There is limited information on outcome, complications and treatments of critically ill COVID-19 patients requiring admission to an intensive care unit (ICU). The aim of this study is to describe the clinical ICU course, treatments used, complications and outcomes, of critically ill COVID-19 patients admitted in seven ICU in Galicia region during the 2020 March–April pandemic peak.
MethodsBetween March 21 and April 19, 2020, we evaluated critically ill COVID-19 patients admitted to the ICU of Anesthesia of seven hospitals in Galicia, northwestern Spain. Outcome, complications, and treatments were monitored until May 6, 2020, the final date of follow-up.
ResultsA total of 97 critically ill COVID-19 patients were included. During ICU stay, mechanical ventilation became necessary in 80 (82.5%) patients, and tracheostomy in 22 (22.7%) patients. Prone position was used frequently in both intubated (67.5%) and awake (27.8%) patients. Medications consisted of antivirals agents (92.7%), corticosteroids (93.8%), tocilizumab (57.7%), and intermediate or high doses of anticoagulants (83.5%). The most frequent complications were ICU-acquired infection (52.6%), thrombosis events (16.5%), and reintubation (9.3%). After a median follow-up of 42 (34–45) days, 15 patients (15.5%) deceased, 73 patients (75.2%) had been discharged from ICU, and nine patients (9.3%) were still in the ICU.
ConclusionsA high proportion of our critically ill COVID-19 patients required mechanical ventilation, prone positioning, antiviral medication, corticosteroids, and anticoagulants. ICU complications were frequent, mainly infections and thrombotic events. We had a relatively low mortality of 15,5%.
Existe poca información sobre la evolución, complicaciones y los tratamientos recibidos por los pacientes críticos con COVID-19 que requieren ingreso en una unidad de cuidados intensivos (UCI). El objetivo de este estudio es describir la evolución clínica, los tratamientos utilizados, las complicaciones y resultados de pacientes críticos COVID-19 ingresados en siete UCI de Anestesiología en la Región de Galicia durante el pico de la pandemia en marzo-abril 2020.
MétodosEntre el 21 de marzo y el 19 de abril de 2020 evaluamos todos los pacientes críticos COVID-19 ingresados en las UCI de Anestesiología de siete hospitales en Galicia, en el Noroeste de España. Los resultados, complicaciones y los tratamientos administrados se registraron hasta el 6 de Mayo de 2020, fecha final del seguimiento.
ResultadosUn total de 97 pacientes críticos COVID-19 fueron incluidos. Durante su estancia en UCI, 80 pacientes (82,5%) necesitaron ventilación mecánica, y 22 pacientes (22,7%) traqueotomía. El decúbito prono se usó frecuentemente en pacientes intubados (67,5%) y despiertos (27,8%). Las medicaciones usadas fueron antivirales (92,7%), corticoides (93,8%), tocilizumab (57,7%), y dosis intermedias y altas de anticoagulantes (83,5%). Las complicaciones más frecuentes fueron infecciones adquiridas en UCI (52,6%), eventos trombóticos (16,5%), y reintubationes (9,3%). Tras un seguimiento medio de 42 (34–45) días, 15 pacientes fallecieron (15,5%), 73 pacientes (75,2%) habían sido dados de alta de UCI y nueve pacientes (9,3%) permanecían todavía en la unidad.
ConclusionesUn alto porcentaje de nuestros pacientes críticos COVID-19 requirieron ventilación mecánica, posición prona, medicaciones antivirales, corticoides y anticoagulantes. Las complicaciones en UCI fueron frecuentes, principalmente infecciones y eventos trombóticos. Tuvimos una mortalidad relativamente baja del 15,5%.
Since its emergence in Wuhan, China, in December 2019, the new coronavirus (SARS-CoV-2) infection has spread rapidly through China and many other countries.1,2 On 31 January 2020, Spain confirmed its first patient with COVID-19, and Galicia, a small region located in the Northwest of the country, did so approximately 1 month later. The number of serious infections increased steadily in Galicia, and by 28 March 2020 many patients had been admitted to the ICU.
Several studies have provided data on critically ill patients with COVID-19.3–14 Most describe the clinical characteristics of patients with COVID-19 in the hospital or ICU, the percentage of patients requiring mechanical ventilation, and mortality rates.4–14 However, many of these studies only included a brief follow-up. Furthermore, they do not provide data on the medications administered, such as anticoagulants, antibiotics, antiviral agents, or corticosteroids, nor on complications such as infections, thromboembolic events, barotrauma or reintubations.
Therefore, we performed an observational, prospective, multicentre study in critically ill patients with COVID-19 infection who required admission to an Anaesthesiology ICU in 7 different hospitals in Northwest Spain. Our objective was to evaluate the clinical course of these patients in the ICU, their outcomes, complications, and the treatments used.
MethodsBetween 21 March and 19 April 2020, we prospectively evaluated all critically ill patients with COVID-19 admitted to the Anaesthesia ICUs of 7 hospitals in Galicia, Northwest Spain. Clinical outcomes were monitored until 6 May 2020, the last date of follow-up. A confirmed case of COVID-19 was defined as a positive result in a reverse transcriptase polymerase chain reaction (RTPCR) test.15 The Galicia Research Ethics Committee (code 2020-188) approved the study, and due to the rapid appearance of this infectious disease the need for informed consent from patients was waived.
