Systemic lupus erythematosus is a complex and chronic disease which impacts on the reproductive function of patients suffering this condition. This assertion is supported by the fact that lupus patients have a smaller family size in comparison to the general population as well as a higher risk of adverse pregnancy outcomes. While this disease per se does not affect fertility, there are several other factors affecting fertility such as age, drugs, disease activity, damage-related disease, and some comorbidities. Currently, there are several interventions to preserve fertility with very good outcomes, among them, cryopreservation or the use of gonadotropin releasing hormone agonists. It is recommended that lupus patients be in low disease activity or in remission for at least six months before conception and pregnancy. If the latter is achieved, multidisciplinary management is very important and recommended, but in particular, physicians must know how to differentiate between a lupus flare and pregnancy-related hypertension. The efficacy and safety of antimalarials throughout pregnancy has been demonstrated so its use must be continued and encouraged. Taking into account all the above, fertility and pregnancy in lupus patients must be an integral part of the management of this disease.
El lupus eritematoso sistémico es una enfermedad compleja y crónica que afecta la función reproductiva de los pacientes que la presentan, considerando que suelen tener un tamaño familiar reducido en comparación con la población general, así como un riesgo más alto de resultados adversos perinatales. Si bien esta enfermedad per se no afecta la fertilidad, existen otros factores que la alteran, como la edad, los fármacos, la actividad de la enfermedad, el daño relacionado con ella y algunas comorbilidades. En la actualidad, existen numerosas intervenciones para preservar la fertilidad, con muy buenos resultados, entre las cuales se encuentran la criopreservación o el uso de análogos de la hormona liberadora de gonadotropinas. Es recomendable que los pacientes lúpicos se encuentren en baja actividad de la enfermedad o en remisión por al menos seis meses antes de la concepción y el embarazo. Si esto se logra, el manejo multidisciplinario es muy importante y recomendado pero, sobre todo, los médicos deben saber cómo diferenciar entre reactivación de la enfermedad y enfermedad hipertensiva del embarazo. Se ha demostrado la eficacia y la seguridad de los antimaláricos a lo largo del embarazo, por lo cual su uso debe ser continuado y aconsejado. Teniendo en cuenta lo señalado en las líneas precedentes, la fertilidad y el embarazo en pacientes lúpicos deben ser parte del manejo integral de esta enfermedad.
Systemic Lupus Erythematosus (SLE) is a complex autoimmune condition of unknown etiology which disproportionally affects women of childbearing age.1 These patients face a chronic disease which impacts their physical and mental health but also their reproductive function. For example, some studies have shown that women with rheumatic diseases, including lupus patients, have a fewer number of births and a smaller family size in comparison to the general population.2–5 On the other hand, although improvements in disease management and perinatal monitoring have occurred over the last five decades resulting in a significant decrease in pregnancy losses in these patients,6 when compared with the general population, lupus patients are still at higher risk of adverse pregnancy outcomes. Therefore, fertility and pregnancy must be part of the management of this disease.
PreconceptionCauses of infertility in lupus patientsIt is important to note that there is no evidence that SLE per se causes primary infertility.4,7–12 Secondary infertility, however, may occur for a number of reasons including advanced age, medications, comorbidities, psychosocial issues, disease activity and damage-related to the disease.
