Systemic lupus erythematosus (SLE) is a systemic disease mediated by immune complexes of unknown aetiology that generates excessive production of autoantibodies against components of the cell nucleus, generating multisystemic involvement affecting organs or systems such as the central nervous, cardiovascular, haematolymphoid, musculoskeletal, kidney, serous, skin and subcutaneous tissue cells, among others. 2-Fluoro-2-deoxyglucose (FDG) is a glucose analogue that has been shown to be a useful diagnostic tool to establish the initial systemic involvement of this disease and response to the various treatments used.
El lupus eritematoso sistémico (LES) es una enfermedad sistémica mediada por inmunocomplejos de etiología desconocida, la cual genera una producción excesiva de autoanticuerpos contra componentes del núcleo celular. Esto último conlleva un compromiso multisistémico que afecta a órganos o sistemas como el nervioso central, el cardiovascular, el hematolinfoide, el musculoesquelético, el riñón, las serosas, la piel y el tejido celular subcutáneo, entre otros. La 2-fluoro-2-desoxiglucosa (FDG) es un análogo de la glucosa que ha mostrado ser una herramienta diagnostica útil para establecer el compromiso sistémico inicial de esta enfermedad y la respuesta a los diversos tratamientos.
Systemic lupus erythematosus (SLE) is an immune complex-mediated disease of unknown etiology, which generates an excessive production of autoantibodies against components of the cell nucleus1 that affects central nervous, cardiovascular, hematolymphoid, musculoskeletal, genitourinary, pleuro-pericardic, skin, and subcutaneous tissue organs or systems, among others2.
Positron emission tomography/computed tomography (PET/CT) is a non-invasive imaging study used as a diagnostic method in different clinical scenarios: detection, classification, staging, prognosis, treatment planning, evaluation of response to therapy, and surveillance in oncological, cardiovascular, neurological, inflammatory, and infectious disorders, among others3.
To obtain the images, the patient is injected with a compound associated with a positron emitter that allows the visualization of normal structures within the biodistribution of this radiopharmaceutical, in addition to those., that are considered pathological according to their location and level of uptake3.
2-Fluoro-2-deoxyglucose (FDG), bound to the positron emitter 18-Fluor (18F), is the most used radiopharmaceutical in PET/CT studies worldwide. It is a glucose analog that is taken up by cells through cell membrane glucose transporters (GLUT). It is subsequently metabolized by hexokinase, which allows it to remain trapped inside cell4.