The following data were collected from all patients upon admission to the ICU: age, sex, height, concomitant diseases and background therapies. We also calculated their acute physiology and chronic health assessment II (APACHE II) score, and measured arterial partial pressure of oxygen (PaO2), percentage of inspired oxygen (FiO2), PaO2/FiO2 ratio, and initial laboratory tests (complete blood count, blood chemistry, myocardial enzymes, interleukin-6, serum ferritin, procalcitonin, lactate dehydrogenase, d-dimer, C-reactive protein). We also determined whether the patients presented coexisting infections, the time from onset of initial symptoms to admission to hospital and ICU, and performed a chest radiograph.
During their ICU stay, we noted their overall outcome, their medication (vasopressors, antibiotics, antivirals, corticosteroids, anticoagulants, etc.), the mode of respiratory support used (invasive mechanical ventilation, non-invasive ventilation, high-flow nasal oxygen therapy), the use of extracorporeal membrane oxygenation (ECMO), the use of renal replacement therapy, the use of prone positioning in awake or intubated patients, the need for tracheostomy for prolonged mechanical ventilation (surgical tracheostomy versus percutaneous dilatation tracheostomy), peak laboratory values during their ICU stay (interleukin-6, serum ferritin, procalcitonin, lactate dehydrogenase, d-dimer, C-reactive protein), complications (ICU-acquired infection, thromboembolic complications, need for reintubation, acute kidney injury requiring renal replacement therapy, pneumothorax, readmission to ICU, etc.) and outcomes in the ICU, including the number of patients who died, were discharged, and those that were still in the unit at the end of follow-up on 6 May 2020.
The authors designed the trial, collected the data, and performed the analysis. All authors reviewed the manuscript, vouched for its accuracy and the integrity of the data, and approved the decision to submit the manuscript for publication.
Statistical analysisThe main objective of the study was to describe the clinical course of critically ill patients with COVID-19 admitted to the Anaesthesia ICUs in Galicia during the peak of the pandemic between March and April 2020, the treatments used, the complications, and the outcomes. Patients and follow-up data were collected until more than 85% of COVID-19 patients experienced the primary end point criteria (died in the ICU or were discharged from the unit). For the secondary end point, the characteristics of patients who survived vs. patients who died during admission to the ICU were compared.
Numerical variables are described by median and interquartile range. Categorical variables are described as frequency and percentage. Based in Levene’s test, the nonparametric null hypothesis was tested because the continuous variables are heteroscedastic, not categorical. Numerical and categorical variables were compared using the Wilcoxon or Kruskal–Wallis paired tests, and the factorial variables were compared with Pearson’s χ2 test. All the p values obtained were penalized with the Benjamini–Hochberg procedure. All analyses were carried out in R v.3.6®.
ResultsBetween 21 March and 19 April 2020, 97 critically ill patients with COVID-19 were admitted to the Anaesthesia ICUs of 7 hospitals in Northwest Spain. Table 1 shows the characteristics of the patients, their concomitant diseases, chronic medication, and the evaluation of the chest X-ray at the time of admission to the ICU. The median age of the patients was 69 (61–73) years. Advanced age was associated with death during the ICU stay (Table 1). The most frequent concomitant pathologies were arterial hypertension (56.7%), dyslipidaemia (43.3%) and obesity (39.2%). A chest radiograph was obtained from all patients, and 85.6% showed bilateral patchy infiltrates. The values of the laboratory tests on admission to the ICU and the peak levels obtained during the ICU stay are shown in Table 1. A lower lymphocyte count at admission and higher procalcitonin levels during ICU stay were associated with a poor prognosis.