As noted, SLE occurs more frequently in reproductive-age women; oftentimes, physicians encourage patients to avoid becoming pregnant until a remission state is achieved, or possible teratogenic medications are safely discontinued. As a consequence, many lupus patients tend to plan a pregnancy relatively late in life which is associated with a decline in fertility, even in healthy women.11,13,14
Primary ovarian failure (POF) is one important cause of infertility in lupus patients; POF is defined as persistent amenorrhea before the age of 40 and is associated with increased levels of circulating follicle-stimulating hormone and hypo-oestrogenism.15 Autoimmune oophoritis may be the cause of POF.16 Likewise, the use of cyclophosphamide (CYC) can cause POF with an incidence that varies between 11% to 59% in all age groups, either if administered orally or intravenously.17 It is known that POF-induced CYC is age- and dose-related. Boumpas et al. found that lupus patients>30 years of age and those who used a larger number of doses of CYC had greater sustained amenorrhea rates.18 Likewise, Ioannidis et al. found that 50% and 90% of lupus patients≥32 years of age had sustained amenorrhea with 8g/m2 and 12g/m2 of CYC cumulative dose, respectively19; in fact, these findings are supported by the fact that SLE patients treated with the Euro-lupus protocol had a lower risk of POF than those treated with the National Institute of Health (NIH) regimen.14 It is also important to note that Hispanic ethnicity, mainly individuals of Mexican ancestry living in the US, was found to be a predictor of POF in the LUMINA (for LUpus in MInorities: NAture versus nurture) cohort.20
Some drugs can cause infertility in lupus patients. As it has been noted already, CYC is one of the main drugs causing POF; however, this drug can also affect male lupus patients due to the fact that it impairs sperm quantity and quality and testis volume.21 Other immunosuppressive drugs such as methotrexate, azathioprine, mycophenolate, cyclosporine A and tacrolimus do not have an effect on fertility in lupus patients13,14,16; that is why, for example, mycophenolate is a good option as induction therapy in lupus patients who desired to become pregnant but its teratogenic risk needs to be considered and thus, not continue it once pregnancy commences. Other drugs which might be associated with infertility include non-steroidal anti-inflammatory drugs (NSAIDs) and glucocorticoids (GC). As to NSAIDs, while their use may interfere with ovulation due to a decrease in prostaglandin production, to date there is lack of rigorous clinical studies about them.11,14 Regarding GC, their association with infertility is controversial due to the difficulty in distinguishing between menstrual irregularities secondary to high GC doses from the effects of disease activity per se.14
Active disease and damage-related disease can influence fertility rates. High disease activity and lupus flares have been associated with infertility in different studies.10,22,23 This might be due to hyperprolactinemia with the consequent reduction in the levels of gonadotropin releasing hormone (GnRH) and, as a result, impaired ovulation.24 Likewise, anti-Müllerian hormone antibody levels, which are indicative of ovarian reserve, have been found lower in non-CYC treated lupus patients than in healthy controls.25 As to damage, renal insufficiency or failure can generate disruption of the hypothalamic–pituitary axis resulting in increased prolactin levels; however, this can be reversed with renal transplantation.10,11,26
Among other causes of infertility are psycho-social issues such as anxiety, depression, fatigue, loss of libido and sexual dysfunction (in females and males), which result in a decreased sexual activity and, consequently, in infertility.5 Some comorbidities might be associated with or increase the risk of infertility; worth mentioning is the anti-phospholipid syndrome (APS),14 Hashimoto's thyroiditis27 and cervico-vaginal inflammation.28 As to autoantibodies, it seems that they do not affect fertility in lupus patients. In the past there has been a controversy about the possible role of anti-phospholipid (aPL) antibodies; however, results from controlled studies have not demonstrated that they, per se, are associated with infertility or poor outcomes in patients undergoing in vitro fertilization (IVF).29
Fertility preservation methodsBecause of the frequency of infertility in lupus patients, strategies to counteract it have been developed. These strategies should be recommended to patients receiving gonadotoxic drugs (as CYC) and patients who must delay a pregnancy because of persistently active disease.
