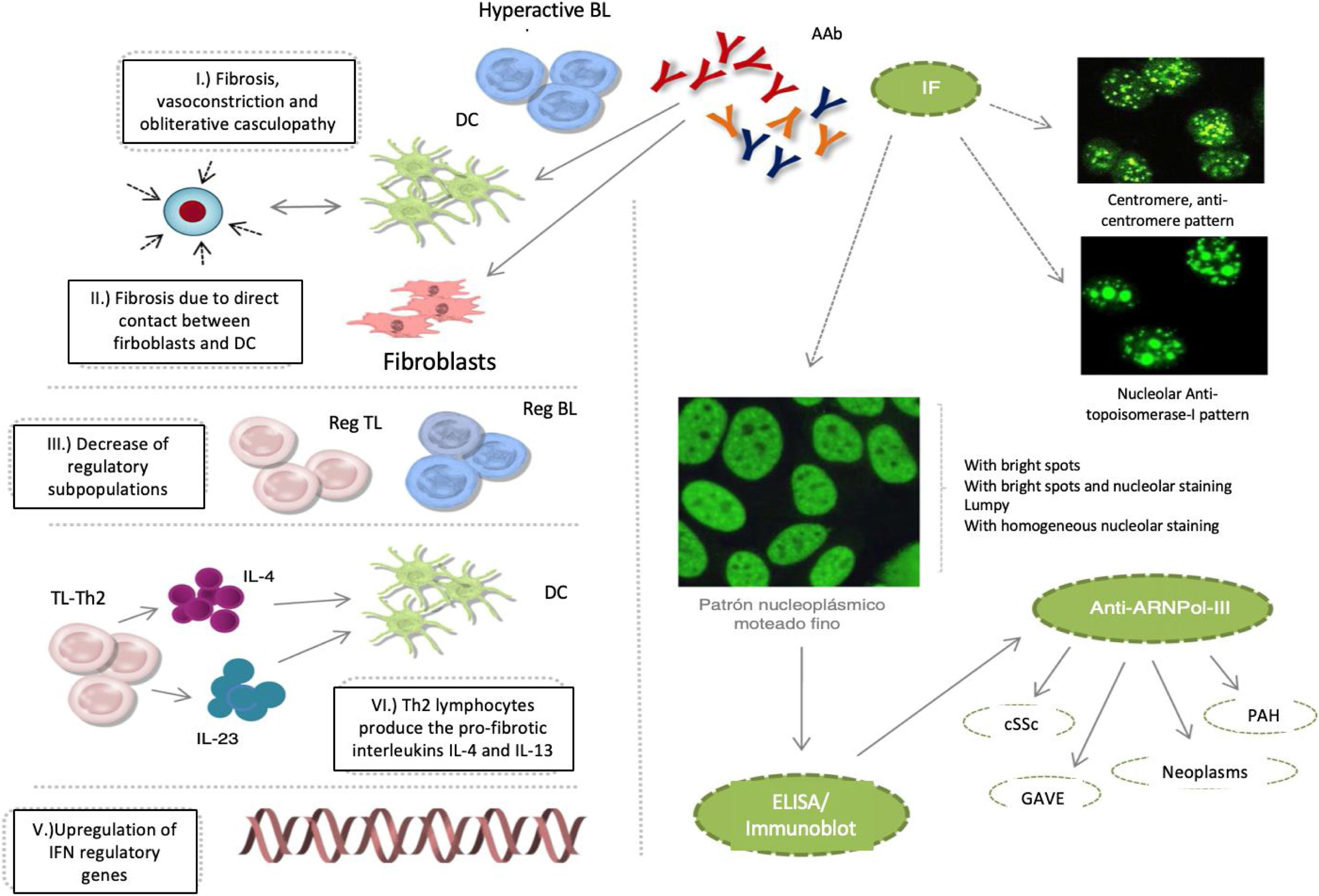
Cutaneous systemic sclerosis (cSSc) is an autoimmune chronic connective tissue disorder, characterized by microvascular lesions, collagen deposition, and skin and visceral fibrosis including: GI tract, lungs, heart, and kidneys. The detection of circulating autoantibodies (AAb) against antinuclear antibodies (ANA) is important in the diagnosis of the disease and of its clinical presentations - diffuse and localized cSSc.
