [0−0] |
| Age at onset of BD-I (years) | 23 [18−27.5] | 15 [14–18] | 22 [18−30.5] | 20 [16–26] |
| Time since onset of BD (years) | 21 [11–29] | 16 [9.5−20.5] | 16 [7.5−26.5] | 17 [9–28] |
| Psychosis | 23 (59) | 10 (52.6) | 5 (26.3) | 38 (49.4) |
| Suicidal behaviour | 10 (25.6) | 13 (68.4) | 4 (21.1) | 27 (35.1) |
| Taking lithium | 23 (59) | 11 (57.9) | 13 (68.4) | 47 (61) |
| Taking valproic acid | 30 (76.9) | 10 (52.6) | 14 (73.7) | 54 (70.1) |
| Taking antipsychotics | 30 (77) | 16 (84.2) | 15 (78.9) | 61 (79.3) |
| Taking benzodiazepines | 12 (30.8) | 10 (52.6) | 6 (31.6) | 28 (36.4) |
| Taking antidepressants | 8 (20.5) | 9 (47.4) | 11 (57.9) | 28 (36.4) |
| Use of psychoactive substances | ||||
| Marijuana | 7 (17.9) | 8 (42.2) | 4 (21.1) | 19 (24.7) |
| Cocaine | 6 (15.4) | 4 (21.1) | 3 (15.7) | 13 (16.8) |
| Other | 8 (20.5) | 8 (42.2) | 4 (21.1) | 20 (26) |
| Alcohol use | 11 (28.2) | 7 (36.8) | 5 (26.3) | 23 (29.5) |
| Smoking | 14 (35.9) | 7 (36.8) | 7 (36.8) | 28 (36.4) |
PP: predominant polarity; DPP: depressive predominant polarity; IPP: indeterminate predominant polarity; MPP: manic predominant polarity.
Values are expressed in terms of n (%) or median [interquartile range].
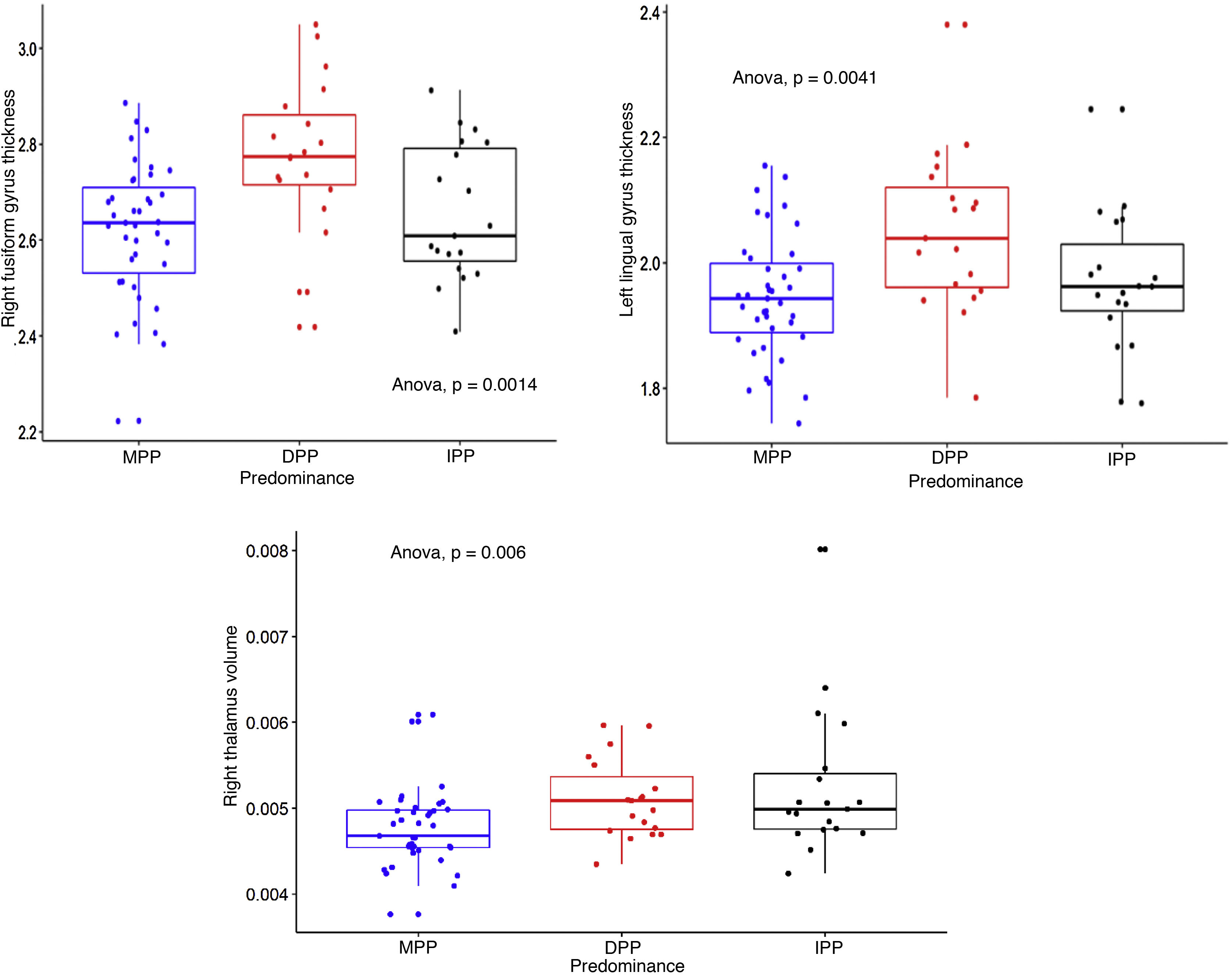
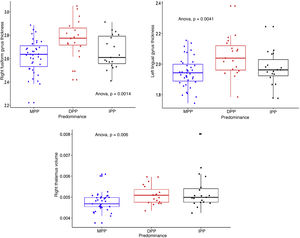
We calculated the volumes of 15 subcortical structures and the thicknesses and areas of 35 cortical structures, right and left (in total, 157 measurements of intracranial structures). Table 2 shows the comparisons between the three groups, the p and s values, with the effect size corrected. The three structures that showed differences with the largest effect size were: the right fusiform gyrus and the left lingual gyrus, the thicknesses of which were greater in the DPP group than in the MPP group: g = 0.92 (95% CI, 0.31–1.46) and g = 0.78 (95% CI, 0.21–1.34) respectively; and the right thalamus, the volume of which was greater in the IPP group than in the MPP group: g = 0.89 (95% CI, 0.3–1.49). Fig. 1 shows the differences in the structures with the largest effect size in the comparison between the PP groups. All the structures evaluated are found in Table 1 of the additional material.
Measurements of the thickness of cortical structures and the volume of subcortical structures, according to the predominant polarity in a group of patients with. BD-I.
| Structures | MPP (n = 39) | DPP (n = 19) | IPP (n = 19) | p | s | SD | DPP vs MPP | IPP vs MPP | ||