Given that POF-induced CYC is age- and dose-related, minimizing the CYC dose using short-induction scheme (Euro-lupus protocol), using other drugs for induction such as mycophenolate or multi-target protocols are indicated.14 However, in some cases, because of severe disease activity using high doses of CYC is necessary; then gonadal protection should be implemented. In this sense, GnRH agonists are drugs with a good safety and efficacy profile in cancer patients30,31; likewise, in patients with SLE, there are some studies with promising data.32–34 This strategy is applied to oral and intravenous CYC; it is recommended to administer GnRH agonists 10–14 days (for leuprolide) or 22 days (for triptorelin) prior to CYC administration and in mid-cycle. The use of testosterone for preservation of male fertility is not recommended.35,36
Cryopreservation is a good option in patients with stable disease due to the fact that ovarian stimulation (OS) is necessary (for the risk of OS vide infra); however, it is difficult to embark on cryopreservation when the patient needs to use CYC for a severe disease.35 Oocyte cryopreservation is the most popular and effective option, no longer considered an experimental technique; it is ideal for women who may not have a partner and it does not require the fertilization of an egg after the procedure.16,37 Embryo cryopreservation is ideal for women who have a partner; however, this technique might bring up some ethical concerns.16 On the other hand, ovarian tissue freezing is an experimental technique in which ovarian tissue is removed through laparoscopic oophorectomy without the need for OS; it might be performed in prepubertal age and in some cases in whom treatment should be initiated at once.11,16,38 Limitations of all cryopreservation techniques are their high cost, the lack of insurance coverage for the procedure, the limited number of centers in which it is available, and the patient's beliefs.
Assisted reproduction techniquesAs noted, lupus patients may not achieve good pregnancy outcomes; that is why assisted reproduction techniques (ART) are a good option for these patients. These techniques include OS, IVF, embryo transfer and oocyte retrieval.
While there is still a concern about the risk of flares or thrombosis due to hormonal stimulation which increases 17β-estradiol levels, some studies have shown that the use of ART is safe and successful. For example, Orquevaux et al. in a study which assessed 37 lupus and APS patients who underwent 97 IVF procedures, found that 26 (70%) patients delivered at least one healthy child; there were complications in only eight IVF cycles (four lupus flares and four thromboembolic events) and there were no cases of ovarian hyperstimulation syndrome (OHS).39 Likewise, Ragab et al. in a study of 65 lupus patients who underwent OS, 20 of them became pregnant and only in four cases it was complicated by OHS.40 Other studies, some of them include patients with other rheumatic diseases, found similar results.38,41,42 It is worth to note that pregnancy rates in lupus patients undergoing ART are similar when compared with the rates observed in the general population (up to 30%).43
Despite these good outcomes, it is important to consider a few additional points. One of them is about a prophylactic approach, especially in patients with aPL antibodies, in whom it is recommended the use of low dose aspirin and/or heparin. In contrast, the empiric increase of the dose of GC as a prophylactic measure in patients undergoing ART is not recommended. About disease activity, it is strongly recommended that women who will undergo ART be in remission or low disease activity (for at least six months).12,35,38 As to ART, it is recommended to use milder hormonal stimulation or GnRH antagonist protocols in order to decrease the risk of flares, thrombosis or OHS.12,44 It is important to note that some patients might be candidates for ART; however, pregnancy may not be recommended in the presence of lupus damage (i.e. severe renal insufficiency, pulmonary hypertension); in these cases, IVF followed by embryo transfer to a gestational carrier (gestational surrogate) could be the preferred alternative.38
Needless to say, a multi-disciplinary approach in lupus patients requesting ART is important if a successful pregnancy outcome is to be achieved.