Clinical characteristics and laboratory parameters on admission to the ICU and highest values during ICU stay of the 97 study patients.
| All patients (n=97) | Deaths in ICU (n=15) | Discharged from the ICU (n=73) | Still admitted to ICU (n=9) | p* | |
|---|---|---|---|---|---|
| Demographic parameters | |||||
| Age, years | 69 [61–73] | 72 [70–76] | 68 [58–71] | 74 [68–75] | 0.00928 |
| Men, n (%) | 62 (63.9%) | 12 (80%) | 45 (61.6%) | 5 (55.5%) | 0.61 |
| Weight, kg | 81 [79–92] | 81 [77–86] | 81 [74–95] | 80 [72–87] | 1 |
| Height, cm | 167 [160–171] | 170 [161–173] | 167 [160–171] | 162 [160–168] | 0.92 |
| BMI, kg/m2 | 29.4 [27.1–32.8] | 29.4 [27.9–31] | 29.1 [26.3–33.4] | 30 [29.3–31.2] | 1 |
| Disease history | |||||
| Hypertension | 55 (56.7%) | 13 (86.6%) | 37 (50.7%) | 5 (55.5%) | 0.1 |
| Dyslipidaemia | 42 (43.3%) | 10 (66.6%) | 28 (38.3%) | 4 (44.4%) | 0.27 |
| Diabetes | 22 (22.7%) | 5 (33.3) | 16 (21.9%) | 1 (11.1%) | 0.87 |
| Asthma | 7 (7.2%) | 1 (6.6%) | 4 (5.5%) | 2 (22.2%) | 1 |
| COPD | 11 (11.3%) | 3 (20%) | 8 (10.1) | 0 | 0.9 |
| Heart disease | 28 (28.9%) | 7 (46.6%) | 19 (26%) | 2 (22.2%) | 0.46 |
| Cancer | 7 (7.2%) | 1 (6.6%) | 5 (6.8%) | 1 (11.1%) | 1 |
| Obesity | 38 (39.2%) | 5 (33.3%) | 30 (41.1%) | 3 (33.3%) | 1 |
| Background therapy | |||||
| ACE inhibitors | 29 (29.9) | 7 (46.6%) | 20 (27.4%) | 2 (22.2%) | 0.57 |
| Anticoagulants | 7 (7.2) | 3 (20%) | 4 (5.5) | 0 | 0.45 |
| Antiplatelet agents | 21 (21.6) | 6 (40%) | 13 (17.8%) | 2 (22.2%) | 0.27 |
| Statins | 36 (37.1) | 10 (66.6%) | 22 (30.1) | 4 (44.4%) | 0.08 |
| Beta-blockers | 14 (14.4) | 6 (40%) | 7 (9.6%) | 1 (11.1%) | 0.05 |
| Corticosteroids | 6 (6.2) | 3 (20%) | 3 (4.1%) | 0 | 0.3 |
| Bronchodilators | 9 (9.3%) | 1 (6.6%) | 8 (10.9%) | 1 (11.1%) | 1 |
| Radiological findings | |||||
| Localized infiltrates | 8 (8.2%) | 1 (6.6%) | 7 (9.6%) | 0 | 0.9 |
| Bilateral infiltrates | 83 (85.6%) | 13 (86.6%) | 63 (83.3%) | 6 (66.6%) | |
| Consolidations | 5 (5.1%) | 1 (6.6%) | 1 (1.4%) | 0 | |
| Others | 1 (1%) | 0 | 1 (1.4%) | 3 (33.3%) | |
| Labs at admission to ICU | |||||
| Leukocytes | 7500 [5600–11,610] | 6660 [4965–7705] | 7500 [5600–11,540] | 14,450 [12,470–16,040] | 0.47 |
| Lymphocytes | 600 [400–960] | 460 [320–520] | 640 [460–1000] | 370 [270–960] | 0.02 |
| C-reactive protein, mg/L | 14.9 [10.3–34.7] | 25 [11.8–43.2] | 14.9 [10.5–32] | 13.8 [9.5–17.1] | 0.75 |
| d-dimer, ng/mL | 1204 [7523 2159] | 1,199 [649–1837] | 1208 [763–2482] | 873 [746–2151] | 0.75 |
| Lactate dehydrogenase, U/L | 454 [361–633] | 517 [385–670.5] | 453 [362–632] | 407 [352 536] | 0.9 |
| Serum ferritin, μg/L | 900 [579–1422] | 1,197 [758–1350] | 894 [566–1478] | 776 [565–1017] | 0.66 |
| Interleukin-6, pg/mL | 63 [36.4–110.5] | 74.8 [42.4–97.5] | 60 [39–102] | 345 [182–507] | 0.98 |
| Procalcitonin, ng/mL | 0.1 [0.1–0.3] | 0.2 [0.1–1.4] | 0.1 [0.1–0.3] | 0.1 [0.1–0.3] | 0.11 |
| Procalcitonin, ≥0.05ng/mL | 21 (21.6%) | 7 (46.6%) | 13 (17.8%) | 1 (11.1%) | 0.14 |
| Peak value during ICU stay | |||||
| C-reactive protein, mg/L | 22 [15.4–37.8] | 26 [20.9–51.5] | 20.3 [14.4–35] | 22 [17.1–26.5] | 0.16 |
| d-dimer, ng/mL | 4959 [2231–8508] | 6486 [5170–13,714] | 4456 [2100–7679] | 4182 [2151–13,830] | 0.1 |
| Lactate dehydrogenase, U/L | 572 [418–849] | 663 [505–804] | 550 [418–910] | 416 [352–629] | 0.75 |
| Serum ferritin, μg/L | 1468 [840–2353] | 1620 [1120−2956] | 1500 [840–2353] | 890 [623–1827] | 0.46 |
| Interleukin-6, pg/mL | 159 [58–463] | 170 [60–323] | 129 [55–463] | 409 [254–1869] | 1 |
| Procalcitonin, ng/mL | 0.4 [0.1–1.3] | 2.8 [1.4–23.1] | 0.3 [0.1–0.7] | 0.2 [0.1–0.4] | 0.00126 |
| Procalcitonin, ≥0.05ng/mL | 42 (43.3%) | 13 (86.6%) | 27 (36.9%) | 2 (22.2%) | 0.00814 |
BMI: body mass index; COPD: chronic obstructive pulmonary disease; ICU: Intensive care unit.
Values are shown as number (percentage) or median [interquartile range].