|---|---|---|---|---|---|---|---|---|---|---|
| SMD (g) | 95% CI | SMD (g) | 95% CI | |||||||
| Cortical, m(SD) (Thickness) | ||||||||||
| Temporal lobe | ||||||||||
| Right fusiform gyrus | 2.62 (0.14) | 2.77 (0.16) | 2.66 (0.14) | 0.001 | 9.97 | 0.16 | 0.92 | 0.34–1.49 | 0.19 | |
| Right entorhinal cortex | 3.41 (0.33) | 3.64 (0.27) | 3.32 (0.27) | 0.003 | 8.38 | 0.32 | 0.72 | 0.15 to 1.28 | −0.31 | −0.87 to 0.23 |
| Left fusiform gyrus | 2.64 (0.13) | 2.75 (0.14) | 2.62 (0.11) | 0.005 | 7.64 | 0.14 | 0.64 | 0.08 to 1.20 | −0.13 | −0.68 to 0.41 |
| Left parahippocampal gyrus | 2.75 (0.28) | 2.93 (0.26) | 2.67 (0.31) | 0.015 | 6.06 | 0.29 | 0.59 | 0.03 to 1.15 | −0.31 | −0.86 to 0.23 |
| Left transverse temporal cortex | 2.17 (0.21) | 2.33 (0.17) | 2.20 (0.19) | 0.015 | 6.06 | 0.21 | 0.72 | 0.15 to 1.28 | 0.06 | −0.48 to 0.61 |
| Left inferior temporal sulcus | 2.37 (0.16) | 2.47 (0.23) | 2.29 (0.19) | 0.018 | 5.79 | 0.19 | 0.39 | −0.16 to 0.94 | −0.42 | −0.97 to 0.13 |
| Left temporal pole | 3.47 (0.29) | 3.65 (0.29) | 3.62 (0.25) | 0.03 | 5.06 | 0.29 | 0.63 | 0.07 to 1.19 0.54 | −0.009 to 1.10 | |
| Frontal lobe | ||||||||||
| Right paracentral lobule | 2.26 (0.10) | 2.38 (0.11) | 2.31 (0.11) | 0.001 | 9.96 | 0.11 | 0.68 | 0.11 to 1.24 | 0.39 | −0.15 to 0.95 |
| Parietal-occipital lobe | ||||||||||
| Left lingual gyrus | 1.95 (0.10) | 2.05 (0.13) | 1.97 (0.11) | 0.004 | 7.96 | 0.11 | 0.78 | 0.21 to 1.34 | 0.14 | −0.40 to 0.69 |
| Right lingual gyrus | 2.01 (0.11) | 2.11 (0.14) | 2.01 (0.16) | 0.018 | 5.79 | 0.14 | 0.46 | −0.09 to 1.01 | −0.07 | −0.62 to 0.47 |
| Right cuneus cortex | 1.78 (0.13) | 1.90 (0.13) | 1.84 (0.17) | 0.013 | 6.26 | 0.15 | 0.48 | −0.07 to 1.04 | 0.41 | −0.14 to 0.96 |
| Left lateral occipital cortex | 2.20 (0.12) | 2.28 (0.15) | 2.17 (0.13) | 0.02 | 5.64 | 0.13 | 0.49 | −0.06 to 1.05 | −0.23 | −0.78 to 0.32 |
| Cingulate cortex | ||||||||||
| Right rostral anterior cingulate cortex | 2.84 (0.23) | 3.01 (0.27) | 2.98 (0.21) | 0.02 | 5.64 | 0.24 | 0.73 | 0.16 to 1.29 | 0.59 | 0.03 to 1.15 |
| Left insular cortex | 3.02 (0.14) | 3.11 (0.18) | 2.98 (0.15) | 0.026 | 5.26 | 0.16 | 0.24 | −0.31 to 0.79 | −0.34 | −0.90 to 0.20 |
| Subcortical, m(SD) (Volume) | ||||||||||
| Right thalamus | 4.76E-03 (4.44E-04) | 5.11E-03 (4.59E-04) | 5.26E-03 (8.65E-04) | 0.006 | 7.38 | 0.00061 | 0.37 | −0.17 to 0.93 | 0.89 | 0.31 to 1.46 |
