Systemic sclerosis is an autoimmune disease whose etiology remains unknown. Some patients prove refractory and require other therapies. Recently, the use of mesenchymal stem cells (MSC) for the treatment of disease refractory to conventional treatments has been considered. We present a case of refractory systemic sclerosis; Wharton's jelly mesenchymal stem cell was given in response. Decrease in perioral wrinkles, reduced telangiectasia and decrease in modified Rodnan skin score were observed two years later. A decrease in brain natriuretic peptide and improved pulmonary function were also found. And improvement of pulmonary fibrosis on high resolution tomography and capillaroscopy changes. In conclusion, MSC infusion seems to be effective and safe treatment of refractory scleroderma.
La esclerosis sistémica es una enfermedad autoinmune de etiología desconocida y difícil manejo. Algunos casos que se tornan refractarios requieren terapias alternativas, como las células madre mesenquimales (MSC). Presentamos un caso de esclerosis sistémica refractaria que se llevó a terapia con MSC de gelatina de Wharton. Tras dos años, se observó disminución en arrugas peribucales, aumento en apertura bucal, reducción de telangiectasias y en Rodnan modificado. También hubo disminución del péptido natriurético cerebral y mejora de pruebas de función pulmonar desde los seis meses de seguimiento, con mejoría en fibrosis pulmonar en tomografía de alta resolución y cambios en la capilaroscopia. En conclusión, el tratamiento con infusión de MSC parece efectivo y seguro en esclerosis sistémica refractaria.
Systemic scleroderma (SSc) is an autoimmune disease affecting the connective tissue; it is characterized by vascular disease secondary to extensive collagen and extracellular matrix deposit, vasculopathy phenomena, fibrotic compromise and inflammation of the skin and organs such as the lungs, digestive tract, heart, blood vessels and kidneys.1 SSc etiology remains unknown and its physiopathology has not yet been fully understood. However, a relationship between SSc and some genetic alterations in IRF5, CD247, BANK1, STAT4, TNFSF4 and BLK genes has been described.2 Likewise, cytomegalovirus, parvovirus B19 and some medications such as bleomycin have even been proposed as environmental triggers which can initiate immune activity.3
The prevalence of SSc ranged from 7/million to 489/million and its incidence from 0.6/million/y to 122/million/y. There were many geographical variations: SSc prevalence was higher in the USA (276/million in 1990) and Australia (233/million in 1999) than in Japan and Europe, where a north-south gradient was also observed (France: 158/million in 2001 and England: 88/million in 2000).4 However, SSc has a 5-year survival rate, varying from 89.9% to 96.7%,5 and a mortality rate associated with complications which has been decreasing from 72% in the 1990s to 48% since 2000.6,7
Two variations of its clinical presentation have traditionally been described; limited SSc, having skin involvement in the limbs’ distal regions, accompanied by esclerodactilia, calcinosis, Raynaud's phenomenon and involvement of the internal organs, i.e. the lungs and kidneys. The anti-centromere antibody in up to 70% of cases is its characteristic serological marker.8 Diffuse SSc is the second variant where skin involvement is proximal in the limbs and includes the trunk and back. Renal, cardiac and pulmonary involvement is frequent and can quickly become progressive Anti-topoisomerase I or anti-SCL70 in up 30% and anti-RNA polymerase III in 20% of cases are the serological markers for diffuse SSc, another biomarker are the anti-nuclear antibodies, that are positive in up 90% of cases with different patterns nuclear and centromeric.9
SSc may display minor symptomatology during its early stages, such as Raynaud's phenomenon and arthralgia; however, it leads to pulmonary, cardiac and even renal complications during advanced stages, with pulmonary involvement being one of the most frequent complications having a 33% 10-year survival rate in patients suffering pulmonary involvement and a 45% 3-year survival rate in pulmonary hypertension (PH) patients10 and that most associated with mortality in this population.11 Such complications make SSc a highly disabling condition affecting patients’ quality of life (QOL) due to limitations in carrying out everyday activities.12 It may also involve direct high costs for any country's healthcare system. Drugs and hospitalizations costs in the USA related to the disease's complications range from $17,365 to $18,396 dollars per year per patient.13
Few therapeutic options controlling the physio-pathological mechanisms favoring the disease's progression.14,15 Some therapies have been studied such as some biological ones, antifibrotic therapies and stem cell transplants, for modifying the pathogenesis and identifying which options can modulate its progression. Regarding cellular therapy, experience concerning stem cell transplantation (HSCT) has been promising, leading to a significant improvement the disease's symptoms and progression,15–17 and the possibility of using mesenchymal stem cells (MSC) has been assessed since 2008.18