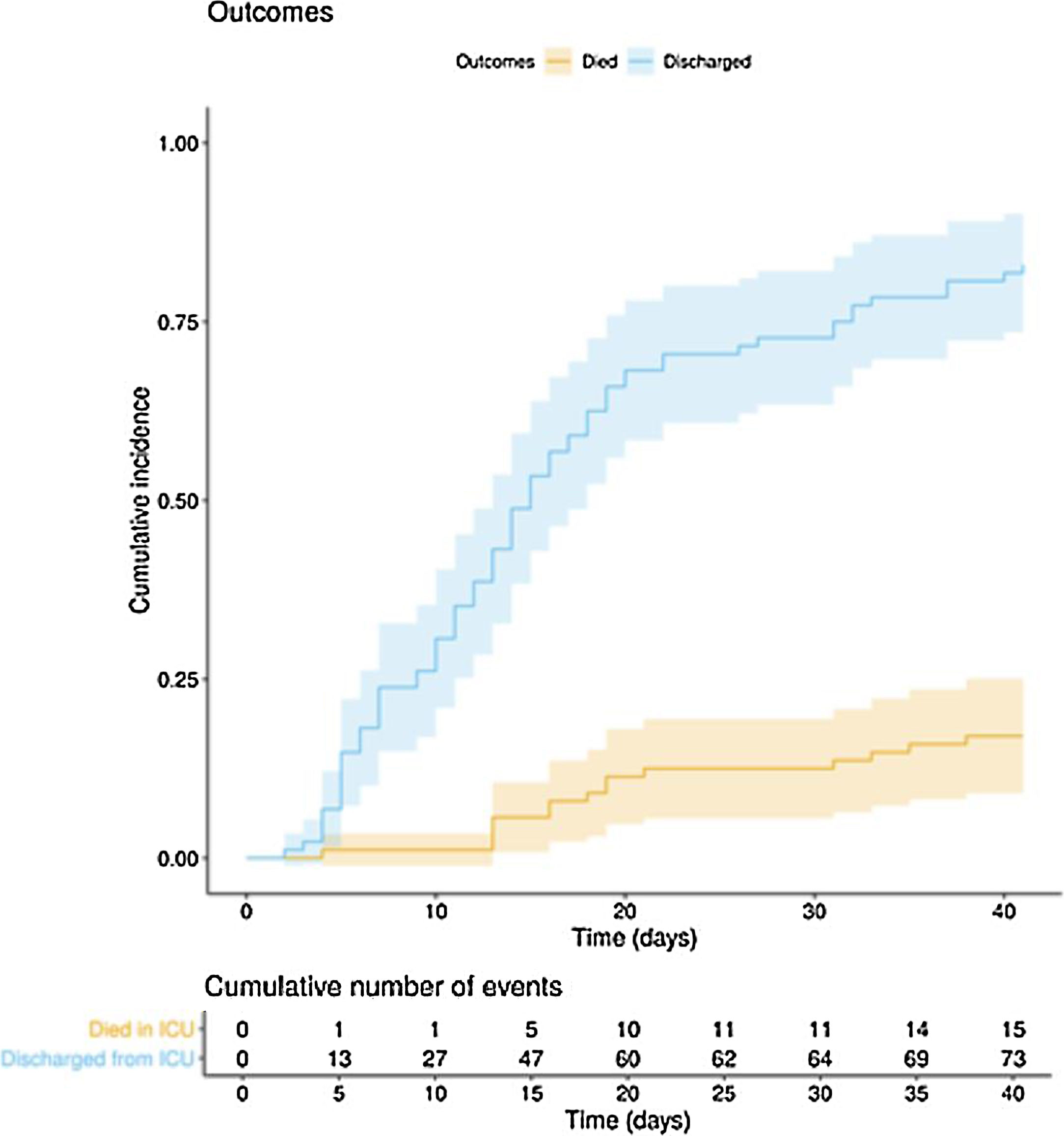
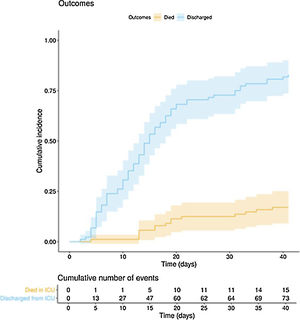
The clinical course and treatment administered during the ICU stay are summarized in Table 2. The median time from onset of symptoms to admission to the hospital and the ICU was 7 (5–10) days and 10 (7–12) days, respectively. The mean APACHE II score in all patients was 15 (12–20). On 6 May 2020, after a median follow-up of 42 (34–45) days, 15 of the total of 97 patients (15.5%) had died, 73 (75.2%) had been discharged from the ICU, and 9 (9.3%) were still in this unit. The cumulative incidence of patients who died in the ICU or were discharged from the ICU is shown in Fig. 1. The mean length of stay in the ICU among non-survivors was 18 (14–28) days. Of the 73 patients discharged from the ICU, 1 died (1.4%) in the hospital outside the ICU, 10 (13.7%) remained hospitalised, and 62 (84.9%) were discharged home. Four patients (5.5%) were readmitted to the ICU during their hospital stay.
Clinical measures and therapies administered to the 97 patients admitted to the Anaesthesia ICU.