MethodsA non-systematic search of the literature was carried out in different medical research databases such as PubMed, Ovid, BMJ, Clinical Key, and Scielo, in which keywords such as: «Systemic Lupus Erythematosus (SLE)», «Myocarditis», « Neuropsychiatric», “Pulmonary”, “Nephritis”, “Panniculitis”, “Lymphadenopathy”, “18F-fluorodeoxyglucose Positron Emission Tomography/Computed Tomography”, “FDG PET”, “Other Tracers”, along with Boolean operators such as AND, OR and NOT were included.
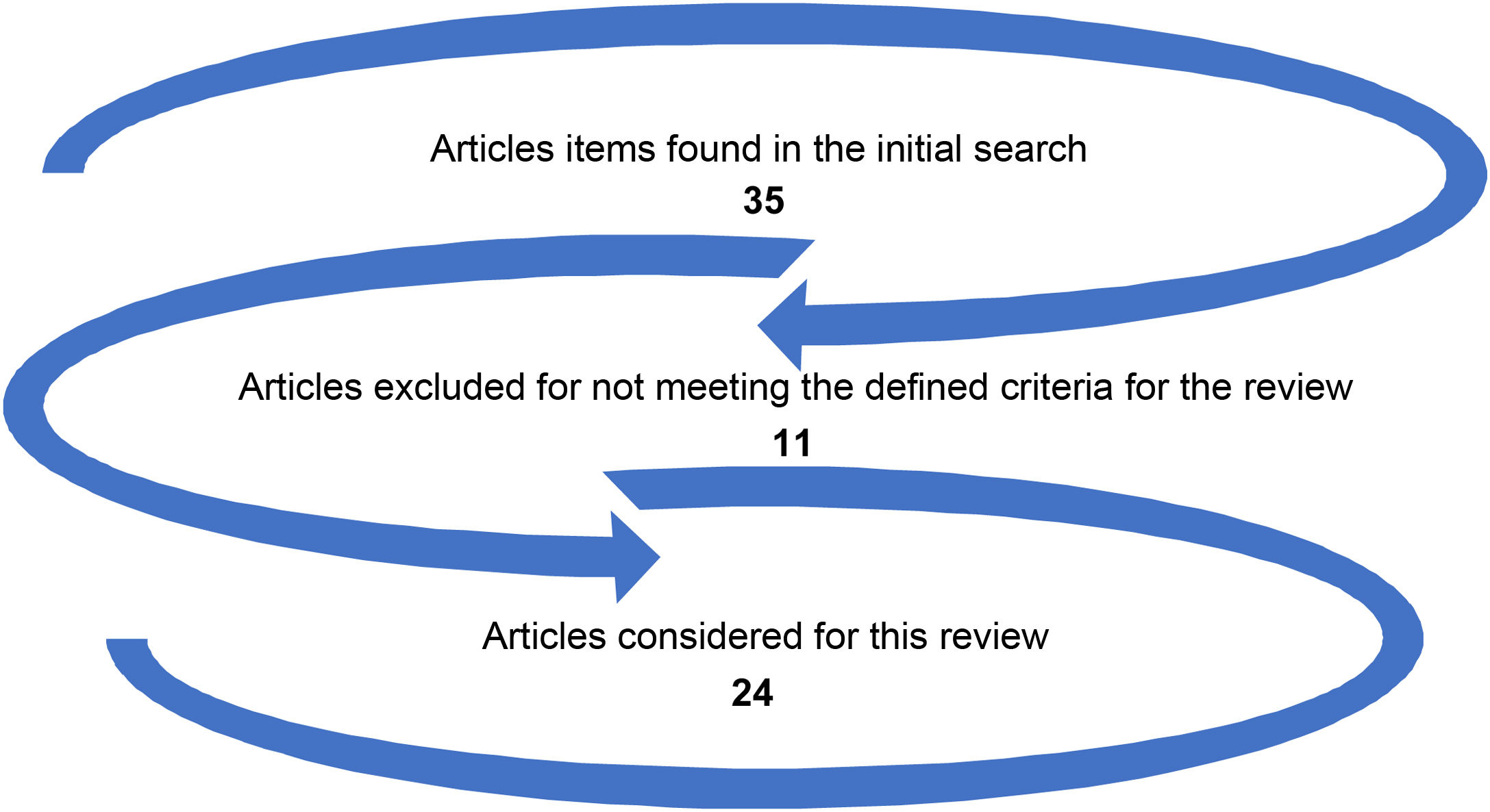
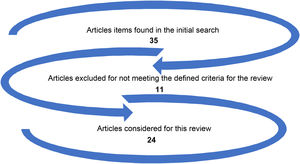
For article choice, the background, methods, results, and discussion were considered, in addition to bibliographic references. Fig. 1 depicts the election of manuscripts for this review.
Physiopathology of the use of 2-(18F) FDG PET/CT in the inflammatory disorder of autoimmune originInflammation is a tissue process made up of a series of phenomena that are generated in the face of an agent that causes tissue damage5.
Within the inflammatory cascade, initially, the damaged tissue generates tissue hyperemia with a subsequent increase in vascular permeability and release of inflammatory mediators, which leads to an increase in blood perfusion. It is at this point that 2-(18F) FDG PET/CT is useful, since a greater release of FDG is observed at the site of the injury, because as the inflammatory cells are recruited, they migrate and proliferate in the place that presents inflammatory changes, and release large amounts of cytokines, with upregulation of GLUT1 and GLUT3. In addition, the activity of hexokinase (subtype A) is increased, which leads to an augmentation in glucose consumption and a consequent FDG uptake in inflammatory cells1,6.
Clinical use of 2-(18F) FDG PET/CT in systemic lupus erythematosusIn 2019, the European League Against Rheumatism (EULAR) and the American College of Rheumatology (ACR) modified the SLE classification criteria, within which 2-(18F) FDG PET/CT was not included as a diagnostic tool7.
Despite the above, in 2020 the Japanese Society of Nuclear Medicine published an expert consensus based on the guidelines indicated in 2013 by the Society of Nuclear Medicine and Molecular Imaging (SNMMI) and the European Association of Nuclear Medicine (EANM)4,8,9, as well as in studies published later to carry out 2-(18F) FDG PET/CT studies in inflammation and infection, using evidence-based medicine scales. This consensus considered that the role of this diagnostic tool in SLE would be level IA when the etiological diagnosis was fever of unknown origin (FUO) or inflammation of unknown origin (IUO), and level IIIC to establish a differential diagnosis of SLE that would guide medical management1.