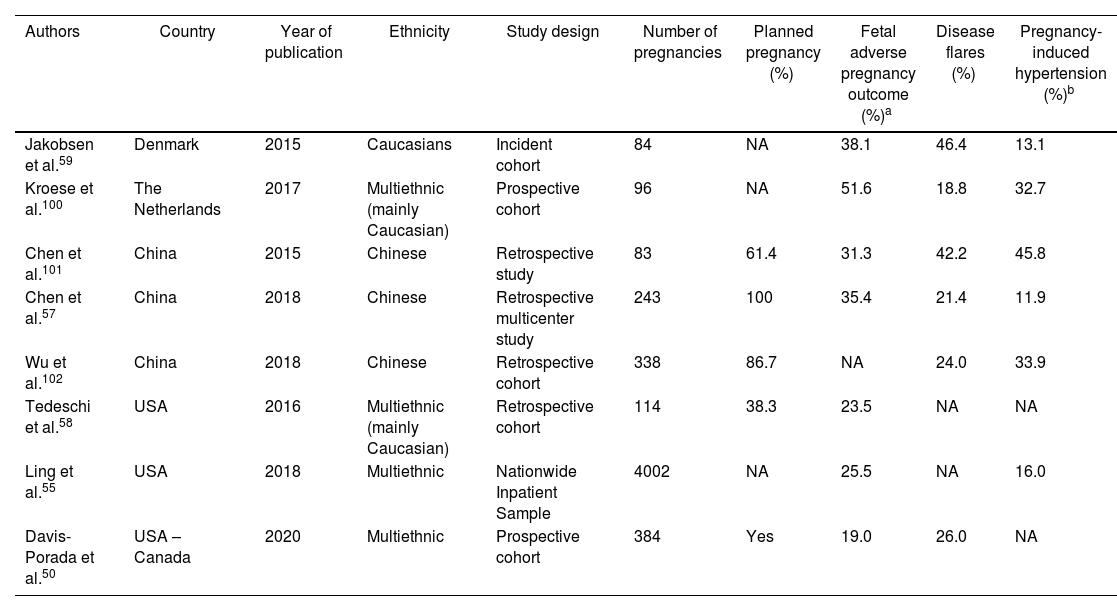
PregnancyOutcomesComparative studies using national registries have reported an increased risk of miscarriages, preterm labor, intrauterine growth restriction (IUGR) and fetal death in lupus patients.45,46 In one study performed in the USA,47 which used the Nationwide Inpatient Sample and compared pregnancies in SLE patients with those in a control group, significantly higher rates of hypertensive disorders [Odds ratio (OR): 3.3 (95% CI 2.8–4.0)], IUGR [OR: 3.5 (95% CI 2.5–4.9)] and cesarean delivery [OR: 1.6 (95% CI 1.4–1.9)] among lupus patients were found; moreover, these patients had an increased length of hospital stay even after adjusting for cesarean delivery. A subsequent study focusing on lupus pregnancy in this database for the years 2000–2003,46 revealed that maternal mortality was 20-fold higher among women with SLE that in those from the general population; likewise, lupus patients had an increased risk of preterm labor, preeclampsia, and cesarean delivery; they were also more likely to have other comorbidities associated with adverse pregnancy outcomes such as diabetes, hypertension and thrombophilia. These poor outcomes have also been well documented in several lupus cohorts48–50 as well as in relatively small medical records review single center studies51–54 in patients from different ethnic background across the world. Although the majority of these studies included adult SLE patients, these findings seem to replicate among adolescent patients.55 Rates of adverse pregnancy outcomes, disease flares during pregnancy and pregnancy induced hypertension published over the past five years are depicted in Table 1. It seems that the occurrence of disease flares increases during SLE pregnancies but decreases if pregnancy is delayed until the disease is quiescent.47
Odds of fetal adverse outcomes, lupus flares during pregnancy and pregnancy-induced hypertension.
| Authors | Country | Year of publication | Ethnicity | Study design | Number of pregnancies | Planned pregnancy (%) | Fetal adverse pregnancy outcome (%)a | Disease flares (%) | Pregnancy-induced hypertension (%)b |
|---|---|---|---|---|---|---|---|---|---|
| Jakobsen et al.59 | Denmark | 2015 | Caucasians | Incident cohort | 84 | NA | 38.1 | 46.4 | 13.1 |
| Kroese et al.100 | The Netherlands | 2017 | Multiethnic (mainly Caucasian) | Prospective cohort | 96 | NA | 51.6 | 18.8 | 32.7 |
| Chen et al.101 | China | 2015 | Chinese | Retrospective study | 83 | 61.4 | 31.3 | 42.2 | 45.8 |
| Chen et al.57 | China | 2018 | Chinese | Retrospective multicenter study | 243 | 100 | 35.4 | 21.4 | 11.9 |
| Wu et al.102 | China | 2018 | Chinese | Retrospective cohort | 338 | 86.7 | NA | 24.0 | 33.9 |
| Tedeschi et al.58 | USA | 2016 | Multiethnic (mainly Caucasian) | Retrospective cohort | 114 | 38.3 | 23.5 | NA | NA |
| Ling et al.55 | USA | 2018 | Multiethnic | Nationwide Inpatient Sample | 4002 | NA | 25.5 | NA | 16.0 |
| Davis-Porada et al.50 | USA – Canada | 2020 | Multiethnic | Prospective cohort | 384 | Yes | 19.0 | 26.0 | NA |
NA: Not available.
