Lithium treatment of bipolar disorder (BD) has been associated with less cognitive impairment and fewer changes in structural brain anatomy compared to other treatments. However, the studies are heterogeneous and few assess whether these effects are related. The objective of this study was to evaluate and relate cognitive performance and structural neuroanatomy in patients treated with and without lithium.
MethodsCross-sectional study that included 48 subjects with BD-I, of which 22 were treated with lithium and 26 without lithium. Performance was assessed on Wechsler III (WAIS III), TMT A and B (Trial Making Test) neuropsychological tests, California verbal learning test (CVLT), Rey complex figure test and Wisconsin card sorting test. Brain structures obtained by magnetic resonance imaging (MRI) were evaluated. The standardised mean difference (SMD) between both groups was calculated, adjusted for confounding variables using a propensity score, and the Spearman correlation coefficient (ρ) was used to assess the relationship between cognitive performance and neuroanatomical regions.
ResultsCompared to the group without lithium, the group with lithium had fewer perseverative errors in the Wisconsin test (SMD = –0.69) and greater left and right cortical areas (SMD = 0.85; SMD = 0.92); greater surface area in the left anterior cingulate (SMD = 1.32), right medial orbitofrontal cortex (SMD = 1.17), right superior frontal gyrus (SMD = 0.82), and right and left precentral gyrus (SMD = 1.33; SMD = 0.98); greater volume of the right amygdala (SMD = 0.57), right hippocampus (SMD = 0.66), right putamen (SMD = 0.87) and right thalamus (SMD = .67). In the lithium group, a correlation was found with these errors and the thickness of the left precentral gyrus (ρ = –0.78), the volume of the right thalamus (ρ = –0.44), and the right amygdala (ρ = 0.6).
ConclusionsThe lithium group had better cognitive flexibility and greater dimension in some frontal and subcortical cortical regions. Furthermore, there was a moderate to high correlation between performance in this executive function and the thickness of the right precentral gyrus, and the volumes of the thalamus and the right amygdala. These findings could suggest a neuroprotective effect of lithium.
El tratamiento del trastorno afectivo bipolar (TAB) con litio se ha relacionado con menos deterioro cognitivo y menores cambios en la anatomía estructural cerebral comparado con otros tratamientos. Sin embargo, los estudios son heterogéneos y son pocos los que evalúan si estos efectos están relacionados. El objetivo de este estudio es evaluar y relacionar el desempeño cognitivo y la neuroanatomía estructural en pacientes tratados con y sin litio.
MétodosEstudio de corte trasversal que incluyó a 48 sujetos con TAB I: 22 tratados con litio y 26 sin litio. Se evaluó el desempeño en las pruebas neuropsicológicas Wechsler III (WAIS III), TMT A y B (Trial Making Test), prueba de aprendizaje verbal de California (TAVEC), prueba de figura compleja de Rey y prueba de clasificación de tarjetas de Wisconsin. Se evaluaron estructuras cerebrales obtenidas por resonancia magnétiva (RM) cerebral. Se calculó la diferencia de medias estandarizada (DME) entre ambos grupos, con ajuste por variables de confusión mediante puntuación de propensión, y se empleó el coeficiente de correlación de Spearman (ρ) para evaluar la relación existente entre el desempeño cognitivo y las regiones neuroanatómicas.
ResultadosRespecto al grupo sin litio, el grupo con litio tuvo menos errores perseverativos en el Wisconsin (DME = –0,69) y mayores áreas corticales derecha e izquierda (DME = 0,85 y DME = 0,92); mayor superficie en el cíngulo anterior izquierdo (DME = 1,32), la corteza orbitofrontal medial derecha (DME = 1,17), el giro frontal superior derecho (DME = 0,82), los giros precentrales derecho e izquierdo (DME = 1,33 y DME = 0,98); mayor volumen de la amígdala derecha (DME = 0,57), el hipocampo derecho (DME = 0,66), el putamen derecho (DME = 0,87) y el tálamo derecho (DME = 0,67). En el grupo con litio, se encontró una correlación con dichos errores y el espesor del giro precentral izquierdo (ρ = –0,78), el volumen del tálamo derecho (ρ = –0,44) y la amígdala derecha (ρ = 0,6).
ConclusionesEl grupo con litio tuvo mejor flexibilidad cognitiva y mayor dimensión en algunas regiones corticales frontales y subcorticales. Además, hubo correlación moderada a alta entre el desempeño en esta función ejecutiva y el espesor del giro precentral derecho, y los volúmenes del tálamo y la amígdala derecha. Estos hallazgos podrían indicar un efecto neuroprotector del litio.
Bipolar disorder (BD) is characterised by episodes of mania, hypomania or depression, and is one of the most common mental disorders, affecting 1–2% of adults worldwide.1–3 According to reports from the World Health Organisation, due to its impact on the functionality and quality of life of the individual due to the chronic nature and intensity of the symptoms, it is the sixth leading cause of disease-related disability.4 One of the factors related to its functional impact is cognitive impairment. Compared with healthy subjects, about 31% of patients with BD, mainly type I (BD-I), may have some type of cognitive impairment.5,6 Various authors have reported that the most affected domains are declarative memory, attention and executive function. This has been demonstrated not only in episodes of mania and depression, but also in euthymia, when patients would be expected to function normally.7–9 Cognitive impairment seems independent of intelligence quotient (IQ), schooling, age or gender.10 It appears also more evident in patients with mania, as their predominant polarity, longer time since onset of the disorder, and a history of psychotic symptoms. Although the cause of cognitive impairment is not fully understood, an association has been found with drug treatment. Anticonvulsants and antipsychotics used for BD as mood stabilisers have been associated with a greater cognitive deficit.11–13 In contrast, less alteration in visual memory, attention and executive function has been found in subjects treated with lithium than in patients not taking lithium.14
It has been suggested that the pathophysiology of cognitive impairment may in part be due to the underlying neurobiological changes typical of BD.15 It is known that, compared to controls, patients with BD show alterations in the structural anatomy on brain magnetic resonance imaging (MRI), such as decreased grey matter with frontal predominance,16,17 increased ventricular volume, and reduced volume in limbic structures such as the amygdala.18,19 A decrease in the volume of white matter adjacent to the cingulate gyrus has also been found.18 Hibar et al.20 found a decrease in grey matter thickness in the frontal region (pars opercularis and left rostral middle frontal cortex) and temporal region (left fusiform gyrus); in this study, subjects treated with lithium had greater thickness, particularly in the left paracentral gyrus and the right and left superior parietal gyri, unlike those treated with anticonvulsants, who had decreased thickness in the lateral occipital gyrus and the paracentral gyrus. A similar phenomenon has been found in subcortical structures: compared to controls, subjects with BD had smaller hippocampus, thalamus and amygdala volumes and bilateral enlargement of the lateral ventricles, with greater impairment in BD-I. Those treated with anticonvulsants had a smaller hippocampus volume than those not on anticonvulsants and those taking lithium, and larger thalamic volume compared to those not on treatment.21 The independent effect of lithium on structural neuroanatomy was observed in a study that showed enlarged hippocampus, thalamus, and amygdala volumes in bipolar patients on lithium monotherapy compared to bipolar patients without treatment.22
According to this, the poorer cognitive performance or the decrease in some cortical and subcortical structures in patients with BD could be attenuated by lithium therapy. Although the impact on cognition and structural anatomy have generally been studied independently, some studies that correlate these variables have found better executive function with greater volume of grey matter in the prefrontal and dorsal and lateral cortex.23 A correlation has also been found between less inhibitory control and smaller volume in parietal regions, such as the cuneus and right inferior parietal lobe.24 Other studies have analysed the correlation of cognitive function with cerebral tractography or functional neuroimaging, with different findings.24,25 The heterogeneity of the studies and the lack of evidence make it difficult to clarify whether the structural findings are related to a significant clinical deterioration in cognition or are findings with no significant clinical impact. Moreover, if the findings are affected by drug treatment, it could act as a predisposing or protective factor for neuroprogression.
