Major neurocognitive disorder (MNCD) affects millions of people worldwide. However, the pharmacological options for its management are limited, ineffective and frequently associated with severe adverse reactions.
Case reportAn 85-year-old man with history of multiple chronic brain injuries (alcohol-use disorder, haemorrhagic stroke, brain trauma, chronic use of benzodiazepines) developed an MNCD, reaching 7 points on the Reisberg Global Deterioration Scale. He had minimal response to antidepressants, antipsychotics and anticholinergic medications. After the use of mother tincture of Indian hemp (cannabis), a significant improvement was found in his cognitive function, ability to carry out activities of daily living and independence.
DiscussionThe endocannabinoid system seems to be implicated in age-related cognitive decline. In addition, the evidence derived from in-vitro and animal models suggest that this system could play an important role in the management of MNCD of different causes.
ConclusionsCannabinoid treatment for MNCD emerges as a promising therapeutic approach that may benefit a growing number of patients who do not have other treatment options. It is therefore necessary to encourage more research efforts that will help to remove political and scientific barriers to its clinical use.
El trastorno neurocognitivo mayor (TNM) afecta a millones de personas a nivel mundial. Sin embargo, las opciones farmacológicas para su manejo son limitadas, poco efectivas y se asocian a importantes reacciones adversas.
Caso clínicoSe presenta el caso clínico de un hombre de 85 años, con antecedente de múltiples lesiones cerebrales crónicas (abuso de alcohol, enfermedad cerebrovascular, traumatismo cerebral, uso crónico de benzodiacepinas), quien desarrolló un TNM clasificado con 7 puntos en la Reisberg Global Deterioration Scale. Tuvo poca respuesta al manejo con antidepresivos, antipsicóticos y anticolinérgicos. Tras el uso de tintura madre de cáñamo índico (cannabis), se evidenció una mejoría en la función cognitiva, la capacidad de cuidado para las actividades de la vida diaria y la independencia.
DiscusiónEl sistema endocanabinoide parece estar relacionado con los procesos de deterioro cognitivo asociados con la edad. Además, la evidencia derivada de modelos in vitro y animales sugiere que podría tener un papel importante en el manejo del TNM de diferentes etiologías.
ConclusionesEl uso de cannabinoides en el TNM se presenta como una pista terapéutica prometedora. Por lo tanto, es necesario promover procesos de investigación que contribuyan a eliminar las barreras políticas y científicas para su uso clínico, beneficiando a un número creciente de pacientes que no poseen opciones terapéuticas eficaces.
Major neurocognitive disorder (MNCD), or dementia, is a complex syndrome having multiple possible etiologies (Alzheimer's disease, frontotemporal lobar degeneration, Parkinson's disease, Lewy body disease, among others). It's core feature is a significant compromise in one or more cognitive domains: memory, complex attention, executive functions, learning, language, perceptual motor skills (praxies) and social cognition.1
According to the World Health Organization, over 47 million people worldwide suffer from dementia, with an expected increase up to 75 million people in 2030. This has situated MNCD as a public health issue.2 Although, the pharmacological options for treating dementias are limited, having indirect therapeutic effects over biochemical modification of secondary impairments due to neuronal loss, regardless of the underlying pathophysiological mechanisms, or any neuroprotective effect.3
In regard to the current dementia treatments, a recent review has shown a discrete evidence for two anticholinergics (donepezil and galantamine), atypical antipsychotics and analgesics over behavioral and psychological symptoms; unfortunately, a wide range of adverse effects have been associated to these medications.3,4 The Clinical Practice Guideline for Dementias of the Colombian Ministry of Health, recommends acetylcholinesterase inhibitors (donepezil, galantamine, and rivastigmine) to control symptoms of mild to moderate Alzheimer's disease, and declares the minimal effect found for memantine in clinical trials.5
In this context, there is a vast number of patients with dementia in front of scarce effective and safe pharmacological interventions. However, new alternatives, such as the use of cannabinoids, have been tested in animal and in vitro models. Flourishing scientific evidence has raised promising expectations in regard to its potential use,6 as well as their neuroprotective role for patients suffering from dementia.7
We present a case report of a patient with dementia, multiple cardiovascular risk factors and chronic brain injuries, who has shown a clinical improvement in cognitive functions and autonomy after the use of cannabinoids. We also present a brief literature review in regard to the potential use of cannabinoids in dementia.