Note: the mean (standard deviation) is shown plus the p value and s value of the comparison between the three groups. Also shown is the SMD adjusted for the use of benzodiazepines, use of alcohol and psychoactive substances, use of lithium, antipsychotics, antidepressants and valproic acid and assuming MPP as the reference group.
Abbreviations: MPP: Manic Predominant Polarity; DPP: Depressive Predominant Polarity; IPP: Indeterminate Predominant Polarity; p: p-value; s: s-value; SD: Standard Deviation; SMD: Standardised Mean Difference; g: Hedges g; 95% CI: 95% Confidence interval.
Dotted box-and-whisker plot showing the distribution of right fusiform gyrus thickness, left lingual gyrus thickness and right thalamus volume, according to predominant polarity (DPP, MPP and IPP). Effect sizes were found comparing DPP > MPP (s = 9.97, SD = 0.16, SMD = 0.92), DPP > MPP (s = 7.96, SD = 0.11, SMD = 0.78) and IPP > MPP (s = 7.38; SD = 0.00061; SMD = 0.89) for the three structures respectively.
Based on analysing the structural brain MRI results of 77 patients with BD-I, our study detected a decrease in the thickness of two cortical structures (right fusiform gyrus and left lingual gyrus) in patients with MPP compared to patients with DPP. In the subcortical structures, however, a decrease in the right thalamic volume was found in the MPP group compared to the IPP group. These differences were corrected for and have a large effect size and narrow confidence intervals. This data suggests that the episodes of mania and, by extension, MPP may be related to structural alterations in patients with BD-I. This would mean that episodes of mania have an effect on these brain structures, in line with what different publications have suggested about a neurodegenerative effect typical of BD. Some studies have attributed this in part to dysfunction of the neuroimmunoendocrine mechanisms that lead to hypercortisolism and a persistent proinflammatory state, commonly observed in patients with affective disorders.29,30 However, attributing the findings solely to neurodegeneration is a complex issue due to the two-way dynamics, still subject to study, between structural changes and their possible causes. In other words, the structural alteration could explain the clinical manifestations, or, on the contrary, the pathophysiological processes of BD-I which are clinically expressed as affective episodes, could be the cause of specific structural alterations; without forgetting many other associated factors.