The MSC have immune regulatory functions, making them potential agents for treating autoimmune conditions, related with its properties anti-proliferative, anti-inflammatory and immunomodulatory. This was due to their ability to adapt to the microenvironment in which they were found, their interaction with cells in their immediate setting and their response to cytokine influx for maintaining homeostasis.19 MSC have low major histocompatibility complex-1 (MHC-I) levels and do not express MHC type-II or costimulatory molecules. They have also proved to be immunosuppressive, self-renewing and are readily available for tissue repair through various mechanisms, including T-cell inhibition and exhibiting antigen-presenting cells through paracrine mechanisms, thereby enabling them to re-establish the immunological microenvironment.20
The immunological benefits of using MSC with SSc patients are currently being studied. Several case reports recording promising results have been published; the first of these published in 2008 was aimed at assessing the response of a patient having acral ulcer-derived cutaneous involvement, with a significant improvement regarding changes in the skin and ulcerous lesions.21 These results led to case series being published regarding patients having skin involvement and organ compromise.22–25 Another current indication of MSC is related with the coronavirus infection disease 19 (COVID-19) in case report wits MSC of umbilical cord with remarkable reversal of symptoms even in severe -critical conditions for its natural mechanisms able to counteract acute inflammatory pneumonia without side effects, representing a new biological approach to treatment.26,27
Regarding adverse events (AE), stem cells have been characterized by having a good safety profile and low AE rate. This has been described in an analysis of safety regarding patients suffering autoimmune diseases (i.e. SLE, SSc and rheumatoid arthritis) who had received an infusion of MSC from different sources (umbilical cord and bone marrow).28
This article presents the case of an SSc patient whose cutaneous manifestation progressed despite conventional treatment with methotrexate, mycophenolate and cyclophosphamide and she have severe compromise in lung and cardiovascular system with decrease in pulmonary capacity and functional test and pulmonary hypertension associated with disease decease a quality of life; a decision to attempt mesenchymal stem cell treatment was thus made.
Case presentationThe case concerned a 45-year-old woman having had a history of Raynaud's phenomenon since 2010, abdominal linear morphea diagnosed through skin biopsy. She had a suitable response after receiving treatment with vasodilators, phototherapy and methotrexate 15mg per week with persistence of morphea. In 2016 she started with face, neck, thorax, abdomen and limbs were affected by skin thickening, increased Raynaud's phenomenon, reduced mouth opening, medium-exertional dyspnea and she was diagnosed with diffuse SSc in 2016, according to 2013 ACR/EULAR classification criteria for scleroderma. During study the patient presented negative antinuclear anti bodies and immunological profile include anticentromeric antibody, Scl-70 antibody, extractable nuclear antibodies, anti-Jo1 antibody, anticiplosmatic neutrophils antibodies and antiphospholipid test. The autoimmune profile was repeated in two occasions and was negative again.
Associated to clinical picture she presented severe pulmonary hypertension (PH) with systolic pulmonary pressure of 100mmHg and biventricular dysfunction, the clinicians rule out a pulmonary thromboembolic disease with ventilation/perfusion scintigraphy and considering that this finding was related with activity of the disease. On that occasion she received treatment involving monthly intravenous (IV) pulse cyclophosphamide (1g) therapy for six months, endothelin receptor antagonists (macitentan), sildenafil and diuretics for acute descompensation cor pulmonale. After six month of treatment she had favorable evolution, skin involvement became stabilized and pulmonary artery pressure decreased. During study she present altered in capillaroscopy related with active pattern in SSc and microvascular compromise and mycophenolate 2g per day was added to the treatment.
In 2017 she was hospitalized again due to small-joint symmetrical polyarthritis, pleural and pericardial effusion, ascites, low-grade fever, general deterioration, increased acute-phase reactants, anemia and increased skin involvement due to severe thickening (modified Rodnan skin score – mRSS 27/50). It was considered that the patient was suffering a rapidly progressive SSc; she was restarted monthly IV cyclophosphamide for six months but until completing nine boluses, she persistence with symptoms and developed respiratory symptoms related with dyspnea, cough and decrease functional capacity with new diagnosis of cardiac dysfunction secondary a pulmonary involvement. In that moment documented pulmonary compromise with interstitial component and suspected of pulmonary fibrosis and she was hospitalized in intensive care unit.