| All patients (n=97) | Deaths in ICU (n=15) | Discharged from the ICU (n=73) | Still admitted to ICU (n=9) | p* | |
|---|---|---|---|---|---|
| Time from onset of symptoms to hospital admission, days | 7 [5–10] | 7 [5–9] | 7 [5–10] | 10 [3–14] | 0.92 |
| Time from onset of symptoms to ICU admission, days | 10 [7–12] | 9 [6–11] | 10 [7–12] | 17 [11–17] | 0.75 |
| APACHE II score on day 1 of ICU admission | 15 [12–20] | 17 [14–22] | 14 [11–19] | 21 [16–28] | 0.16 |
| PaO2/FiO2 at ICU admission, mmHg | 128 [100–170] | 111 [75–141] | 130 [101–170] | 115 [89–168] | 0.21 |
| PaO2 at ICU admission, mmHg | 81 [68–98] | 73 [59–92] | 84 [70–99] | 78 [69–90] | 0.51 |
| FiO2 at ICU admission, mmHg | 60 [50–100] | 80 [50–100] | 60 [50–100] | 60 [50–80] | 0.63 |
| Hospital and ICU admission on the same day | 23 (23.7%) | 6 (40%) | 15 (20.5%) | 2 (22.2%) | 0.46 |
| ICU stay, days | 14 [9–21] | 18 [14–28] | 13 [7–19] | 34 [21–35] | 0.11 |
| Co-infection on ICU admission | 20 (20.6%) | 2 (13.3%) | 15 (20.5%) | 3 (33.3%) | 1 |
| Oxygen therapy | |||||
| High flow nasal cannula | 7 (7.2%) | 1 (13.3%) | 6 (8.2%) | 0 | 1 |
| Non-invasive mechanical ventilation | 11 (11.3%) | 0 | 9 (12.3%) | 2 (22.2%) | 0.66 |
| Invasive mechanical ventilation | 80 (82.5%) | 14 (93.3%) | 57 (78.1%) | 9 (100%) | 0.64 |
| Duration of mechanical ventilation, days | 12 [7–18] | 18 [14–28] | 10 [6–14] | 35 [23–37] | 0.00075 |
| Prone positioning in all patients | 56 (57.5%) | 14 (93.3%) | 35 (47.9%) | 7 (77%) | 0.73 |
| Prone positioning in awake patients | 27 (27.8%) | 2 (26.6%) | 21 (28) | 4 (44.4%) | 0.32 |
| Prone positioning in ventilated patients | 54/80 (67.5%) | 12/14 (85.7%) | 39/57 (68.4%) | 3/9 (33.3%) | 0.32 |
| Tracheotomy | 22 (22.7%) | 6 (40%) | 10 (13.7%) | 6 (66.6%) | 1 |
| Surgical tracheotomy | 16 (16.5%) | 3 (20%) | 8 (10.9%) | 5 (55.5%) | 0.9 |
| Percutaneous dilatational tracheotomy | 6 (6.2%) | 3 (20%) | 2 (2.7%) | 1 (11.1%) | 0.16 |
| Time from mechanical ventilation to tracheotomy, days | 16 [12–18] | 17.5 [15–21] | 16 [13–16] | 13 [11–15] | 0.49 |
| Renal replacement therapy | 8 (8.2%) | 3 (20%) | 4 (5.5%) | 1 (11.1%) | 0.45 |
| Extracorporeal membrane oxygenation | 1 (1%) | 0 | 1 (1.4%) | 0 | 1 |
| Extracorporeal CO2 removal | 1 (1%) | 0 | 1 (1.4%) | 0 | 1 |
| Drugs | |||||
| Lopinavir–ritonavir | 90 (92.7%) | 14 (93.3%) | 68 (93.1%) | 8 (88.8%) | 1 |
| Hydroxychloroquine | 96 (98.9%) | 14 (93.3%) | 73 (100%) | 9 (100%) | 0.7 |
| Azithromycin | 93 (95.9%) | 15 (100%) | 69 (94.5%) | 9 (100%) | 1 |
| Interferon B | 38 (39.2%) | 9 (60%) | 26 (35.6%) | 3 (33.3%) | 0.4 |
| Remdesivir | 1 (1%) | 0 | 1 (1.4%) | 0 | 1 |
| Tocilizumab | 56 (57.7%) | 10 (66.6%) | 42 (57.5%) | 4 (44.4%) | 0.95 |
| Corticosteroids | 91 (93.8%) | 14 (93.3%) | 68 (93.1%) | 9 (100%) | 1 |
| Antibiotics | 87 (89.7%) | 13 (86.6%) | 67 (91.8%) | 7 (77.7%) | 1 |
| Neuromuscular blockers | 56 (57.7%) | 13 (86.6%) | 38 (52.1%) | 5 (55.5%) | 0.11 |
| Vasopressors | 63 (64.9%) | 14 (93.3%) | 42 (57.5%) | 7 (77.75) | 0.09 |
| Prophylactic-dose anticoagulants | 16 (16.5%) | 3 (20%) | 12 (16.4%) | 1 (11.1%) | 1 |
| Intermediate-dose anticoagulants | 38 (39.2%) | 5 (33.3%) | 31 (42.4%) | 2 (22.2%) | 0.95 |
| High-dose anticoagulants | 43 (44.3%) | 7 (46.7%) | 30 (41.1%) | 6 (66.6%) | 1 |
APACHE II: acute physiology and chronic health assessment scale II; FiO2: fraction of inspired oxygen; ICU: Intensive care unit; PaO2: arterial partial pressure of oxygen;
Values are shown as number (percentage) or median [interquartile range].
The mean length of ICU and hospital stay was 14 (9–21) and 27 (19–38) days, respectively. During the ICU stay, 80 patients (82.5%) received mechanical ventilation, 63 (64.9%) required vasopressors, and 8 (8.2%) were treated with renal replacement therapy (Table 2). Among those on mechanical ventilation, 14 (17.5%) died, 57 (71.2%) were discharged from the ICU, and 9 (11.2%) remained in the ICU at the end of follow-up. The median duration of invasive mechanical ventilation was 12 (7–18) days. Twenty-two patients (22.5%) required tracheostomy for prolonged mechanical ventilation. Prone ventilation was frequently used in both intubated and spontaneously ventilated patients (Table 2). Most of the 97 patients received antiviral medication, antibiotics, anticoagulants, and corticosteroids (Table 2). Tocilizumab was administered in 56 (57.3%) patients.