Attempting to compare our results with the available literature, we found few studies that specifically analyse neuroimaging and PP findings. In one such study, Kim et al31 evaluated the MRI of 35 patients with BD-I and compared them with those of 35 healthy controls. They found that patients with BD-I had a more marked decrease bilaterally in prefrontal, insular, temporal and parietal grey matter. In addition, those with MPP also had a decrease in the volume of the superior frontal gyrus greater than those in the DPP group or the healthy controls. Janiri et al32 evaluated MRI images of 175 outpatients with BD, regardless of subtype, and compared them with 150 healthy controls. They focused on the hippocampal structure and made comparisons according to the PP, and found volume reductions in specific portions of the hippocampus in patients with BD, even more accentuated in those who also belonged to the DPP subgroup. Although it might seem that the results of these studies do not directly relate to ours, as the differences were not found in the same PP, some degree of concordance is likely, mainly due to the temporal location of the structural findings in these two studies. We found differences with a large effect size in the right fusiform gyrus (inferior temporal location) and in the left lingual gyrus (medial occipitotemporal location) in the MPP subgroup. Although based on our findings it is perhaps premature to assert that there are precise associations, they do give cause for considering the temporal lobe and some of its structures as relevant to understanding BD, an idea that has already been raised by other authors.33,34
One study sought to identify different temperament-associated and neuroanatomical neurocognitive phenotypes, their heritability, and their association with BD-I severity in two closely related and genetically isolated populations, the Central Valley of Costa Rica and the Antioquia region of Colombia, where the population included in our study is from. Among other findings, reductions in thalamic volume and cortical thickness were observed in the fusiform and lingual gyri. The alterations in these three structures are heritable in this population, and those in the fusiform and lingual gyri also associated with the clinical severity of BD-I.35 The results of this study, which highlight these three structures as neuroimaging phenotypes of BD-I, are consistent with our findings.
Other studies that evaluated neuroanatomy and BD without considering the PP also showed a certain degree of agreement with our observations: that the right fusiform gyrus, the left lingual gyrus and the right thalamus, although they are not the only structures involved, have relevance in the regulation of emotions and mood.36,39 For example, one study evaluated differences in voxel-based morphometry examinations between patients with BD, patients with unipolar depression, and a group of healthy controls. They found that the group of patients with BD had a marked bilateral reduction in grey matter volume in the hippocampi up to the fusiform gyri, dorsal prefrontal cortex, insula, amygdala, caudate nuclei, putamen, thalami and lingual gyri.37 A meta-analysis, also of ENIGMA (Enhancing Neuroimaging Genetics through Meta-Analysis), which evaluated cortical structures in adults with BD, identified that they had thinning of the inferior parietal and temporal regions, including the fusiform and middle temporal gyri, which are related to processing and visuospatial awareness.40 This finding was in turn correlated with decreased metabolism of the right fusiform gyrus in patients with BD-I and a history of psychotic symptoms, and it was suggested that alterations in this structure could be considered a biomarker of psychosis in patients with BD.41 This appears in line with our study, where the alterations in the right fusiform gyrus were significant in the MPP subgroup, which is the group with the highest rate of previous psychotic symptoms over the course of the disorder.
In terms of the left lingual gyrus, in a study that compared patients with BD and patients with unipolar depression, those with BD had decreased grey matter volume in 12 brain regions: lobule VIII of the right cerebellum; the right and left putamen; the left hippocampus; right precuneal cortex; left and right superior frontal gyri; left precentral gyrus; left calcarine cortex; left inferior temporal gyrus; right fusiform; and left lingual.42 The authors found a higher prevalence of overweight/obesity and higher concentrations of proinflammatory cytokines in the group of patients with BD. Similar observations have been obtained in other studies on BD,43 although with the involvement of other structures, with these data suggesting a neuroinflammatory and endocrine mechanism in the pathophysiology of the disorder.