Our aim was to study the differences in cognitive performance and volume and the correlation between the two in patients with BD-I living in the region of Antioquia in Colombia being treated with and without lithium.
MethodsCross-sectional study derived from the PRISMA project26 of the Grupo de Investigación en Psiquiatría (GIPSI) [Psychiatry Research Group] at the University of Antioquia. The study was endorsed by the Bioethics Committee of the University of Antioquia's Faculty of Medicine and the Hospital San Vicente Fundación in Medellín. It complies with the principles that protect the rights of the participants according to resolution No. 008430 of 1993 of the Ministry of Health of the Republic of Colombia, and with the Declaration of Helsinki of 2013.
Only subjects who had signed the informed consent form for PRISMA were included in the study. The anonymity of the participants was guaranteed during the statistical analysis with a code that allowed identification of different subjects.
ParticipantsOf the PRISMA sample of 302 patients, 198 had a diagnosis of BD-I. We analysed the data from 48 participants who met the eligibility criteria. We included patients aged 18–60, with reports from neuropsychological tests and MRI performed around the same time, who were in euthymia at the time of assessment, defined as a Young Mania Rating Scale score <6 points and a score <7 on the Hamilton Rating Scale for Depression. We excluded those with psychosis and schizophrenia spectrum disorder, intellectual disability, minor and major cognitive impairment, severe head injury (loss of consciousness >30 min) and use of benzodiazepines at doses >2 mg lorazepam (or equivalent), and those who had undergone electroconvulsive therapy (ECT) in the 2 years prior to the assessment. Once we had our sample of patients, we divided them into 2 groups according to whether or not they had received lithium treatment for at least 12 consecutive months prior to the assessment, based on the medical records or verbal report from the patient.
Instruments and proceduresSociodemographic and clinical variablesTrained psychiatrists performed the clinical assessment using the Diagnostic Interview for Genetic Studies (DIGS) version 3.0 validated in Colombia.27 From this they were able to obtain the sociodemographic variables age, marital status, schooling, number of school years passed and occupation. Also the clinical variables age at diagnosis, time since onset of BD, number of affective episodes, predominant polarity, history of psychosis, suicide attempts, type of drug treatment for the disease (lithium, antipsychotics, benzodiazepines and antidepressants), treatment time in continuous weeks and doses (in chlorpromazine-equivalent milligrams for antipsychotics and lorazepam-equivalent for benzodiazepines). For the evaluation of affective symptoms, we used the Hamilton Rating Scale for Depression and the Young Mania Rating Scale. Functionality was assessed with the Global Assessment of Functioning (GAF) scale and the Functional Assessment Staging Tool (FAST).
Neuropsychological assessmentA trained psychologist conducted the neuropsychological assessment. According to each domain, the following tests and subtests were performed:
• Intelligence: Wechsler Adult Intelligence Scale III (WAIS III), which assesses verbal, performance and full-scale intelligence. Attention: The Trail Making Test A (TMT-A), which assesses motor skills, visuospatial skills and sustained attention. Memory: WAIS, assesses logical, delayed and recognition memory, and California Verbal Learning Test (CVLT), which assesses the learning curve and susceptibility to interference. Executive Functions: Wisconsin Card Sorting Test (WCST), which assesses abstract reasoning and flexibility, and TMT-B, which assesses flexibility. Language: semantic and phonological verbal fluency. Gnosis: Rey-Osterrieth Complex Figure Test, which evaluates recall and errors with time and copying percentage.28
• Evaluation of brain structure: MRI were performed with a Philips Achieva 3 Tesla Ingenia resonator, with which axial volumetric images were obtained in T1 with an isotropic voxel size of 1 mm3. For image processing, the Freesurfer software29,30 was used with the following workflow: first, an affine registration with the anatomical space of Talairach was made, followed by initial volumetric labelling or segmentation, then correction of the image intensity variations due to magnetic field heterogeneities (bias field correction); subsequently, a skull extraction algorithm was applied, a nonlinear alignment was performed for the Talairach space, and final volumetric labelling was assigned for each structure, which included white matter (WM) and grey matter (GM) segmentation. We analysed cortical and subcortical structures bilaterally. We used the Desikan and Killiany atlas of brain regions of interest.31 Data from 68 cortical structures (34 from each hemisphere) and 14 subcortical structures (7 from each hemisphere) were obtained. We created a database with the measurements of thickness (mm) and area (mm2) of the cortical structures and the volume (mm3) of the subcortical structures obtained from Freesurfer. To normalise the volume of brain structures and enable comparison, we used the equation v′ = v/ICV, where v’ is the normalised volume, v is the non-normalised volume, and ICV, the total intracranial volume.32 The Freesurfer surface and cortical thickness results were plotted in MATLAB.33
Statistical analysisA database was constructed in Excel with the data obtained. The data were processed with R version 3.6.3 and R studio version 1.2.503334,35 and Package “esc” version 0.5.1.36 The categorical variables are described as frequency and percentages. For the quantitative variables, we used measures of central tendency (mean ± standard deviation in the volumetric tests) and median [interquartile range] in the other variables of the study.
To compare cognitive performance and volumetry results, we calculated the crude and adjusted standardized mean difference (SMD). In view of the small sample size, the adjustment was made using a propensity score,37 calculated using logistic regression as the likelihood of receiving lithium according to age, gender, educational level, history of cardiovascular disease (hypertension, dyslipidaemia, diabetes, obesity), time since onset of BD, number of manic episodes, history of psychosis, suicide attempt, taking of benzodiazepines and consumption of alcohol and other psychoactive substances. The results were presented with the respective 95% confidence interval (95%CI), as well as the p value calculated with the Student’s t test for independent samples in the case of volumetric tests, and with the Mann–Whitney U test for the case of neuropsychological variables. Given the limitations of the p value and following the suggestions of Greenland et al, we calculated the s value (Shannon information or surprise value) as s = –log2(p), where p is the value of the statistical test used.38,39 The s value should be interpreted as a continuous measure of the amount of information or bits provided by the statistical test against the contrasted hypothesis (in our case, the hypothesis of no differences between those who receive lithium and those who do not). The SMD was calculated with its respective confidence interval using the online application of the Campbell Collaboration.40 SMD values above 0.80, 0.50 or 0.20 were interpreted as large, moderate or small differences respectively.41
We examined the relationship between performance in neuropsychological tests by cognitive domain (attention, memory, processing speed, intelligence) and corrected brain volume of specific areas defined a priori by Spearman’s correlation coefficient (ρ), considering correlations as moderate with ρ >0.4 and high with ρ >0.7.42,43 This was done with the entire sample and depending on whether they were taking lithium or not.
ResultsSociodemographic and clinical characteristicsWe obtained a sample of 48 individuals with BD-I who met the eligibility criteria. We found that 45.6% were taking lithium. The patients on lithium were younger, a higher percentage were employed, fewer had hypertension, and their onset of BD was at an earlier age. The predominant polarity was manic in both groups, but it was more common in the group without lithium, as was a history of psychosis.
In the lithium group, 36% were on monotherapy and the rest were on other treatments, including anticonvulsants (27%), antipsychotics (36%), antidepressants (9%) and benzodiazepines (9%). In the group without lithium, anticonvulsants were the most common treatment (92%).