Complications are summarized in Table 3. The most frequent complications were ICU-related infections (52.6%), thromboembolic events (16.5%), reintubation (9.3%), acute kidney injury requiring renal replacement therapy (8.2%), and pneumothorax (7.2%).
Complications and outcomes of the 97 COVID-19 patients treated in the Anaesthesia ICU.
| All patients (n=97) | Deaths in ICU (n=15) | Discharged from the ICU (n=73) | Still admitted to ICU (n=9) | p* | |
|---|---|---|---|---|---|
| Pneumothorax | 7 (7.2%) | 2 (13.3%) | 1 (1.4%) | 4 (44.4%) | 0.36 |
| Reintubation | 9 (9.3%) | 1 (6.7%) | 6 (8.2%) | 2 (22.2%) | 1 |
| Intestinal perforation | 2 (2.1%) | 0 | 1 (1.4%) | 1 (11.1%) | 1 |
| Gastrointestinal bleeding | 2 (2.1%) | 0 | 1 (1.4%) | 1 (11.1%) | 1 |
| Acute kidney injury requiring RRT | 8 (8.2%) | 3 (20%) | 4 (5.5%) | 1 (11.1%) | 0.45 |
| Thrombotic events | 16 (16.5%) | 4 (26.6%) | 8 (10.9%) | 4 (44.4%) | 1 |
| Stroke | 2 (2.1%) | 1 (6.7%) | 0 | 1 (11.1%) | 1 |
| Pulmonary embolism | 7 (7.2%) | 2 (13.3%) | 4 (5.5%) | 1 (11.1%) | 0.9 |
| Others | 7 (7.2%) | 2 (13.3%) | 3 (4.1%) | 2 (22.2%) | 0.75 |
| Readmission to ICU | 4 (4.1%) | 0 | 4 (5.5%) | 0 | 1 |
| ICU-related infection | 51 (52.6%) | 12 (80%) | 30 (41%) | 9 (100%) | 0.07 |
ICU: intensive care unit; RRT: renal replacement therapy.
Values are shown as number (percentage) or median [interquartile range].
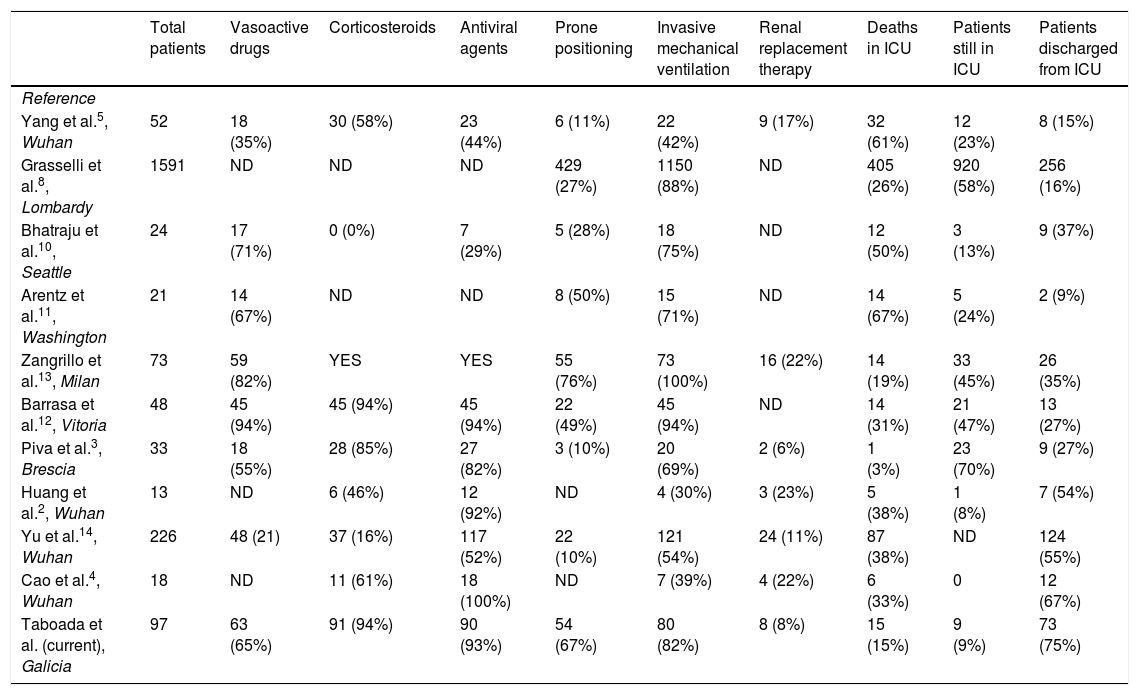
Table 4 summarizes the outcomes and treatments administered in the principle studies performed in critical patients with COVID-19 admitted to ICUs in different countries.