The thalamus is a structure with multiple connections to other brain regions, such as the orbital and medial prefrontal cortex, and other limbic structures involved in emotional regulation. It has been pointed to as a determining structure in the regulation of mood, behaviour and response to environmental stimuli. There is evidence of volumetric abnormalities in the thalamus, as well as patterns of activation and connectivity in affective disorders when compared to healthy controls.38,39,44 Haznedar et al45 evaluated thalamic volumes in various groups of patients with BD-I, BD-II and cyclothymia, and in healthy individuals. They found volumetric abnormalities in the thalamus according to the clinical subtype, mainly in patients with BD-II, in whom asymmetries were observed between the right and left thalami, and in patients with cyclothymic disorder, who showed bilateral reduction in thalamic volume. These findings may be due to the characteristics of each disorder, a greater number of episodes and difficulties in the timely diagnosis of BD-II, or the effect of drug treatments which can mitigate the expected loss or decrease in volume of different structures, for example in BD-I.45 More recent studies evaluating the effect of recurrent affective episodes have compared functional neuroimaging of patients in their first episode with patients with multiple affective episodes. It was found that patients with a chronic course and multiple decompensations have less activation in the prefrontal-striatal-amygdala tracts, and bilaterally in the thalamus, ventrolateral prefrontal, orbitofrontal and anterior cingulate cortices, putamen, caudate, amygdala, Brodmann's area 22, and right posterior parietal regions. It is likely that neurophysiological changes associated with a greater number of affective episodes lead to a decrease in the activation of neural networks involved in affect regulation in patients with BD,46 and in turn have a theoretical correlation with structural changes that may vary according to the PP.
All these observations corroborate the complexity of the disorder, and the fact that neuroimaging research may be a useful instrument for proposing new hypotheses and providing important information for a better understanding of the underlying neurophysiological and pathological processes. This is precisely why neuroimaging biomarkers are proposed as potential specifiers in BD.47,48
This research in particular, which was based on structural neuroimaging, proposes the presence of a neurobiological substrate for PP, which gains strength as a potential specifier of the clinical course and prognosis of BD-I. Analysis of the utility of the results of studies such as ours should also consider previous studies in the same line of research, which have shown heterogeneous and sometimes contradictory results, with structures involved that differ from one study to another, possibly due to the influence of multiple factors. On the one hand, there are factors inherent to the disorder (clinical phenotypes, specifiers), the individual (genetic factors) and the environment (stressful or important life events, epigenetic factors) and the influence of medications such as lithium, which has been related to changes in some brain structures49–51 and could theoretically have a neuroprotective role against the disorder. On the other hand, the methodology of each study with the population studied, the size and characteristics of the sample, the follow-up or lack thereof, and the processing of the neuroimaging are also important.40 All these elements, which differ from one study to another, influence the results and lead to little agreement, even though the studies may be similar.
Despite the diversity of studies on the neuroanatomy of BD, few have specifically investigated the relationship between structural neuroimaging findings and PP. We therefore consider one of the strengths of our study to be our extensive measurement of multiple cortical and subcortical structures, which can add to the body of data on structural neuroanatomy and facilitate quantitative synthesis. Another advantage is the statistical adjustment for confounding variables when analysing the results, which reduces the interference of variables that affect the neurobiology. Among the weaknesses is the small sample size, which could have led to type II errors in identifying regions of interest. It would be beneficial to perform more studies that reproduce ours, but with a larger sample size. Another limitation is the possible measurement bias in some variables, such as substance use or treatment received, as, although DIGS 3.0 includes these variables, there could be inaccurate responses from the participants or recording errors from the evaluator.
ConclusionsOur study found large effect size differences consistent with reduced thickness of the right fusiform gyrus and left lingual gyrus, and reduced right thalamic volume in patients with MPP compared to the DPP and IPP groups. This suggests that PP has a plausible neurobiological correlate, which, in view of the structural differences found, has possible imaging biomarkers for BD-I with potential utility in the diagnostic process, as a predictor of clinical course or in making therapeutic decisions. Further studies with larger samples and the possibility of follow-up are required to evaluate how neuroanatomical changes occur over time in patients with particular clinical manifestations, such as the predominance of each affective episode.
FundingThe data to carry out this research were obtained from the PRISMA research study sample, financed by Colciencias. Code: 501153730798, contract 351-2011, funding call 537 DE 2011. There was no additional sponsorship.
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: Ruiz GC, Ospina JPZ, Vargas C, Acevedo DCA, López-Jaramillo C. Neuroimagen estructural y polaridad predominante en pacientes con trastorno afectivo bipolar tipo I de Antioquia. Rev Colomb Psiquiat. 2022;51:123–132.