Of importance, SLE disease activity, the presence of proteinuria, previous and/or active lupus nephritis and arterial hypertension have been associated with small for gestational age and pre-term delivery.48,56–58 In the same way, high disease activity at the time of conception (or in the months preceding it) is recognized as an independent predictor for increased risk of adverse pregnancy outcomes, including prematurity, IUGR and fetal losses.56,57,59 Moreover, variables such as baseline arterial hypertension and the presence of aPL antibodies, particularly the lupus anticoagulant49,60,61 as well as triple positivity, have been associated with fetal losses.56,62,63 Other variables, such as the use of high doses of GC, low platelet counts, complement activation, serositis and fewer years of education have been also associated with adverse pregnancy outcomes.48,49,58,64 More recently, the effect of higher levels of cholesterol and body mass index have been also associated with poorer pregnancy outcomes.65 Conversely, pregnancy outcomes are better and disease flare rates are not increased if patients become pregnant while in remission or with low levels of disease activity.49,50 The accumulated knowledge has led to the current recommendation that lupus patients should be advised to consider pregnancy only during periods of inactive or stable disease,46,48 the so-called “planned pregnancy”, and achieve at least six months of quiescent disease prior to attempting conception.50 Unfortunately, many pregnancies in lupus patients are either unplanned or conception occurs against medical advice.
It is important to note that the probability of having a small for gestational age newborn was reduced by 85% in women who received hydroxychloroquine (HCQ) therapy throughout the pregnancy. Furthermore, the benefits of HCQ include maintaining a good disease control throughout the pregnancy.66–68 However, some physicians, including rheumatologists and obstetricians, may recommend their patients to discontinue its intake69,70; unfortunately, once a woman is told not to take a medication because it may affect the outcome of her pregnancy, she may be reluctant to re-initiate it despite being presented with solid data in favor of doing so. Thus, physicians treating pregnant lupus patients, should be encouraged to recommend the use of HCQ before, during and after pregnancy.
Differences between lupus flare and pre-eclampsiaA systematic literature review including 37 studies of pregnancy outcomes in women with SLE and a meta-analysis about the association of lupus nephritis with adverse pregnancy outcomes, showed that lupus flares occurred in 25.6% of the patients, hypertension in 16.3%, nephritis in 16.1% and pre-eclampsia in 7.6%.71 Even for patients in whom nephritis is clinically quiescent, past kidney involvement raises concerns regarding the occurrence of renal flares and overall pregnancy outcomes.
It is difficult to differentiate between lupus flare and pre-eclampsia due to some overlapping features. For example, thrombocytopenia, edema, proteinuria and hypertension could be found in both. Hypertension, however, appears throughout pregnancy in lupus flares while in preeclampsia, it does not appear before 20 weeks and in the majority of patients appears after 34 weeks of gestation. Laboratory findings are useful in monitoring not only SLE activity but also in the differentiation between lupus flare (such as leukopenia, low complement levels, increasing anti-dsDNA titers, active urine sediment) and preeclampsia (elevated uric acid levels, elevated liver enzymes).72 As commented, although an active serology is helpful in differentiating lupus flare from preeclampsia, sometimes it can be difficult to distinguish both, particularly if patients present with severe features such as the HELLP (for Hemolysis, Elevated Liver enzymes and Low Platelet) syndrome; this is usually the case in patients presenting with lupus hepatitis or nephritis. SLE renal flares are often associated with increases in proteinuria and/or an active urinary sediment (hematuria, cellular casts) and significant elevations in serum creatinine level whereas hypertension, although present, may be less pronounced compared with preeclampsia with severe features/HELLP syndrome. Acute onset of accelerated hypertension is more likely to be due to preeclampsia than to lupus flare. Thrombocytopenia and hemolytic anemia, on the other hand, may be more difficult to attribute to either a lupus flare or HELLP syndrome. Treatment is totally different among these disorders: immunosuppressive drugs for lupus flares and terminating the gestation for those with preeclampsia are currently recommended.