Clinical caseDJ, a 85-years-old man from Pereira (Colombia) attended multiple psychiatry outpatient consultations due to memory loss, behavioral changes and decreased self-care abilities. He has a family history of alcohol-use disorder. The patient also had a history of alcohol-use disorder; he had started drinking heavily in his 20s until he was 78 years old. He was a heavy smoker as well (48 pack-year index).
The patient suffered a myocardial infarction 30 years ago, requiring a revascularization intervention. At that moment, he started to refer insomnia and was prescribed with triazolam during 5 years. Few months later, the patient was switched to lorazepam. Fifteen years after the myocardial infarction, DJ had a hemorrhagic stroke without surgical indication. Psychiatric symptoms emerged since then: disinhibition, irritability, paranoid delusions, obsessive cleaning ideas, and ritualistic grooming behaviors.
Despite the psychiatric symptoms, the patient was able to keep his job for 3 more years until he started to exhibit a clear loss of memory and a decline in his abilities for instrumental activities. When DJ was 75 years-old, he was partially dependent for daily life basic activities, self care, and outside home activities. He underwent psychiatry treatment 2 years later, reporting a MiniMental test score of 26/30, mainly due to memory failures.
DJ had two head traumas related to alcohol-use: the first episode did not induce any structural sequelae or bleeding; in the second, the patient suffered a skull fracture and two hematomas. He then exhibited a clear cognitive and basic daily life activities worsening, along with difficulties to recognize family and friends. Two years later he required diaper and had complete dependence for self-care and mobilization, with persistent aggressive behaviors.
Four years before, he required Intensive Care Unit intervention for pulmonary embolism, community-acquired pneumonia, and digestive bleeding. At that time, the Reisberg Global Deterioration Scale (GDS) was 7. Agitation, insomnia and the cognitive decline worsen despite pharmacological treatment. By that time, the patient was receiving quetiapine titrated up to 600mg/day, escitalopram 20mg/day, lorazepam 6mg/day without clinical improvement. Later, memantine 10mg/day was added, with only partial improvement of agitation episodes after one year of treatment; although, no evidence of cognitive or autonomy recovery was observed.
Mother tincture of Indian hemp in a master formula was added when the patient was 82 years old (three years before the present report). After 4 months of use, DJ have shown a significant improvement on major behavioral symptoms (irritability and agitation), cognitive and motor abilities. DJ significantly recovered his autonomy, only requiring punctual help for daily life activities. He was able again to spontaneously initiate conversations and recall family or social recent events. Aggression episodes disappeared. There has been a gradual deprescription of medicines; DJ is currently taking quetiapine 50mg/day, lorazepam 2mg/day, escitalopram 20mg/day, memantine 10mg/day and Indian hemp tincture 10 drops/day.