The dose of valproate was similar in both groups, but those on lithium had been taking anticonvulsants for longer. The dose of antipsychotic was higher and the treatment duration longer in the group without lithium. The use of benzodiazepines was not common in either group, but the rate was higher in the group without lithium (Table 1).
Sociodemographic and clinical characteristics of patients with type I bipolar affective disorder.
| Characteristic | With lithium (n = 22; 45.6%) | Without lithium (n = 26; 54.2%) | Total (n = 48; 100%) |
|---|---|---|---|
| Age (years) | 36 [28–46] | 49 [38–53] | 43 [30–52] |
| Education (years) | 13.0 [11–15] | 12 [10–15] | 13.0 [11–15] |
| Level of schooling | |||
| None | 0 | 1 (3.8) | 1 (2.1) |
| Primary | 5 (22.7) | 6 (23.1) | 11 (22.9) |
| Secondary | 7 (31.8) | 13 (50) | 20 (41.7) |
| Undergraduate | 9 (40.9) | 5 (19.2) | 14 (29.2) |
| Postgraduate | 1 (4.5) | 1 (3.8) | 2 (4.2) |
| Female | 15 (68.2) | 16 (61.5) | 31 (64.6) |
| Marital status | |||
| Single | 15 (68.2) | 17 (65.4) | 32 (66.7) |
| Married | 6 (27.3) | 4 (15.4) | 10 (20.8) |
| Separated/divorced | 1 (4.5) | 4 (15.4) | 5 (10.4) |
| Widowed | 0 | 1 (3.8) | 1 (2.1) |
| Occupation | |||
| Unemployed | 2 (9.1) | 5 (19.2) | 7 (14.6) |
| Managers/professionals | 3 (13.6) | 3 (11.5) | 5 (10.4) |
| Technical, sales, administrative | 4 (18.2) | 3 (11.5) | 7 (14.6) |
| Service/housekeeper | 8 (34.3) | 8 (30.7) | 16 (33.3) |
| Different trades | 3 (13.3) | 4 (25.3) | 7 (14.5) |
| Student | 2 (9.1) | 3 (11.5) | 5 (10.4) |
| Medical comorbidity | |||
| Thyroid disease | 13 (59.1) | 15 (57.7) | 28 (58.3) |
| Epilepsy | 0 | 1 (3.8) | 1 (2.1) |
| Traumatic brain injury | 1 (4.5) | 0 | 1 (2.1) |
| Hypertension | 1 (4.5) | 6 (23.1) | 7 (14.6) |
| Diabetes | 0 | 2 (7.7) | 2 (4.2) |
| Obesity | 1 (4.5) | 2 (7.7) | 3 (6.2) |
| Dyslipidaemia | 0 | 2 (7.7) | 2 (4.2) |
| Other | 14 (63.6) | 13 (50.0) | 27 (56.2) |
| Psychiatric comorbidity | |||
| Anxiety | 1 (4.5) | 1 (3.8) | 2 (4.2) |
| Eating disorder | 1 (4.5) | 0 | 1 (2.1) |
| Alcohol use | 6 (27.3) | 5 (19.2) | 11 (22.9) |
| Substance use (other than alcohol) | 6 (27.3) | 7 (26.9) | 13 (27.1) |
| GAF | 81 [73–89] | 81 [80–88] | 81 [77–90] |
| FAST | 24 [13–34] | 29 [19–38] | 28 [15–36] |
| Age at start of treatment (years) | 21 [15–25] | 21 [17–27] | 21 [17–25] |
| Time since onset of BD (years) | 14 [9–23] | 22 [8–19] | 15 [8–27] |
| Manic episodes | 2 [1–5] | 4 [2–5] | 3 [1–5] |
| Depressive episodes | 2 [0–3] | 1 [0–2] | 1 [1–2] |
| Predominant polarity | |||
| Depressive | 6 (27.3) | 2 (7.7) | 8 (16.7) |
| Manic | 10 (45.5) | 20 (76.9) | 30 (62.5) |
| None | 6 (27.3) | 4 (15.4) | 10 (20.8) |
| History of psychosis | 9 (40.9) | 16 (61.5) | 25 (52.1) |
| Previous hospital admissions | 4.45 (4.72) | 3.35 (3.60) | 3.85 (4.14) |
| Previous suicide attempt | 8 (36.4) | 8 (30.8) | 16 (33.3) |
| Other treatments | |||
| Anticonvulsants | 6 (27.3) | 24 (92.3) | 30 (62.5) |
| Valproate | 6 (27.3) | 21 (80.8) | 27 (56.2) |
| Carbamazepine | 0 | 3 (11.5) | 3 (6.25) |
| Antipsychotics | 8 (36.4) | 17 (65.4) | 25 (52.1) |
| Antidepressants | 2 (9.1) | 2 (7.7) | 4 (8.3) |
| Benzodiazepine | 2 (9.1) | 1 (3.8) | 3 (6.2) |
| Treatment dosage (mg) | |||
| Lithium | 1,130 [900–1,200] | N/A | 1,130 [900–1,200] |
| Valproate | 1,000 [750–1,500] | 1,000 [1,000–1,000] | 1,000 [1,000–1,380] |
| Carbamazepine | N/A | 300 [250–750] | 300 [250–750] |
| Antipsychotic | 150 [75.6–344] | 275 [125–400] | 275 [100–400] |
| Benzodiazepine | 0.800 [0.6–0.9] | 1.50 [1.2–1.7] | 1.00 [0.8–1] |
| Treatment duration (weeks) | |||
| Lithium | 312 [165–715] | N/A | 312 [165–715] |
| Valproate | 624 [403–767] | 260 [156–312] | 312 [156–520] |
| Carbamazepine | N/A | 24.0 [18–116] | 24.0 [18–116] |
| Antipsychotic | 36.0 [15–108] | 156 [40–208] | 144 [24–208] |
FAST: Functional Assessment Scale Tool; GAF: Global Assessment of Functioning scale.
Values are expressed in terms of n (%) or median [interquartile range].