Treatment of lupus flares during pregnancyAccording to the recommendations published in 2017 by the European League Against Rheumatism (EULAR),12 the following drugs may be used for the prevention and management of SLE flares during pregnancy:
- •
HCQ, oral GC, azathioprine, cyclosporine A and tacrolimus.
- •
Moderate-to-severe flares can be managed with additional strategies, including intravenous GC pulse therapy, intravenous immunoglobulin and plasmapheresis.
- •
Other drugs such as mycophenolate, CYC, leflunomide and methotrexate should be avoided.
These data are supported by a few high-quality studies, mostly, however, being uncontrolled. There is only one randomized, placebo-controlled study that supports the beneficial role of HCQ66 in controlling disease activity and preventing flare-ups during pregnancy; therefore, and as noted, its use is recommended throughout pregnancy. HCQ may also reduce the probability of cardiac heart block occurrence in fetuses exposed to maternal anti-Ro/SSA antibodies73,74 as well as the odds of prematurity and IUGR.67 However, a recent meta-analysis failed to prove the efficacy of HCQ in the prevention of prematurity as well as of IUGR in SLE pregnancies.75 Some uncontrolled studies suggest an acceptable benefit/risk ratio of oral GC,76,77 azathioprine78,79 and calcineurin inhibitors.80
Benefits of antimalarials in pregnancy outcomesAntimalarial (AM) medications, such as HCQ and chloroquine, should be continued during pregnancy, because they reduce the risk of fetal and maternal complications. About fetal complications, those pregnancies without exposure to HCQ had a higher risk of preterm delivery [relative risk (RR): 6.0 (95% CI 1.6–22.0)] in a retrospective analysis of all SLE patients admitted to deliver after 22 weeks of gestation to a Bordeaux hospital.67 Another study from The Netherlands found that among preterm live births, pregnancy duration was longer in HCQ users.81 However, a meta-analysis including six studies failed to prove the efficacy of HCQ in the prevention of prematurity; however, as the studies included in this meta-analysis were heterogeneous, the meta-analysis results should be interpreted cautiously.82 Additionally, in a Korean retrospective cohort, HCQ use was associated with a lower risk of preeclampsia [OR: 0.11 (95% CI 0.02–0.67)].83 Similarly, in a study from Mexico, AMs use was associated with a lower risk of preeclampsia [RR 0.21 (95% CI 0.08–0.53)].84
A lower percentage of patients with active disease during pregnancy (defined as a SLEDAI≥4) has been reported in patients who continue taking HCQ during their pregnancy (52%) than in those who never used it (62%) and those who stopped it (84%), p=0.0075; these data come from a longitudinal analysis carried out on the Hopkins Lupus Pregnancy cohort.68 Similarly, in another Korean retrospective cohort, HCQ discontinuation was associated with a higher frequency of flares.85 Even more, the HCQ use could mitigate the risk of flares during and after pregnancy,86 and their use was associated with an improvement of disease activity during the pregnancy, compared to placebo, in a small clinical trial.66
Lactation and family planningDrugs and lactationThere are only scanty and small reports about drugs safety in lactation, and they have been summarized by the British Society for Rheumatology and British Health Professionals in Rheumatology guidelines87,88; they have also been included in the EULAR recommendations.89
Non-selective COX inhibitors (classical NSAIDs) can be detected in a minimal concentration, they could be used during breastfeeding, but short-life agents should be preferred.87,89 Among selected COX-II inhibitors, only celecoxib has been studied90,91; its concentration on milk is minimal, so it could be used. Other Cox inhibitors are not recommended due to the lack of information about them.87,89
About GC, they can be detected in breast milk, however, in a minimal concentration. They can be used safely if the dose is lower than 50mg/d, but if the dose is equal or higher than 50mg/d, a 4-h delay before breastfeeding should be considered.89,92,93 AMs can be detected in breast milk in a minimal concentration; however, they can be safely used and there has not been any adverse event reported.89,92–94 About mepacrine, there are no data published about its safety, and it is therefore not recommended.