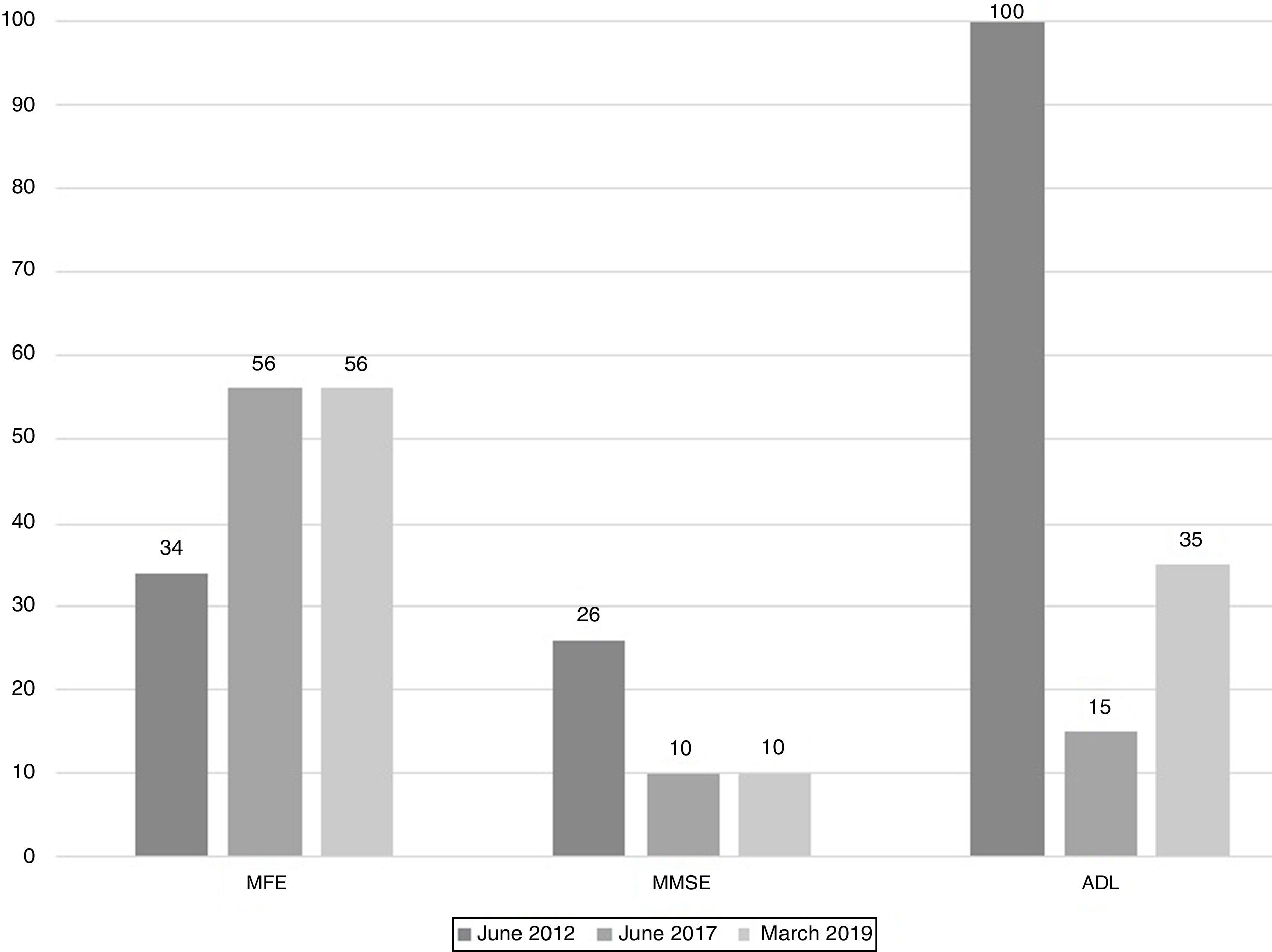
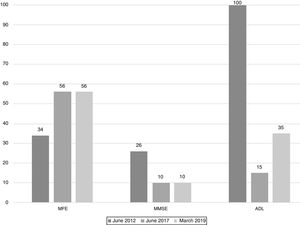
The scores evolution of the patient's Memory Failures of every day (MFE; score range 0 to 56)), Mini-Mental State Examination (MMSE; maximum score 30), and Barthel Index for Activities of Daily Living (ADL; maximum score 100) are shown in Figure 1. All scales scores have been translated into performance percent in order to facilitate the visualisation of the evolution in time.
The patient's family signed informed consent for the publication and dissemination of the clinical case for academic purposes, preserving the patient's identity and privacy.