The following sections discuss the findings of 2-(18F) FDG PET/CT in the various organ involvements of SLE.
2-(18F) FDG PET/CT findings in neuropsychiatric systemic lupus erythematosusPsychiatric and neurological manifestations in SLE occur in 15%–75% of patients affected by this disease10 and affect multiple cognitive domains, which generates high morbidity and decreased quality of life in the patient11.
Within the pathophysiology, neuronal damage is due to the chronic neurotoxic effects of autoantibodies against N-methyl-d-aspartate receptors, ribosomal P antigen, and neuronal surface, in addition to the direct effects of cytokines11,12.
2-(18F) FDG PET/CT allows evaluation of brain metabolism. According to pathophysiology descriptions, different studies carried out on these patients agree on the documentation of hypometabolism in the frontal, temporal, parietal, and occipital lobes10, in addition to hypermetabolism in the temporal lobe towards the hippocampus region, the orbitofrontal cortex, and the basal ganglia. In symptomatic patients, hypometabolism is observed in at least two brain regions and the cerebellum8,10–12.
2-(18F) FDG PET/CT findings in SLE-associated myocardial involvementMyocardial involvement by SLE, also called lupus myocarditis, occurs in up to 57% of the patients13.
The pathogenesis of this type of manifestation involves the presence of antibodies against the myocardium, which triggers an inflammatory reaction that in late stages can lead to fibrosis14. Similarly, literature reports that cardiovascular signs and symptoms are clinically evident in the late stages; that is why diagnostic images are required for early assessment of this complication.
In studies conducted with 2-(18F) FDG PET/CT in subjects with lupus myocarditis, it has been reported that the affected areas present hypermetabolism with an active inflammatory process; additionally, it is possible to make objective evaluations of the changes due to the availability of quantitative measures of inflammation (standardized uptake value –SUV–)13,14.
2-[18F] FDG PET/CT findings in SLE-induced lung affectionPulmonary involvement occurs in up to 80% of SLE patients15. The pathophysiology is related to antibodies generated in SLE that affect the pulmonary vasculature leading to vasculitis, thrombosis, and interstitial pulmonary fibrosis that can conduct to pulmonary hypertension15,16.
Hypermetabolism has been described in studies performed with 2-[18F] FDG PET/CT in the affected lung parenchyma when the disease is active, with high SLEDAI scores16.
2-(18F) FDG PET/CT findings in other systemic lupus erythematosus involvementMesenteric panniculitis is an infrequent inflammatory disease of the adipose tissue of the mesentery that occurs in various disorders, SLE among them. It may correspond to an incidental finding in 2-(18F) FDG PET/CT, after finding multiple hypermetabolic foci in the mesenteric fat17.
Another infrequent finding in SLE is the presence of adenopathies, which can be identified in up to 5%, showing a generalized increase in 2-(18F) FDG uptake in different lymph node chains in patients with active SLE, as well as thymic uptake8,18.
Similarly, it has been described that patients with SLE who have undergone 2-(18F) FDG PET/CT may present a diffuse increase in bone marrow uptake in the context or not of hematological alterations19.
For subcutaneous cellular tissue, the presence of panniculitis in the context of SLE can be a frequent finding on 2-(18F) FDG PET/CT, which at this level manifests as hypermetabolism20.
Potential false positives of 2-(18F) FDG PET/CT in the context of systemic lupus erythematosusMultiple inflammatory diseases can reveal findings like those described in SLE when 2-(18F) FDG PET/CT is used, among which are reactive follicular hyperplasia, Kikuchi-Fujimoto disease, as well as inflammation and lymphoid hyperplasia of the spleen21.
Other types of PET/CT tracers in systemic lupus erythematosusIn 2010 Alexánderson et al. carried out a case-control study in which they used (13N) ammonium in 32 female patients diagnosed with SLE to determine the presence of endothelial dysfunction and found that those who were cardiovascular asymptomatic in the absence of active disease presented abnormal coronary flow22.
On the other hand, (68Ga) Ga-deferoxamine-folate and (18F)1,4,7-triazacyclononane-N,N′,N″-folate, conjugated with triacetic acid (18F-FOL) PET/CT have been used, experimentally, to evaluate active states of autoimmune diseases such as SLE, based on the principle that active macrophages overexpress folate receptors23,24.
ConclusionSLE is an inflammatory disease of autoimmune origin in which 2-(18F) FDG PET/CT is clinically useful to determine the extension of organic affection and, potentially, as an evaluator of response to treatment.
Conflict of interestsThe authors declare the absence of a conflict of interest.