For critical ill stated of patient and unresponsive of treatment, it was decided to start treatment with Wharton's jelly-derived (WJ) multipotent mesenchymal stromal cells (MSC) in view of the extensive skin involvement and its rapid progression (related donor 3/6 HLA). Compliance with International Society of Cellular Therapy (ISCT) cell characteristics criteria were verified.
The patient authorized the intervention jointly with her family, due to the failure of multiple lines of management (methothrexate, mycophenolate and cyclophosphamide) and deterioration of her quality of life, which had limited the development of her work and family role.
The patient entered the experimental intervention protocol with compassionate intervention for orphan disease, with approval from the ethics committee in 2017 and underwent the MSC infusion.
Expanded MSC were for passages 4 and then cryopreserved and qualities garantized; they were verified as being negative for mycoplasma, endotoxins, aerobic and anaerobic germ and karyotype normal with the aim of prevent infections and mutation. Two doses of 1×106MSC/kg were infused; they were administered in 100ml saline solution with 5% human serum albumin (HSA) and IV infusion at one-month intervals of cyclophosphamide cycles 8 and 9 and 10 days after the drug had been administered.
The patient tolerated the infusion of Wharton's jelly-derived allogenic MSCs. The only adverse effect observed was fever 2h after the first infusion; this subsided without treatment and not was related with infection.
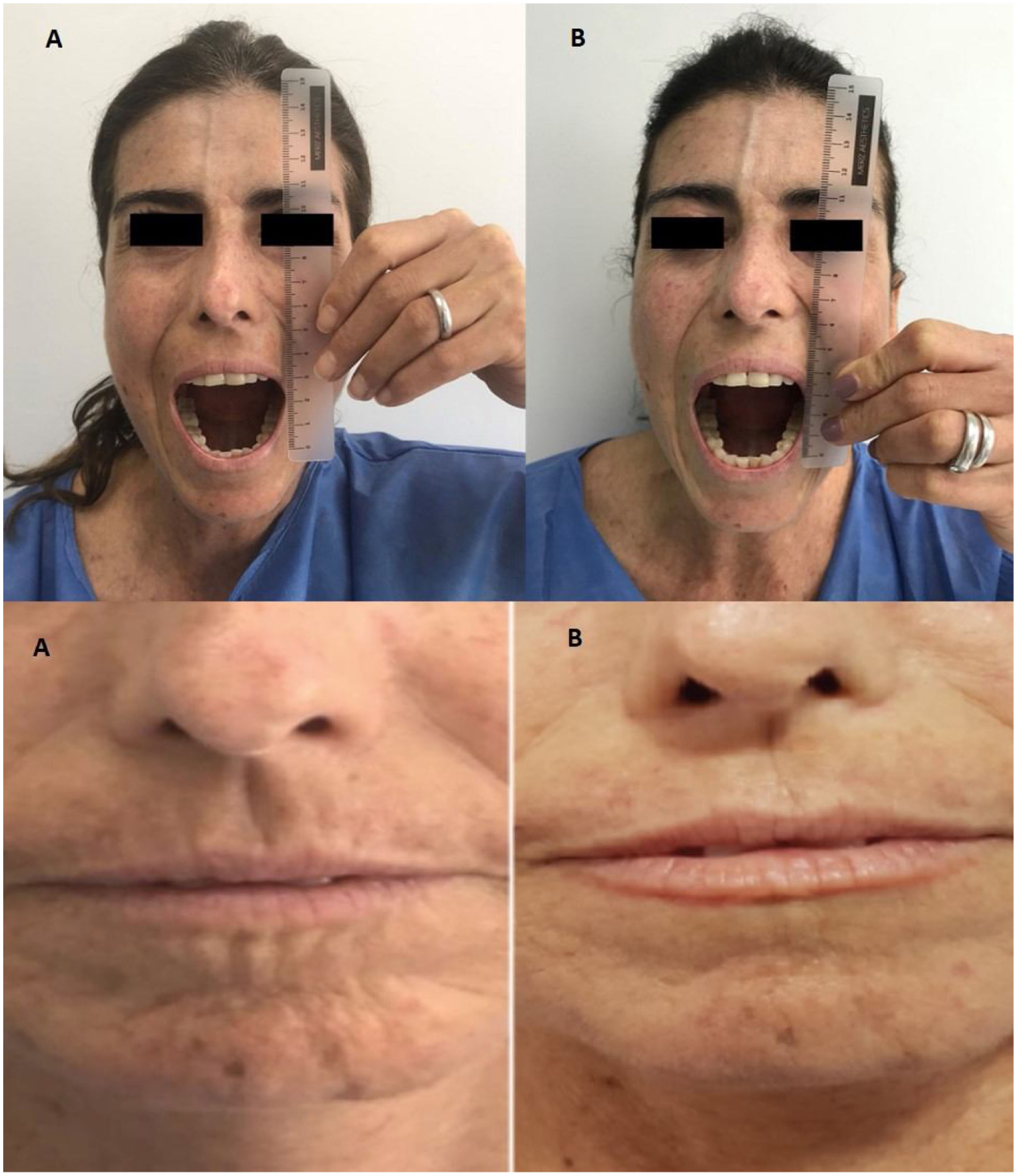