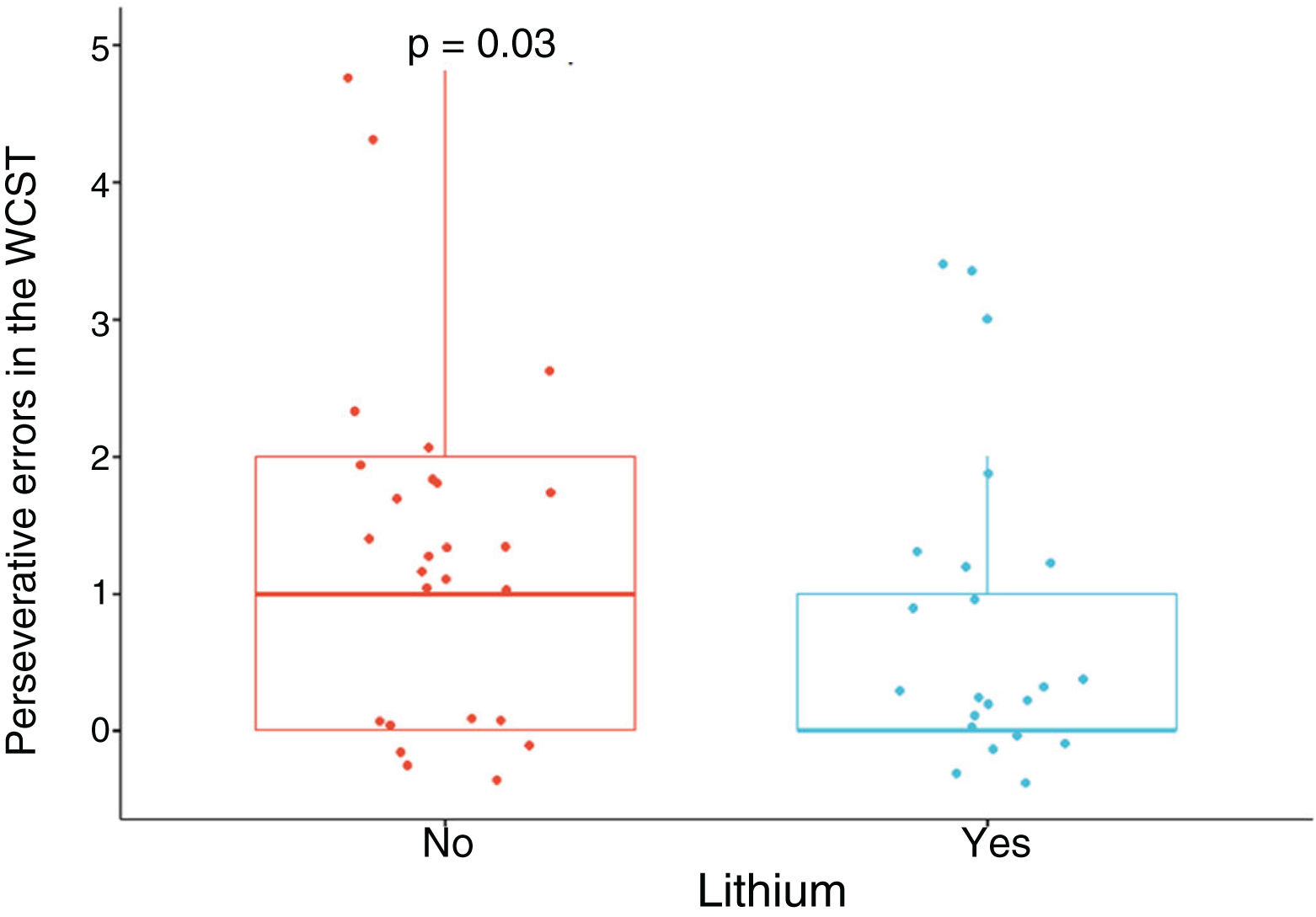
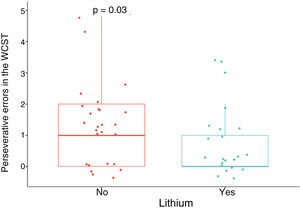
Patients treated with lithium had better performance in executive function, specifically in flexibility, in view of the lower number of perseverative errors in the WCST, with a moderate SMD, when adjusting for age, gender, education, cardiovascular disease, number of manic episodes, history of psychosis, suicide attempt, and use of benzodiazepines, alcohol and other psychoactive substances (SMD = –0.69; 95% CI, –1.28 to –0.11; p = 0.03) (Fig. 1). No significant differences were found in the other neuropsychological variables (Appendix B Table 1 of the additional material).
Brain structureSurface area and thickness of cortical regionsOn average, a greater bilateral cortical area was found in the group taking lithium (Table 2), with no significant differences in total cortical thickness between the two groups (Table 3).
Surface area of the cortical regions of interest of patients with type I bipolar affective disorder according to each cerebral lobe.
| Region | With lithium (n = 22; 45.6%) | Without lithium (n = 26; 54.2%) | SMD | aSMD | 95% CI | s | p |
|---|---|---|---|---|---|---|---|
| Frontal | |||||||
| Left anterior cingulate | 875 ± 142 | 730 ± 114 | 1.12 | 1.32 | 0.7–1.95 | 13.29 | <0.01 |
| Left caudal middle frontal gyrus | 2,233 ± 446 | 1,941 ± 276 | 0.79 | 0.81 | 0.22–1.41 | 6.97 | 0.01 |
| Right superior frontal gyrus | 6,773 ± 930 | 6,132 ± 589 | 0.82 | 0.85 | 0.25–1.44 | 7.38 | 0.01 |
| Left superior frontal gyrus | 6,841 ± 735 | 6,351 ± 693 | 0.69 | 0.88 | 0.28–1.47 | 5.51 | 0.02 |
| Right lateral orbitofrontal cortex | 2,499 ± 397 | 2,303 ± 286 | 0.57 | 0.48 | –0.1 to 1.05 | 4.24 | 0.05 |
| Left lateral orbitofrontal cortex | 2,530 ± 335 | 2,331 ± 303 | 0.63 | 0.5 | –0.08 to 1.08 | 4.8 | 0.04 |
| Right medial orbitofrontal cortex | 1,854 ± 180 | 1,649 ± 172 | 1.17 | 1.08 | 0.47–1.69 | 9.97 | <0.01 |
| Pars opercularis, right | 1,376 ± 252 | 1,231 ± 184 | 0.66 | 0.67 | 0.08–1.25 | 5.27 | 0.03 |
| Pars orbitalis, left | 642 ± 105 | 570 ± 73 | 0.8 | 0.78 | 0.19–1.37 | 6.97 | 0.01 |
| Pars triangularis, right | 1,498 ± 275 | 1,333 ± 214 | 0.67 | 0.77 | 0.18–1.36 | 5.38 | 0.02 |
| Right precentral gyrus | 4,821 ± 526 | 4,424 ± 452 | 0.81 | 1.33 | 0.71-1.97 | 7.16 | 0.01 |
| Left precentral gyrus | 4,828 ± 634 | 4,406 ± 456 | 0.77 | 0.98 | 0.38–1.58 | 6.64 | 0.01 |
| Parietal | |||||||
| Right inferior parietal cortex | 5,332 ± 580 | 4,737 ± 596 | 1.01 | 1.33 | 0.7–1.95 | 9.97 | <0.01 |
| Left inferior parietal cortex | 4,526 ± 513 | 4,056 ± 571 | 0.87 | 0.97 | 0.37–1.57 | 7.64 | 0.01 |
| Temporal | |||||||
| Left fusiform gyrus | 3,174 ± 388 | 2,881 ± 295 | 0.85 | 0.89 | 0,3–1.49 | 7.64 | 0.01 |
| Left insula | 2,144 ± 224 | 1,983 ± 159 | 0.83 | 1.26 | 0.64–1.88 | 7.64 | 0.01 |
| Right parahippocampal gyrus | 681 ± 102 | 629 ± 61 | 0.62 | 0.72 | 0.13–1.31 | 4.88 | 0.03 |
| Right inferior temporal gyrus | 2,958 ± 349 | 2,729 ± 366 | 0.64 | 0.87 | 0.27–1.46 | 4.92 | 0.03 |
| Left inferior temporal gyrus | 3,137 ± 423 | 2,824 ± 402 | 0.76 | 0.82 | 0.23–1.41 | 6.38 | 0.01 |
| Right middle temporal gyrus | 3,246 ± 431 | 3,008 ± 379 | 0.59 | 0.63 | 0.05–1.2 | 4.38 | 0.05 |
| Left middle temporal gyrus | 2,930 ± 453 | 2,556 ± 327 | 0.95 | 1 | 0.4–1.6 | 8.97 | <0.01 |
| Right superior temporal gyrus | 3,509 ± 411 | 3,283 ± 276 | 0.65 | 0.79 | 0.21–1.38 | 5.16 | 0.03 |
| Left superior temporal gyrus | 3,704 ± 401 | 3,434 ± 432 | 0.65 | 0.65 | 0.07–1.24 | 5.01 | 0.03 |
| Occipital | |||||||
| Right cuneus | 1,497 ± 215 | 1,377 ± 173 | 0.62 | 0.66 | 0.08–1.24 | 4.76 | 0.04 |
| Left cuneus | 1,424 ± 169 | 1,300 ± 187 | 0.7 | 0.66 | 0.08–1.24 | 5.57 | 0.02 |
| Right lateral occipital cortex | 4,510 ± 588 | 4,163 ± 518 | 0.63 | 0.69 | 0.1–1.27 | 4.84 | 0.04 |
| Left lateral occipital cortex | 4,655 ± 557 | 4,211 ± 506 | 0.83 | 0.87 | 0.28–1.47 | 7.38 | 0.01 |
| Left lingual gyrus | 3,103 ± 421 | 2,818 ± 418 | 0.68 | 0.57 | –0.01 to 1.15 | 5.38 | 0.02 |
| Right cortical area | 82,363 ± 7,754 | 76,957 ± 6,894 | 0.74 | 0.85 | 0.25–1.44 | 6.16 | 0.01 |
| Left cortical area | 82,432 ± 7,889 | 76,089 ± 6,907 | 0.86 | 0.92 | 0.33–1.52 | 7.64 | 0.01 |
SMD: standardised mean difference; aSMD: SMD adjusted for age, gender, schooling, cardiovascular disease, number of manic episodes, history of psychosis, suicide attempt, and use of benzodiazepines, alcohol, and other psychoactive substances.