Notwithstanding the fact that these AAbs participate in the pathophysiology of cSSc, the specific mechanisms that promote the deregulation of the immune system have not been described. It is currently said that: 1) fibrosis is promoted by hyperactive autoantibody producing B lymphocytes, the maturation of fibroblasts, or the inhibition of the degradation of the extracellular matrix, in addition to vasoconstriction and obliterative vasculopathy events; 2) B-lymphocytes promote fibrosis through direct cell contact of fibroblasts and dendritic cells; 3) the reduction of B and T lymphocytes with a regulatory profile, favors the development of tissue fibrosis; 4) Th2 type T lymphocytes produce profibrotic interleukins IL-4 and IL-13, which induce the maturation of dendritic cells and reinforce this profibrotic microenvironment; and 5) the upregulation interferon regulatory genes, induces tissue fibrosis (Fig.1).1–3
cSSc etiopathological events, IF patterns, and production of autoantibodies: anticentromere, antitopoisomerase-I, and anti-ARNPol-III.
AAb: autoantibodies; DC: denditric cell; SRC: scleroderma renal crisis; GAVE: gastric antral vascular ectasia; PAH: pulmonary arterial hypertension; IF: immunofluorescence; IFN: interferon; IL: interleukin; BL: B lymphocyte; TL: T lymphocyte; Reg: regulator.
The clinical (Raynaud’s phenomenon), histological (microvasculopathy), cellular (apoptosis of the endothelial cells) o autoimmune (development of AAb) events may present years before the manifestation of cutaneous fibrosis.1,2 Between 80–98 % of the patients with AAb exhibit one or several of these patterns of immunofluorescence in the ANA test: centromeric, nucleolar or nuclear fine speckled. It has been noted that these AAb are specifically targeted against centromeric proteins (anticentromere), enzymes such as topoisomerase-I (anti-Scl-70) or enzymes responsible for the transcription of DNA into RNA, such as the nucleotidyltransferase polymerase enzyme of the ribonucleic III acid (anti-ARN Pol-III). Other AAb that may develop, though less frequently, are the so called anti-ribonucleoproteins Th/To, anti-PM-Scl and anti-fibrillarin (U3-RNP).4–7
The importance of such specificity is due to the fact that the frequency of each one of these AAb varies depending on ethnicity and on the geographical background of patients; they have also been associated with different clinical profiles and do not usually coexist in the same patient, or change over the course of the disease. For all of these reasons, their identification at the time of making a diagnosis is extremely valuable to predict which organs will be affected, and the clinical evolution of our patients.
The antitopoisomerase and anticentromere antibodies are associated with diffuse cSSc and localized cSSc, respectively. Anti-Th/To, anti-PM-Scl and antifibrillarin antibodies have been identified in patients with 1) fibrosis and pulmonary hypertension or polymyositis, 2) Systemic Lupus Erythematous, and 3) overlap syndrome, respectively.6,8 Lazzaroni et al., in study promoted by European Scleroderma Trials and Research have shown that the scleroderma renal crisis, gastric antral vascular ectasia, the rapid progression of the skin thickness, pulmonary arterial hypertension and neoplasms, are all independent clinical characteristics associated with the presence of anti-ARNPol-III.9 The significance of these associations has led to the inclusion of anti-ARNPol-III among the most recent criteria for the classification of cSSc, developed by an expert consensus of the American College of Rheumatology and the European League against Rheumatism.10 Therefore, in the usual clinical practice, in addition to the determination of antitopoisomerase-I and anticentromere AAbs, it would be extremely valuable and highly recommended, to always have the test available for the detection of anti-ARNPol-III. Similarly, including this test into the design of clinical trials and into the follow-up protocols would be of critical importance for our patients.
Please cite this article as: Hernández-Flórez D, Genaro Susan, Martínez-Barrio J, López-Longo FJ, Valor L. Hiperactivación del linfocito B y producción de los autoanticuerpos anticentrómero, antitopoisomerasa-I, anti-ARNPol-III en lafisiopatología de la esclerosis sistémica cutánea. Rev Colomb Reumatol. 2020;27:68–70.