Outcome and follow-upAfter two MSC IV infusion the clinical improve was evident, the patient recovery cardiovascular and pulmonary function and she come back home after treatment. In the Table 1 are presented the variables evaluated with quality of skin and life compromise before the infusion and two years later. A marked decrease in peribucal wrinkles and 1,5-cm increase in mouth-opening (incisor-incisor), reduced telangiectasias in the trunk, and a 16-point decrease in mRSS (i.e., 31/47) were observed six months later and these findings remained present two years after the infusion. These changes are shown in Fig. 1 The patient scored 11 on the CAMPHOR scale prior to stem-cell treatment (3 points for symptoms, 5 for activity limitation and 3 for QOL) and then scored 2 after 6 months treatment (0 for symptoms, 0 for activities and 2 for QOL) and two years later. This scale is specific for pulmonary hypertension, one of the patient's complications, therefore showing improvement of her symptoms.29
Skin and quality of life compromise.
| Test | Before therapy | After 6 months | After one year | After two years |
|---|---|---|---|---|
| RODNAN score | 47 | 31 | 7 | 5 |
| CAMPHORa | 11 | 2 | 0 | 0 |
| Distance between incisors teeth | 3cm | 3.5cm | 4.5cm | 4.5cm |
In Table 2 are presented the pulmonary function and echocardiography; a decrease in brain natriuretic peptide (from 128.8pg/ml to 36pg/ml) and increase regarding the 6-minute walk were also noted. The diffusing capacity of the lungs for carbon monoxide (DLCO) increased from 76% at the beginning of treatment to 94% after one year. The pulmonary artery pressure, measured by echocardiogram, remained stable two years without relapse. Before treatment patient realized a right cardiac catheterization with documented RVP 584 din, and Pulmonary artery pressure Systolic/Diastolic/Media: 109/67/71mmHg but the patient doesn’t have after treatment right cardiac catheterization.
Pulmonary functional test and echocardiography.