Values are expressed as or mean ± standard deviation in millimetres.
Thickness of the cortical regions of interest of patients with type I bipolar affective disorder according to each cerebral lobe.
| Region | With lithium (n = 22; 45.6%) | Without lithium (n = 26; 54.2%) | SMD | aSMD | 95% CI | s | p |
|---|---|---|---|---|---|---|---|
| Frontal | |||||||
| Right anterior cingulate | 2.98 ± 0.22 | 2.84 ± 0.25 | 0.60 | 0.42 | 1.15–0.99 | 4.41 | 0.04 |
| Left paracentral lobule | 2.37 ± 0.13 | 2.26 ± 0.14 | 0.75 | 0.28 | –0.28 to 0.85 | 6.15 | 0.01 |
| Pars triangularis, left | 2.45 ± 0.17 | 2.34 ± 0.16 | 0.68 | 0.30 | –0.26 to 0.87 | 5.38 | 0.02 |
| Left precentral gyrus | 2.57 ± 0.13 | 2.44 ± 0.13 | 0.97 | 0.43 | –0.14 to 1.01 | 8.96 | <0.01 |
| Parietal | |||||||
| Right isthmus | 2.56 ± 0.22 | 2.37 ± 0.20 | 0.87 | 0.31 | –0.25 to 0.88 | 7.96 | <0.01 |
| Right superior parietal cortex | 2.18 ± 0.11 | 2.12 ± 0.11 | 0.58 | 0.25 | –0.31 to 0.82 | 4.26 | 0.05 |
| Right postcentral gyrus | 2.10 ± 0.11 | 2.03 ± 0.11 | 0.68 | 0.37 | –0.20 to 0.94 | 5.38 | 0.02 |
| Left postcentral gyrus | 2.12 ± 0.11 | 2.04 ± 0.11 | 0.75 | 0.43 | –0.13 to 1.01 | 6.38 | 0.01 |
| Temporal | |||||||
| Left superior temporal gyrus | 2.74 ± 0.15 | 2.63 ± 0.14 | 0.72 | 0.33 | –0.23 to 0.90 | 5.87 | 0.01 |
| Right transverse temporal cortex | 2.30 ± 0.22 | 2.16 ± 0.22 | 0.64 | 0.36 | –0.21 to 0.93 | 4.96 | 0.03 |
| Left transverse temporal cortex | 2.29 ± 0.15 | 2.13 ± 0.18 | 0.96 | 1.07 | 0.46–1.68 | 8.96 | <0.01 |
| Occipital | |||||||
| Right cuneus | 1.50 ± 0.22 | 1.38 ± 0.17 | 0.98 | 0.63 | 0.05–1.21 | 8.96 | <0.01 |
| Left cuneus | 1.42 ± 0.17 | 1.30 ± 0.19 | 0.70 | 0.48 | –0.10 to 1.05 | 5.64 | 0.02 |
| Right lateral occipital cortex | 4.51 ± 0.59 | 4.16 ± 0.52 | 1.01 | 0.41 | –0.15 to 0.99 | 9.96 | <0.01 |
| Left lateral occipital cortex | 4.65 ± 0.56 | 4.21 ± 0.51 | 0.70 | 0.07 | –0.49 to 0.63 | 5.64 | 0.02 |
| Left pericalcarine cortex | 1.37 ± 0.22 | 1.26 ± 0.25 | 0.71 | 0.56 | –0.02 to 1.13 | 5.79 | 0.01 |
| Right precuneus | 2.33 ± 0.13 | 2.26 ± 0.11 | 0.59 | 0.24 | –0.32 to 0.81 | 4.47 | 0.04 |
| Right cortical thickness | 2.48 ± 0.10 | 2.40 ± 0.11 | 0.68 | 0.09 | –0.47 to 0.65 | 5.38 | 0.02 |
| Left cortical thickness | 2.49 ± 0.11 | 2.41 ± 0.10 | 0.70 | 0.09 | –0.47 to 0.66 | 5.64 | 0.02 |
SMD: standardised mean difference; aSMD: SMD adjusted for age, gender, schooling, cardiovascular disease, number of manic episodes, history of psychosis, suicide attempt, and use of benzodiazepines, alcohol, and other psychoactive substances.
Values are expressed as or mean ± standard deviation in millimetres.