Summary of the therapies administered and outcomes of different studies in critical patients with COVID-19.
| Total patients | Vasoactive drugs | Corticosteroids | Antiviral agents | Prone positioning | Invasive mechanical ventilation | Renal replacement therapy | Deaths in ICU | Patients still in ICU | Patients discharged from ICU | |
|---|---|---|---|---|---|---|---|---|---|---|
| Reference | ||||||||||
| Yang et al.5, Wuhan | 52 | 18 (35%) | 30 (58%) | 23 (44%) | 6 (11%) | 22 (42%) | 9 (17%) | 32 (61%) | 12 (23%) | 8 (15%) |
| Grasselli et al.8, Lombardy | 1591 | ND | ND | ND | 429 (27%) | 1150 (88%) | ND | 405 (26%) | 920 (58%) | 256 (16%) |
| Bhatraju et al.10, Seattle | 24 | 17 (71%) | 0 (0%) | 7 (29%) | 5 (28%) | 18 (75%) | ND | 12 (50%) | 3 (13%) | 9 (37%) |
| Arentz et al.11, Washington | 21 | 14 (67%) | ND | ND | 8 (50%) | 15 (71%) | ND | 14 (67%) | 5 (24%) | 2 (9%) |
| Zangrillo et al.13, Milan | 73 | 59 (82%) | YES | YES | 55 (76%) | 73 (100%) | 16 (22%) | 14 (19%) | 33 (45%) | 26 (35%) |
| Barrasa et al.12, Vitoria | 48 | 45 (94%) | 45 (94%) | 45 (94%) | 22 (49%) | 45 (94%) | ND | 14 (31%) | 21 (47%) | 13 (27%) |
| Piva et al.3, Brescia | 33 | 18 (55%) | 28 (85%) | 27 (82%) | 3 (10%) | 20 (69%) | 2 (6%) | 1 (3%) | 23 (70%) | 9 (27%) |
| Huang et al.2, Wuhan | 13 | ND | 6 (46%) | 12 (92%) | ND | 4 (30%) | 3 (23%) | 5 (38%) | 1 (8%) | 7 (54%) |
| Yu et al.14, Wuhan | 226 | 48 (21) | 37 (16%) | 117 (52%) | 22 (10%) | 121 (54%) | 24 (11%) | 87 (38%) | ND | 124 (55%) |
| Cao et al.4, Wuhan | 18 | ND | 11 (61%) | 18 (100%) | ND | 7 (39%) | 4 (22%) | 6 (33%) | 0 | 12 (67%) |
| Taboada et al. (current), Galicia | 97 | 63 (65%) | 91 (94%) | 90 (93%) | 54 (67%) | 80 (82%) | 8 (8%) | 15 (15%) | 9 (9%) | 73 (75%) |
ND: no data; v: percentage of patients who received invasive mechanical ventilation; YES: administered, but without giving the percentage of patients.
In this multicentre observational study we describe the clinical course in the ICU of 97 critically ill patients with COVID-1: 46 days after the first ICU admission and after a mean follow-up of 42 (34–45) days, 15 (15.5%) patients had died, 73 (75.5%) had been discharged and 9 (9.3%) were still in the ICU.
The mortality rate was similar to that previously reported in a large tertiary hospital in Milan,13 but lower than in other hospitals (Table 4).3,14 The lower mortality rate observed in our study compared to previous studies may be due to several factors.
First, the healthcare system in Northwest Spain was under less pressure than those in Northern Italy,8 Wuhan,2,4,5,14 Seattle10 or Washington11 (Table 4). Doctors in these regions have probably been stretched to the limits of their abilities. The rapid increase in the number of cases frequently exceeded the number of ICU beds or ventilators available, thus reducing the chances of obtaining a good outcome in these critically ill patients.16 In the 7 hospitals participating in this study, only about 70% of ICU beds were occupied, so the doctors were able to work under good conditions with no shortage of resources. Another reason for the lower mortality rate in our patients could be the use of medications such as corticosteroids and anticoagulants, in addition to the early use of protective mechanical ventilation in patients with ARDS and prone positioning in both intubated and spontaneously breathing patients.
Most COVID-19 patients have only mild symptoms; however, some deteriorate and develop respiratory failure due to severe ARDS that requires intubation and mechanical ventilation. In this study, 80 patients (82.5%) required mechanical ventilation after admission to the ICU. Prolonged cycles of prone ventilation (≥16h/day) were used in 67.5% of patients, following the recommendations of the World Health Organization (WHO).15 Prone positioning is a strategy that improves oxygenation and survival in patients with ARDS.15,17,18 A number of explanations have been put forward for this: reduction of ventilation/perfusion mismatch, a more homogeneous distribution of transpulmonary pressure along the ventral-to-dorsal axis compared to supine positioning, and recruitment of nonaerated dorsal lung regions of the lung.19 In our ICU patients, prone positioning was used more frequently (67.5%) than recently described for other similar patient populations: 27% in Lombardy,8 28% in Seattle10 and 11% in Wuhan5,14 (Table 4). This difference might be due to differing workloads in different hospitals or regions. Prone positioning in an intubated patient is complicated, and 4 or 5 people are needed to turn the patient. After achieving significant improvement in oxygenation in initially intubated COVID-19 patients, this manoeuvre was also used to improve oxygenation and avoid intubation in awake patients with moderate or severe ARDS. Many of the mechanisms that explain improved oxygenation in the prone position in intubated patients may also, theoretically, be applicable to non-intubated patients.20,21 Prone positioning is easier to perform in awake patients. Patients can place themselves face down and maintain this position for as long as possible. Patients with COVID-19 and mild to moderate ARDS can be placed prone even outside the ICU, for example in general wards. This will improve oxygenation and reduce the number of ICU admissions in hospitals with a limited number of ICU beds.13