| Hemodynamic test | Before therapy | After 6 months | After one year | After two years |
|---|---|---|---|---|
| BNPb (pg/ml) | 128.80 | 62.30 | 30 | 36 |
| 6min walking testa (m) | 572 | 625 | 639 | 615 |
| PASPc (mmHg) | 103 | 51 | 45 | 42 |
| DLCOd – ml/min/mmHg (PV%) | 16.4 (57) | 22.9 (94) | 23.0 (94) | |
| DLCO adj (PV%) | 14.3 (66) | 19.9 (62) | 20.3 (88) | |
| DLadj+VA – ml/min/mmHg (PV%) | 4.31 (75) | 5.37 (98) | 5.3 (98) |
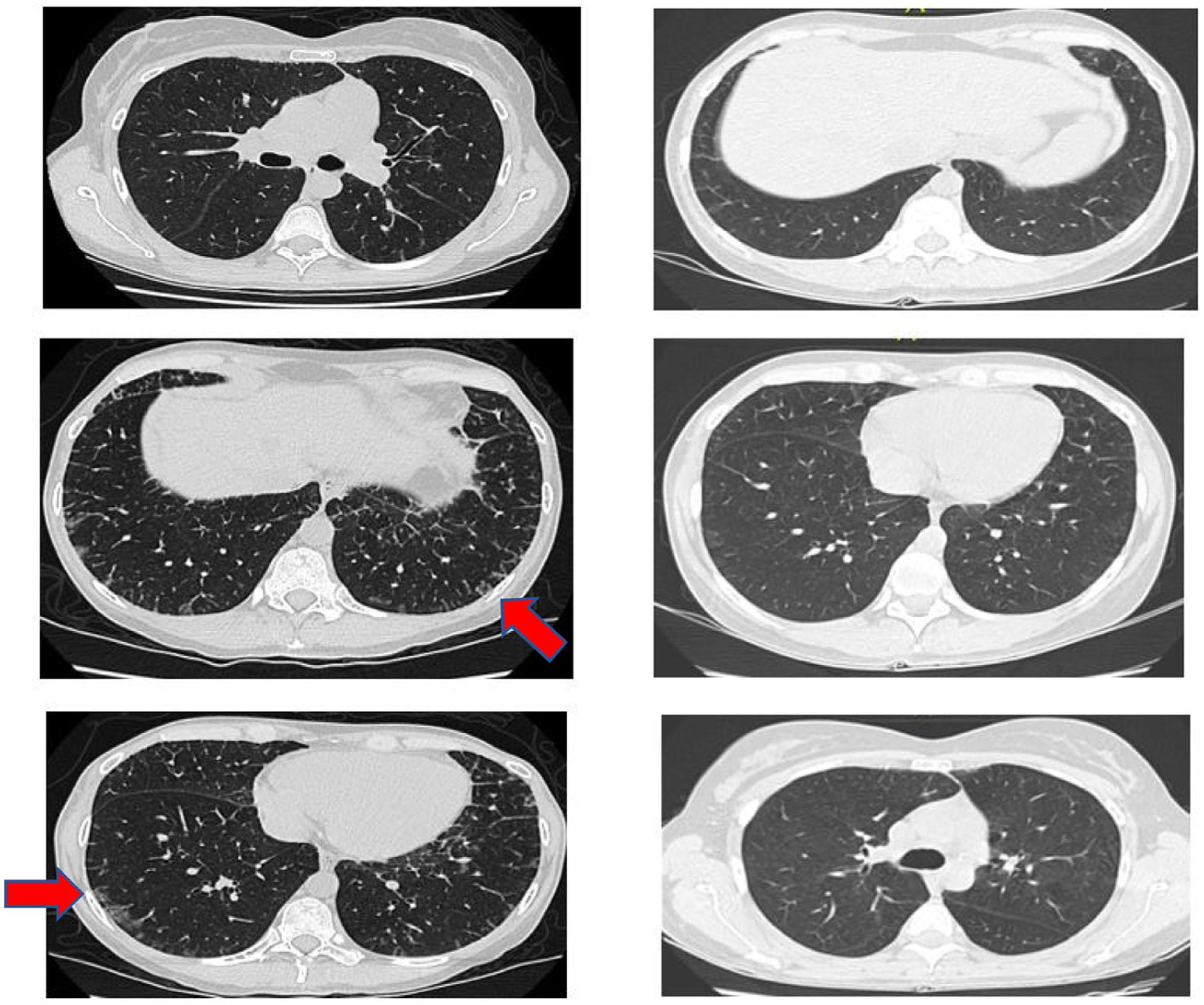
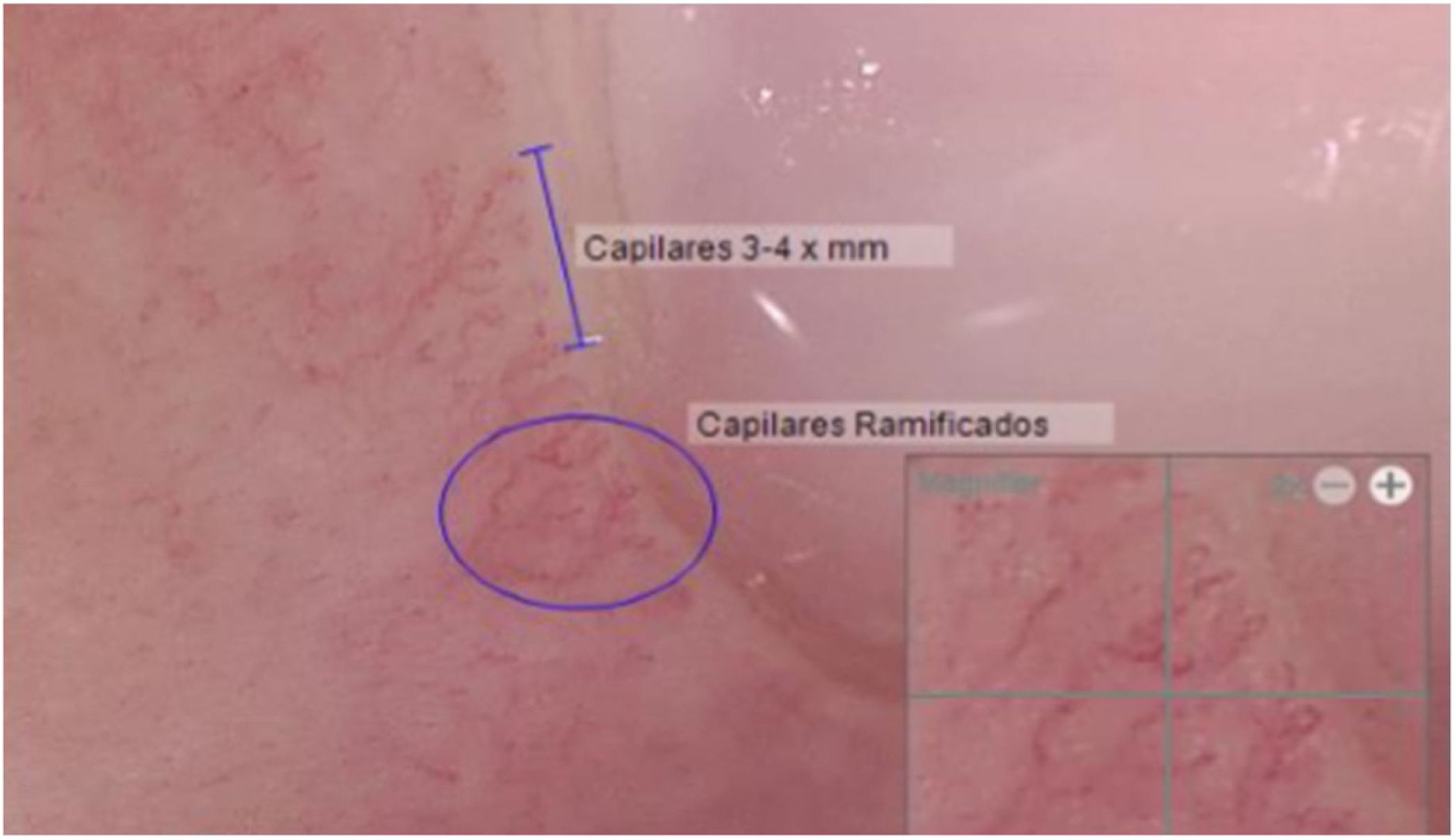
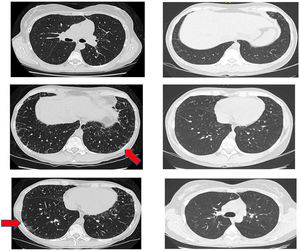
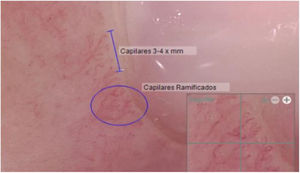
Another positive finding was the improvement and stopped progression in interstitial compromise in high resolution tomography (Fig. 2), where disappear the initial lessons and this is correlated with pulmonary function recovery. Respect a capillaroscopy change, after two years of follow up, the microvascular compromise was stopped and patient recovery a normal capillary morphology. Before treatment the findings in capillaroscopy were described: “Capillary density 4–5×mm, discrete avascular areas, neoangiogenic capillaries +++, giant capillaries: yes, capillary dilations ++, hemorrhages: no”. The results of test after two years of follow-up are shown in Fig. 3.