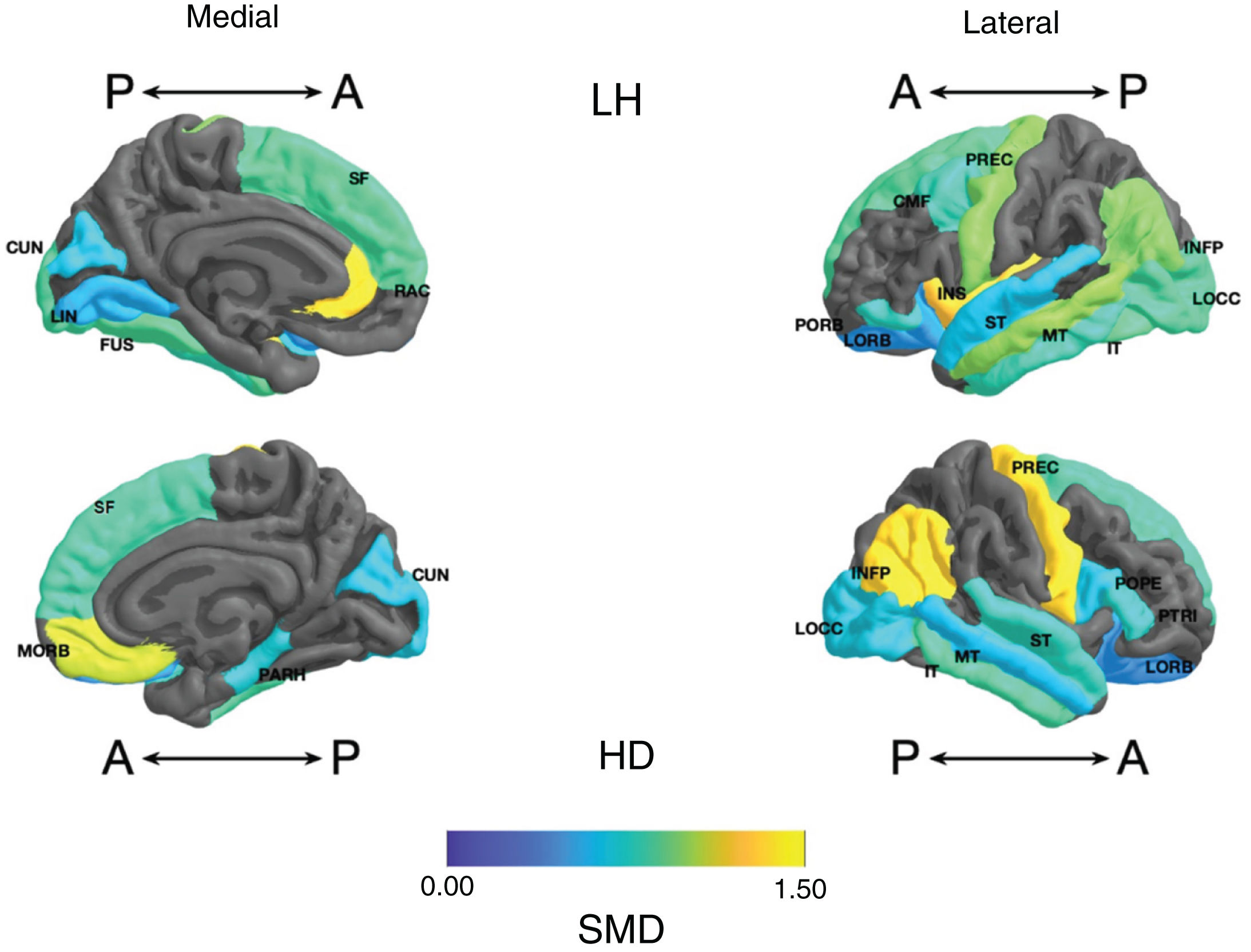
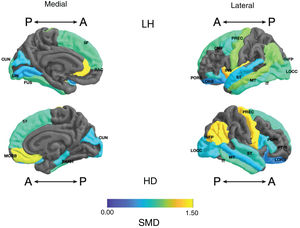
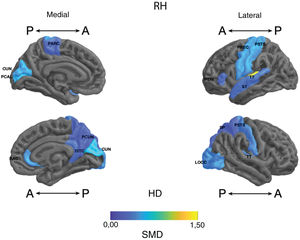
Frontal. Greater surface area was found in the lithium group in the left anterior cingulate, left caudal middle frontal gyrus, right and left superior frontal gyri, right medial orbitofrontal cortex, and precentral gyrus bilaterally (large SMD). There was also greater surface area in the left lateral orbitofrontal cortex, the left pars orbitalis the right pars opercularis and triangularis (moderate SMD) (Table 2 and Fig. 2). Greater thickness was found in the right anterior cingulum and the left precentral gyrus (small SMD) (Fig. 3 and Appendix B Table 2 of the additional material).
Standardised mean difference (SMD) of the surface area of the cortical regions of interest in patients with type I bipolar affective disorder treated with lithium and without lithium, according to right (RH) and left (LH) hemispheres with anterior (A) and posterior (P) views. CMF: caudal middle frontal; CUN: cuneus; FUS: fusiform; INFP: inferior parietal; INS: insula; IT: inferior temporal; LIN: lingual; LOCC: lateral occipital; LORB: lateral orbitofrontal; MORB: medial orbitofrontal; MT: middle temporal; PARH: parahippocampal; POPE: pars opercularis; PORB: pars orbitalis; PREC: precentral; PTRI: pars triangularis; RAC: rostral anterior cingulate; SF: superior frontal; ST: superior temporal.
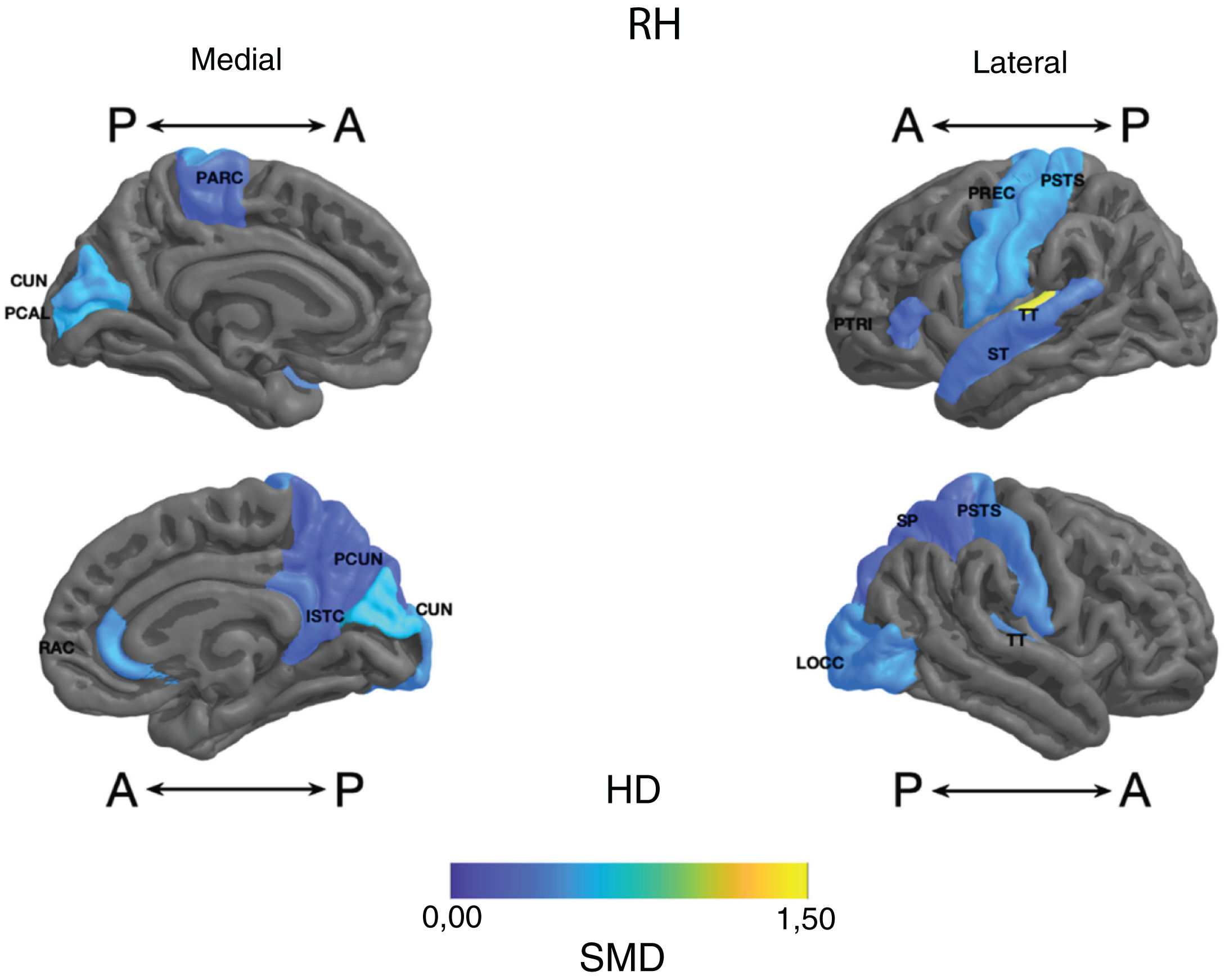
Standardised mean difference (SMD) of the thickness of cortical regions of interest in patients with type I bipolar affective disorder treated with lithium and without lithium, according to right (RH) and left (LH) hemispheres with anterior (A) and posterior (P) views. RAC: rostral anterior cingulate; PARC: paracentral lobule; PTRI: pars triangularis; PREC: precentral; ISTC: isthmus cingulate; SP: superior parietal; PSTS: postcentral; ST: superior temporal; TT: transverse temporal; CUN: cuneus; LOCC: lateral occipital; PCAL: pericalcarine; PCUN: precuneus.