Among conventional immunosuppressive drugs, azathioprine (dose<2m/kg/d), cyclosporine A and tacrolimus are compatible with breastfeeding. But, methotrexate, leflunomide, CYC and mycophenolate are contraindicated.88 Biologics like rituximab or belimumab are not recommended88; however, their absorption is unlikely due to their low bioavailability.89 Based on the pharmacological properties of biologics, lactation should not be discouraged when using these agents, if no other options are available.89 Intravenous immunoglobulin administration is safe and it is compatible with breastfeeding.88
Recommendations about family planningPlanned pregnancyBased on the data presented, the key for a successful pregnancy in lupus patients is a multidisciplinary approach with close medical, obstetric and neonatal monitoring. This entails: (a) a preconception evaluation to establish and inform women about pregnancy risks; (b) planning pregnancy during inactive lupus nephritis, maintained it inactive with the lowest possible dosage of allowed drugs; (c) adequate treatment of known risk factors (arterial hypertension, overweight, cholesterol level, smoking, aPL antibodies); (d) close monitoring during and after pregnancy to rapidly identify and treat SLE flares and obstetric complications.56
Contraceptive methodsWomen with SLE should be counseled about contraception, in particular those with active disease or who are using teratogenic drugs. The World Health Organization includes SLE as one of the conditions with specific recommendations95 and EULAR has published also its recommendations for these patients.12
Contraceptive measures should be discussed based on the risk factors, including general risk factors (like hypertension, obesity, tobacco use, family history of hormonal-dependent cancers) and disease related factors, in particular disease activity, damage and thrombosis risk.12,95
An intrauterine device (IUD) can be offered to all patients, unless there is a gynecological contraindication. Copper IUD could be used in all patients whereas levonorgestrel-containing IUD could be recommended only if the benefits of the hormone outweigh the risk of thrombosis.12,95 In a small retrospective cohort, disease activity did not increase in those patients who started levonorgestrel-containing IUD, but two patients presented arterial thrombosis (both patients were positive for aPL antibodies).96 With the exception of severe thrombocytopenia, copper IUD is the best rated contraceptive method for SLE patients due to its safety95; however, infections could occur more frequently compared to those patients using oral contraceptives97; however, this has not been consistently reported.98
The safety of oral combined contraceptives97–99 and progestin-only formulations97 has been evaluated in randomized clinical trials, but these studies excluded patients with severe disease activity, history of thrombosis, presence of aPL antibodies, among other contraindications. Additionally, there is an increased risk of thrombosis in women with aPL antibodies.98 Based on these data, these contraceptives should be used only in patients with inactive or stable active disease, without a history of thrombosis and who lack aPL antibodies. In patients with aPL antibodies or history of thrombosis, the use of hormones (oral contraceptives, vaginal ring and transdermal patch) should be strongly discouraged.12,95,97,99
Vaginal ring and transdermal patch have a lower level of evidence, and they are recommended in the same situations as the oral contraceptives.95
ConclusionsFertility in lupus patients is a big challenge. However, currently there are several options to preserve it according to some patient's characteristics (age or degree of disease activity) and which could also improve the probability of a successful pregnancy. It is important to advice lupus patients to get pregnant when their disease is in low disease activity or remission for at least six months. While some drugs are contraindicated during pregnancy, it is important to note the efficacy and safety of HCQ in particular, and AMs in general, throughout pregnancy so its use must be encouraged. Team management of the lupus patient, prior to, during and post-pregnancy is strongly recommended.
Conflict of interestsThe authors declare that they have no conflict of interest.