There is currently limited data on the best medications for treating COVID-19 patients, particularly when to start administration. Antiviral, anti-inflammatory, anticoagulant, and immunosuppressive treatments have been suggested. At an early stage of viral infection, that is, in the first 5–8 days from the onset of the disease, antiviral therapy should probably be recommended; later, that is, 8–20 days after the onset of the disease, immunomodulatory agents can be used to reduce the over-active immune response that leads to hyperinflammation.21,22 As patients were admitted to the ICU on average 10 (7–12) days after the onset of symptoms, and the mean length of stay in the ICU was 14 (9–21) days, it is likely that most of our critically ill patients were in the hyperinflammation stage. Therefore, a good approach was to treat the immune response with agents such as tocilizumab and/or a corticosteroid.21,22 In this study, corticosteroids were administered in 93.8% of cases, a similar rate reported in a previous study,13 but higher than other investigations5,10 (Table 4). The WHO15 does not recommend routine use of corticosteroids in COVID-19 patients outside of clinical trials, and the use of corticosteroids in COVID-19 patients is widely debated.23–29 Studies conducted in patients with H1N1 infection and SARS found that corticosteroids reduced mortality rates and shortened the length of hospital stay in critically ill patients.24,25 A recent retrospective study of 201 COVID-19 patients in China revealed that in those with ARDS, methylprednisolone lowered the risk of mortality.7 Therefore, several medical societies and guidelines now recommend the use of corticosteroids in COVID-19 patients with ARDS.24,27,28 Members of the Chinese Thoracic Society have published an expert consensus statement on the use of corticosteroids in 2019-CoV pneumonia.24 The Italian National Institute of Infectious Diseases “Lazzaro Spallanzani” also recommends the use of corticosteroids in patients presenting ARDS.27 Post mortem findings such as pulmonary oedema and hyaline membrane formation support the use of corticosteroids.29 Recently, the RECOVERY Collaborative Group observed that the use of dexamethasone in hospitalized COVID-19 patients receiving invasive mechanical ventilation or oxygen reduced the 28-day mortality rate.30 Future clinical trials will no doubt clarify how these drugs should be administrated, the efficacy of this treatment, the right time to start it, and the right dose and duration of therapy.30,31
Recent studies have reported the presence of thromboembolic events in patients with COVID-19.32–34 Because of this, most of the patients in this study received intermediate or high doses of low molecular weight heparins. Thrombotic complications occurred in 16.5% of cases. In severely ill COVID-19 patients, an increased level of d-dimer or an elevated level of pro-inflammatory cytokines such as IL-6 were associated with a poor prognosis.35 These findings may prove the relationship between thrombosis and inflammation, two mutually reinforcing processes. Several authors have observed that COVID-19 can predispose to venous and arterial thromboembolism due to excessive inflammation, hypoxia, immobilization, and diffuse intravascular coagulation.32–34 The authors of a recent study in twelve patients who died from COVID-1932 found a high incidence of deep vein thrombosis (58%). In one third of their patients, pulmonary embolism was the direct cause of death. Thrombotic complications have been described by various authors. Klok et al.32 reported a 31% incidence of thrombotic complications in ICU patients with COVID-19. Similarly, Helms et al.34, in a multicentre prospective cohort study in 4 participating hospitals observed that 42% of their COVID-19 patients with ARDS developed life-threatening thrombotic complications. In these studies, the authors suggest using higher than normal doses of anticoagulant medication in critically ill COVID-19 patients. In this context, some authors36,37 and various scientific societies38,39 have already made recommendations for antithrombotic therapy in COVID-19 patients. Randomised trials are needed to determine whether systemic anticoagulants provide a survival benefit in hospitalized patients with COVID-19.
This study has some limitations. We only included critically ill patients admitted to the Anaesthesiology ICU of hospitals located in Northwest Spain, where overall pressure on the health system was lower than in other Spanish regions and other countries. Therefore, the results may not reflect the experience of other ICUs in different regions. Furthermore, the study was observational and the interventions described were not part of a randomised controlled trial. Finally, 9 patients (9.3%) were still in the ICU at the end of follow-up – 6 May 2020 – so their final outcome data were not available.
In conclusion, critically ill patients with COVID-19 admitted to the Anaesthesiology ICUs of 7 hospitals in Northwest Spain between March and April 2020 presented a high percentage of complications, mainly infections and thrombotic events. Our mortality rate of 15.5% was relatively low. The most frequent critical care interventions consisted of mechanical ventilation and prone ventilation, together with various different therapies, including antiviral agents, antibiotics, corticosteroids, and anticoagulants. Randomized clinical trials are urgently needed to determine the most effective treatments for critically ill COVID-19 patients.
Final messageIn this prospective multicentre study in critically ill patients with COVID-19 requiring admission to an intensive care unit (ICU), we observed a high rate of complications and a relatively low mortality rate of 15%. A large percentage of our patients received invasive mechanical ventilation, prone positioning, corticosteroids, and anticoagulants.
FundingNo funding was received for this study.
Conflict of interestsThe authors have no conflict of interest to declare.
The authors would like thank the doctors and nurses of the participating hospitals.
Please cite this article as: Taboada M, Rama P, Pita-Romero R, Moreno E, Leal S, Varela M, et al. Pacientes críticos COVID-19 atendidos por anestesiólogos en el Noroeste de España: estudio multicéntrico, prospectivo, observacional. Rev Esp Anestesiol Reanim. 2021;68:10–20.