Parietal. The surface area of the right and left inferior parietal cortices was greater in the lithium group (large SMD) (Table 2 and Fig. 2). Postcentral gyrus thickness was greater bilaterally in those in the lithium group (small SMD) (Fig. 3 and Appendix B Table 2 of the additional material).
Temporal. In the lithium group, a greater surface area was found in the left fusiform gyrus, the left insula, the inferior temporal gyrus bilaterally and the left middle temporal gyrus (large SMD), and in the right parahippocampal gyrus and superior temporal gyrus bilaterally (moderate SMD) (Table 2 and Fig. 2). Greater cortical thickness was found in the left transverse temporal cortex (large SMD) (Fig. 3 and Appendix B Table 2 of the additional material).
Occipital. The lithium group had a greater surface area in the left lateral occipital cortex (large SMD) and the bilateral cuneus, the left lingual gyrus, and the right lateral occipital cortex (moderate SMD) (Table 2 and Fig. 2). In the lithium group, cortical thickness was greater in the bilateral cuneus and right pericalcarine cortex (moderate SMD) (Fig. 3 and Appendix B Table 2 of the additional material).
Volume of subcortical regionsIn the lithium group, greater volume was found in the right amygdala, right hippocampus, right putamen and right thalamus (moderate SMD) and in the right and left caudates, left hippocampus and left thalamus (small SMD). No differences were identified in the volume of the right and left lateral ventricles or in total brain volume (Table 4).
Normalised volume* of subcortical regions of interest of patients with type I bipolar affective disorder.
| Region | With lithium (n = 22; 45.6%) | Without lithium (n = 26; 54.2%) | SMD | aSMD | 95% CI | s | p |
|---|---|---|---|---|---|---|---|
| Right nucleus accumbens | 0.000439 ± 0.000068 | 0.000428 ± 0.00005 | 0.18 | –0.11 | –0.68 to 0.46 | 0.89 | 0.54 |
| Left nucleus accumbens | 0.000477 ± 0.000042 | 0.000456 ± 0.00007 | 0.37 | 0.05 | –0.52 to 0.61 | 2.2 | 0.22 |
| Right amygdala | 0.001362 ± 0.000154 | 0.001268 ± 0.000173 | 0.57 | 0.6 | 0.02–1.17 | 4.18 | 0.05 |
| Left amygdala | 0.001202 ± 0.000149 | 0.00115 ± 0.000149 | 0.35 | 0.18 | –0.39 to 0.75 | 2.1 | 0.23 |
| Right caudate nucleus | 0.002463 ± 0.000319 | 0.002406 ± 0.000223 | 0.21 | 0.29 | –0.28 to 0.86 | 1.08 | 0.47 |
| Left caudate nucleus | 0.002487 ± 0.000288 | 0.00235 ± 0.000225 | 0.53 | 0.49 | –0.09 to 1.06 | 3.8 | 0.07 |
| Right hippocampus | 0.003327 ± 0.000175 | 0.003189 ± 0.000206 | 0.72 | 0.66 | 0.09–1.25 | 5.88 | 0.02 |
| Left hippocampus | 0.003285 ± 0.000222 | 0.003053 ± 0.000381 | 0.75 | 0.37 | –0.2 to 0.93 | 6.06 | 0.02 |
| Right globus pallidus | 0.000985 ± 0.000118 | 0.000967 ± 0.000143 | 0.14 | 0.04 | –0.53 to 0.6 | 0.66 | 0.63 |
| Left globus pallidus | 0.00079 ± 0.000142 | 0.000829 ± 0.0002 | 0.23 | –0.17 | –0.74 to 0.4 | 1.16 | 0.45 |
| Right putamen | 0.003875 ± 0.000365 | 0.003613 ± 0.000467 | 0.62 | 0.87 | 0.28–1.46 | 4.72 | 0.04 |
| Left putamen | 0.003952 ± 0.000532 | 0.00375 ± 0.000545 | 0.38 | 0.2 | –0.37 to 0.77 | 2.3 | 0.2 |
| Right thalamus | 0.005067 ± 0.000505 | 0.004694 ± 0.000367 | 0.84 | 0.67 | 0.08–1.25 | 7.64 | 0.01 |
| Left thalamus | 0.00608 ± 0.000759 | 0.005611 ± 0.000631 | 0.67 | 0.47 | –0.11 to 1.03 | 5.38 | 0.02 |
| Right lateral ventricle | 0.005746 ± 0.003123 | 0.007071 ± 0.003119 | 0.43 | 0.13 | –0.44 to 0.7 | 2.74 | 0.15 |
| Left lateral ventricle | 0.00598 ± 0.003333 | 0.008028 ± 0.003369 | 0.61 | 0.06 | –0.63 to 0.5 | 4.64 | 0.04 |
| Total brain volume | 1,384,034.09 ± 163,362.63 | 1,337,168.08 ± 154,505.21 | 0.3 | 0.36 | –0.21 to 0.94 | 1.68 | 0.31 |
SMD: standardised mean difference; aSMD: SMD adjusted for age, gender, schooling, cardiovascular disease, number of manic episodes, history of psychosis, suicide attempt, and use of benzodiazepines, alcohol, and other psychoactive substances.
Values are expressed as or mean ± standard deviation in millilitres.
In those taking lithium, an inverse correlation was found between the perseverative errors of the WCST and the thickness of the left precentral gyrus (ρ = –0.78) which was not detected in the group without lithium (ρ = 0.12). No correlation was found between the perseverative errors of the WCST and other cortical regions.
Subcortical regionsIn those taking lithium, a moderate inverse correlation was found between the perseverative errors of the WCST and the volume of the right thalamus (ρ = –0.44) which was not detected in the group without lithium (ρ = 0.03). There was also a positive correlation between the perseverative errors of the WCST and the volume of the right amygdala (ρ = 0.6), which was not detected in the group not taking lithium (ρ = –0.1). No correlation was found between the perseverative errors of the WCST and other subcortical regions.
DiscussionIn this study, we compared the cognitive performance and structure of different cortical and subcortical regions of interest in patients with BD, depending on whether they were taking lithium as drug treatment, and correlated these findings. We found better performance in executive function, indicated by fewer perseverations, in those taking lithium, and this correlated with greater thickness of the left precentral gyrus and larger volume of the right thalamus. In addition, a positive correlation was found with the volume of the right amygdala and the number of perseverative errors in those treated with lithium. The results were robust when adjusting for clinical variables such as the use of psychoactive drugs, cardiovascular history, time since onset of the disease, number of episodes, and suicide attempt, which have previously been shown to influence cognition and neuroanatomy.23,44–49
Executive function has been described as the ability to respond and adapt to new situations. One of its components is flexibility to change, i.e. making new decisions when environmental factors have changed. A larger number of perseverations suggests more difficulty in changing or alternating tasks, in other words less flexibility.28 Previous studies have shown poorer performance in executive function, not only in flexibility, but also in planning and the inhibitory response in bipolar patients compared to healthy controls,50 and in memory and attention. Moreover, when compared to lithium, patients treated with anticonvulsants have worse cognitive performance.5,6,11,51–53 In our study, when comparing the two treatment groups, we found differences in flexibility measured by the perseverative errors of the WCST, with fewer errors in the lithium group. There were no significant differences in other WCST variables. The TMT-B time was shorter in the lithium group, which indicates better executive function in this group, but with p > 0.05. Other tests which indirectly assess executive function, such as the Corsi block-tapping task and verbal fluency, were similar in both groups. No differences were found for the other cognitive domains.