DiscussionThere is an over increasing evidence regarding the neuroprotective role of the endocannabinoid system, and its potential benefits on neurodegenerative diseases. Strong empirical evidence has suggested that the functional endocannabinoid system declines with age, becoming one of the key factors that may explain the therapeutic positive response to medical cannabis for dementia.8
The brain aging process is not yet fully understood. Although, some neural mechanisms have been linked to age-related functional decline, such as synaptic dysfunctions and neuronal plasticity sluggishness.9 Many of the cellular and molecular mechanisms leading to age related decline, are also linked to the endocannabinoid system, such as oxidative stress, accumulation of damaged macromolecules, modulation of mitochondrial activity, regulation of glial activity, synaptic plasticity loss and the effect of the Brain-Derived Neurotrophic Growth Factor.10
Animal models studies have revealed significant reduction on CB1 receptors density reduction over the cerebral cortex and cerebellum, modifications on CB1 genes expression pattern on distinct brain structures, and lower G protein coupling capacity of CB1 receptor.10
Moreover, know-out mice models for CB1 receptors, have been successful to induce learning and memory impairments, which is considered as a proper replication of what is observed during normal aging. These changes are related to neuronal density loss10,11 and neuro-inflammatory alterations in the hippocampus. Therefore, it has been postulated that CB1 receptors are a clue factor for neuroprotective processes against aging.10
Initially, it was considered that the CB2 receptor, unlike CB1, did not have a major role in the aging cognitive decline.10 However, Alzheimer disease post-mortem studies have found a CB2 receptors up-regulation in the hippocampus, frontal and entorhinal cortex, which could pinpoint an adaptive response against the inflammatory process related to this particular disease.12
In vitro and animal models have demonstrated that cannabinoids exhibit a neuroprotective effect, mediated through antioxidant, anti-inflammatory, and anti-apoptotic properties; Furthermore, cannabinoids are crucial for excitotoxicity regulation, featuring a protective role against mitochondrial toxins.13 In neurodegenerative models, this effect seems to act in synergy with distinct mechanisms through cell proliferation induction and neurogenesis, leading to attenuate cell damage due to hypoxia, multiple sclerosis, iron overload, Alzheimer's disease, Parkinson's disease and Huntington's disease.13
In early-stage Alzheimer's disease murine models, the combination of 9-tetrahydrocannabinol (Δ9-THC) and cannabidiol reduces pathological expression and reverse the physiopathological mechanisms involved at neuronal level.14 In the advanced stage models, there is a symptomatic improvement, although there is no modification of the underlying physiopathological process.15
In addition, selective pharmacological stimulation of the CB2 receptor in Alzheimer's disease murine models, it has been shown a reduced production of β-amyloid, whilst modulating COX-2 and tumor necrosis factor alpha expression by glial cell, being related to cognitive improvement.12
These potentially neuroprotective effects add to previous existing evidence that cannabinoids reduce the Tau protein hyperphosphorylation, the inhibition of the formation of Hungtingtin aggregates, inhibit the Glutamate presynaptic release, may block voltage-dependent calcium channels, lower mitochondrial calcium output and help to improve the cerebral blood flow.16
In regard to cannabinoids treatment in humans, studies on elderly population have reported an overall safe and effective use in different neurodegenerative diseases.17 A study involving 2736 elders (mean age of 74.5 years) reported that 93.7% of patients claimed an improvement of their conditions after 6 months of treatment, particularly pain reduction. The most common adverse events were dizziness (9.7%) and dry mouth (7.1%).17 Moreover, during the 6 months follow up, 18% of the patients stopped taking opioid analgesics or had reduced their dose.17 This data is highly relevant for countries having opioid analgesics dependence and abuse, such as the United States. The cannabinoids efficacy, mainly for pain relief, may account for the over increasing consumption on the elderly. Users over 65 years-old account for 7% to 33% of medical cannabis users in different countries; the wide range has been attributed to singularities depending on the reported country and legislations. In the United States, for example, the elderly represent 14% of the total population and use more than 30% of all the prescription medications.17
ConclusionsCannabinoids have a neuroprotective effect and promote neuroplasticity in in-vitro and animal models. Growing evidence suggests a potential use of cannabinoids for neurodegenerative diseases, including different types of dementia. In these cases, there could be an improvement of cognitive symptoms and attenuation of the underlying pathophysiological process, as the one concerning the present case report.
Despite the great potential of medical marijuana in the elderly, there is still a high resistance for its use. Even in states or countries where cannabis is legal, it is not easy to obtain medication. Evenly, many geriatric home care and doctors seem to disapprove its use, supported by the lack of representative studies on the effects of medicinal cannabis in this population, lack of information or prejudices related to recreational marijuana.
It is necessary to overcome the political, clinical and scientific barriers to generate adequate evidence regarding the efficacy and safety of cannabinoids in different types of dementia.
Conflict of interestThe authors declare they have no competing interest.