Research continues regarding systemic scleroderma treatment; new therapeutic options have currently emerged for cases where conventional management is not enough. Our case has dealt with therapy involving MMSC and combined intravenous methotrexate and cyclophosphamide for refractory management. Although HSCT has been progressively used for this type of patient, it has been associated with high mortality rates which, despite being lower in some studies such as the SCOT study in 2005, (6% in 6 years).30
MSC cells were used for managing our patient, along with cyclophosphamide treatment, which differs regarding the need for myeloablation, thereby reducing possible adverse effects. Five articles describing the cases of 10 patients were found after reviewing the pertinent literature; just like our case, their patients received IV MSC cell infusion without the need for myeloablation.21–25
A 10-point decrease in Rodnan skin score was recorded in our case and the patient managed to increase her mouth-opening by 5mm 6 months after having been intervened. Such results were similar to those provided by the case report described by Christopeit et al., who recorded their patient's clinical improvement regarding Rodnan skin score 6 months after treatment was begun.31 Guiducci et al., found revascularization improvement and decreased skin thickness.32
Concerning pulmonary function, our patient had more than 50% decrease in BNP, a 53-meter increase in the 6-minute walk, as well as a 4-point DLCO improvement and 18% increase in predicted QOL value. Wehbe et al., reported two cases in which patients suffered pulmonary alterations due to SSc; pulmonary pressure became normalized regarding both patients and their mobility, function and QOL improved.20
Regarding adverse events, our patient only suffered a single episode of fever when infused (i.e. described in the literature as an allogenic reaction),33 whereas the pertinent literature contains a case report by Chakr et al., which involved a 36-year-old female patient (10-year history of diffuse SSc manifested by severe scleroderma (Rodnan score 47/51)). This patient suffered respiratory failure 3 weeks after having been infused (although fatality was not attributed to MSC infusion).34
Methotrexate, mycophenolate and cyclophosphamide are currently being used for treating SSc regarding skin improvement and cyclophosphamide pulses are being used for pulmonary involvement.35 HSCT is one of the new therapies for managing SSc; this is mainly used for refractory cases and has managed to improve subjects’ complication-free time to 54 months by 79%, compared to the 50% improvement rate for patients treated with cyclophosphamide.36
We considered the case reported here to be relevant because it was decided to use IV MSC treatment with the expectation of involving fewer adverse reactions as may happen with HSCT involving myeloablation. Our patient underwent suitable evolution at various levels, i.e. skin (improved Rodnan skin score and mouth opening), pulmonary function tests (6-minute walk and DLCO) and BNP level.
Due to the lack of right cardiac catheterization, it was not possible to establish whether the improvement in pulmonary hypertension was related to the control of inflammation due to group 1 pulmonary hypertension (according to Dana Point 2008) or to the arrest of disease progression. interstitial in relation to group 3. However, its improvement and the impact on functional capacity are relevant.
The patient was already undergoing pulmonary hypertension treatment with macitentan and sildenafil when her QOL was measured on the CAMPHOR scale; even though such measurement was not made prior to stem cell infusion, it was considered that QOL improvement after infusion was clinically relevant.
Our literature review revealed that not many case reports have involved using this therapy. However, such reports have described similar findings to ours, thus highlighting the question of whether IV administration of mesenchymal stem cells is a safer (and eventually just as effective) option than transplantation.
It is important to note that MSC therapy requires quality raw material and either autologous or by donation, cells must be expanded taking into account good manufacturing practice (specialized Human resource, sterile infrastructure, GMP reagents and studies of the cellular safety profile). Currently in Colombia, advanced cell therapies (they are not drugs) are carried out within the framework of human research that in Colombia is governed by resolution 8430 of the Ministry of Health or Compassionate Therapy, which must comply with article 37 of the Declaration of Helsinki. in Germany in cell therapy centers the costs for two doses vary from 9000 to 15,000 euros.
Ethical aspectsThe protocol had the approval of the ethics committee “Centro de Investigación de Oncología Clínica San Diego, CIOSAD”, through Act No. 023 of March 6, 2018. Prior to the procedure, the patient signed an informed consent authorizing the publication of clinical, laboratory information and the photographs disclosed in this writing.
FundingThe author(s) received no financial support for the research, authorship, and/or publication of this article.
Conflict of interestThe authors declare that there is no conflict of interest.
The authors wish to thank Ana M. Santos, Claudio Villaquirán, Gonzalo Gutiérrez-García and Edwin Jáuregui for providing technical assistance and general support.