The frontal lobes, the striatum and the thalamus, have been considered the neurobiological substrate of executive functions. The orbitofrontal cortex and the anterior cingulate cortex are the most closely associated with flexibility.54 In this study, when comparing treatment with and without lithium, differences were found precisely in the dimensions of frontal regions involved in executive processes. Interestingly, the correlation was stronger with the left precentral gyrus, which contains the primary motor area responsible for motor function, than with the prefrontal structures or the cingulum. A correlation was also found with the thalamus, which is the anatomical region for the integration of cortical information.55
One finding which was striking in our study was the positive correlation between amygdala volume and perseverative errors in those who used lithium. The amygdala is the substrate for the processing of primary emotions.56 Animal studies have shown that mesial lesions which include the amygdala generate a decrease in conditioned fear, and in humans, such lesions reduce autonomic reactivity to stimuli.57,58 With this in mind, it has been suggested that the role of the amygdala, in addition to that of the orbitofrontal cortex, is involved in decision-making, as it is a process dependent on emotions.59 In view of our results, one hypothesis is the possible relationship between the volume of the amygdala and greater emotional interference in executive function in those taking lithium, but this effect would be dampened by the greater volume of frontal structures already described.
Our study also found a decrease in structural dimensions in the parietal, temporal and occipital regions. The parietal lobe is involved in cognitive processes such as attention, memory, language and praxis; in this study, there were no significant differences between the groups in the first three and praxis was not included in the protocol; another inferior parietal function is sensorimotor integration and mirror neurons, involved in affective processes, but we did not assess these cognitive areas in this study. It is not the first time parietal involvement has been discussed in the literature; a previous study showed greater deterioration in the inferior parietal region of bipolar patients compared to healthy controls, but this effect was not seen when adjusting for treatment, and neuropsychological variables were not taken into account.20 Other studies have also found temporal and occipital involvement, but without taking into account a neuropsychological correlation, which could be plausible as temporal impairment has been associated with memory and occipital impairment, with processes of perception and visual retention.60 Despite the structural differences in these groups, no relationship was found with changes in the cognitive profile.
The correlation between neuropsychological and volumetric variables is a valuable contribution, as most of the studies we found evaluate cognitive function and structural neuroanatomy in BD separately. From the cognitive point of view, alterations in executive function, verbal memory and attention have been reported5,11,51–53; some studies have shown poorer cognitive performance with taking anticonvulsants such as valproic acid and antipsychotics.11 Hibar et al.20 evaluated brain structure, and found changes in predominantly frontal and temporal cortical regions, particularly decreased thickness, with greater structural involvement in BD patients taking atypical anticonvulsants and antipsychotics than in those taking lithium, in whom a larger precentral gyrus was found, similar to that described in our study; in that study it was not correlated with cognitive performance. Moore et al.61 reported that the effects of lithium on the structural neuroanatomy of bipolar patients could be seen after four weeks of treatment, and they described an increase in the volume of the prefrontal grey matter. Another study conducted here in the Antioquia region compared patients with BD-I treated with lithium and those without treatment, and found an increase in the volume of the hippocampus, thalamus and amygdala when adjusting for blood lithium levels which was positively correlated with the increase in the amygdala,22 although no neuropsychological tests were included. There seem to be few studies in the literature looking at correlation between cognitive profile and structural neuroanatomy. Abé et al.62 analysed the relationship between executive function and brain structure, and found a positive relationship between cortical thickness and executive function, but without differences when adjusting for drug treatment, this being quite different from the findings in our study. Better executive function has also been found, with greater volume of grey matter in the prefrontal, dorsal and lateral cortices,23 as well as a correlation with less inhibitory control and less volume in the parietal regions, such as the cuneus and the right inferior parietal lobe.24
We believe that a reasonable way to interpret our findings is that lithium as drug treatment for patients with BD-I could have a neuroprotective effect, the theoretical basis of which is due to different mechanisms. It has been suggested that it inhibits glycogen synthase kinase 3β (GSK-3β), a factor involved in the control of gene expression, cell behaviour, cell adhesion, neuronal polarity and neuronal plasticity. That inhibition is associated with less clinical deterioration in patients with BD. In addition, the decrease in the action of GSK-3β has been associated with an increase in the volume of grey matter in the right frontal lobe, specifically in the orbitofrontal cortex. It is also though to alter cell signalling and interact with the action of Wnt/β-catenin, brain-derived neurotrophic factor (BDNF), Nrf2 and NFkB, as well as neurogenic, cytoprotective, antioxidant and anti-inflammatory effects.61 In contrast, other treatments for BD may induce cytotoxicity. Valproic acid causes greater mitochondrial damage and has pro-apoptotic effects on neurons,63,64 similar to antipsychotics.65,66 However, the evidence from human studies is not strong.
This study provides new evidence supporting a role for lithium in cognition and structural neuroanatomy. Studying the factors related to the causes of deterioration in BD and their relationship with drug treatment will provide additional evidence to support us when prescribing medications. This needs to be accompanied by studies addressing the impact that may transcend functionality. It has been reported, for example, that in patients with BD, impaired executive function is related to occupation, quality of life and suicidal risk, which are important clinical outcomes in this population.67–69
One of the strengths of our study is that it is based on homogeneous data, with the basis obtained by expert assessment, with eligibility criteria aimed at reducing confusion. However, we must recognise that this is a cross-sectional study, so it was not possible to establish differences between the precise moments of measurement. Causality cannot therefore be established and it should be interpreted as purely descriptive. Moreover, it was carried out with a small sample, partly due to the control of confusion from the screening of the patients; even so, the groups were subjected to different drug groups, in addition to lithium, and different duration times and doses. One option for future research would be comparison with healthy controls. One of the limitations of our study is that the subjects received non-psychiatric drug treatments for comorbidities which could have also affected neurobiology, and this was not taken into account. Additionally, the data on psychiatric treatment came from the patients’ reports, and the taking of lithium or anticonvulsants was not confirmed by serum measurements.
Future studies in this line of research should include a larger sample and longer follow-up in order to establish the evolution of the changes and their relationship with drug treatment. Another variable to take into account is the functional MRI which assesses connectivity, as it allows functional changes to be explored even in the absence of structural changes.
ConclusionsOur study found fewer perseverative errors and larger size in frontal cortical regions such as the left anterior cingulate, right medial orbitofrontal cortex, right and left superior frontal gyri, and right and left precentral and subcortical regions, such as the amygdala, hippocampus, right putamen and thalamus of patients with BD treated with lithium. A moderate to high correlation was established between performance in this executive function and the thickness of the left precentral gyrus, and the volume of the thalamus and the right amygdala in patients with BD treated with lithium, which was not found in those who did not take lithium.
Greater surface area and thickness were also found in some parietal and temporal structures, but without significant differences between groups in performance in cognitive tests related to these areas.
Although judging by the literature the neurobiological factors involved in cognition may be multiple, these findings are compatible with a neuroprotective role of lithium, as it seems to improve or at least not worsen cognition and the neuroanatomical dimensions of cortical and subcortical structures.
Conflicts of interestThe authors have no conflicts of interest to declare.
We thank all the people who made this research possible: the patients and their families, the professionals related to the GIPSI who helped collect the data analysed, and Johana Valencia Echeverry, neuropsychologist, for her support and her recommendations.
Please cite this article as: Díaz Ortiz AC, Vargas Upeguí C, Zapata Ospina JP, Aguirre Acevedo DC, Pineda Zapata JA, López Jaramillo CA. Correlación entre el desempeño cognitivo y la neuroanatomía estructural en pacientes con trastorno afectivo bipolar tipo I tratados con y sin litio. Rev Colomb Psiquiat. 2022;51:133–145.