The infection by the coronavirus SARS-CoV-2, which causes the disease called COVID-19, mainly causes alterations in the respiratory system. In severely ill patients, the disease often evolves into an acute respiratory distress syndrome that can predispose patients to a state of hypercoagulability, with thrombosis at both venous and arterial levels. This predisposition presents a multifactorial physiopathology, related to hypoxia as well as to the severe inflammatory process linked to this pathology, including the additional thrombotic factors present in many of the patients.
In view of the need to optimise the management of hypercoagulability, the working groups of the Scientific Societies of Anaesthesiology-Resuscitation and Pain Therapy (SEDAR) and of Intensive, Critical Care Medicine and Coronary Units (SEMICYUC) have developed a consensus to establish guidelines for actions to be taken against alterations in haemostasis observed in severely ill patients with COVID-19. These recommendations include prophylaxis of venous thromboembolic disease in these patients, and in the peripartum, management of patients on long-term antiplatelet or anticoagulant treatment, bleeding complications in the course of the disease, and the interpretation of general alterations in haemostasis.
La infección por el coronavirus SARS-CoV-2, causante de la enfermedad denominada COVID-19, provoca alteraciones fundamentalmente en el sistema respiratorio. En los pacientes graves, con frecuencia la enfermedad evoluciona a un síndrome de distrés respiratorio agudo que puede predisponer a los pacientes a un estado de hipercoagulabilidad, con trombosis tanto a nivel venoso como arterial. Esta predisposición presenta una fisiopatología multifactorial, relacionada tanto con la hipoxia como con el grave proceso inflamatorio ligado a esta patología, además de los factores trombóticos adicionales presentes en muchos de los pacientes.
Ante la necesidad de optimizar el manejo de la hipercoagulabilidad, los grupos de trabajo de las Sociedades Científicas de Anestesiología-Reanimación y Terapéutica del Dolor (SEDAR) y de Medicina Intensiva, Crítica y de Unidades Coronarias (SEMICYUC), han desarrollado un consenso para establecer unas pautas de actuación frente a las alteraciones de la hemostasia observadas en los pacientes graves con infección por COVID-19. Estas recomendaciones incluyen la profilaxis de la enfermedad tromboembólica venosa en pacientes graves y en el periparto, el manejo de los pacientes en tratamiento crónico con fármacos antiagregantes o anticoagulantes, de las complicaciones hemorrágicas en la evolución de la enfermedad y de la interpretación de las alteraciones generales de la hemostasia.
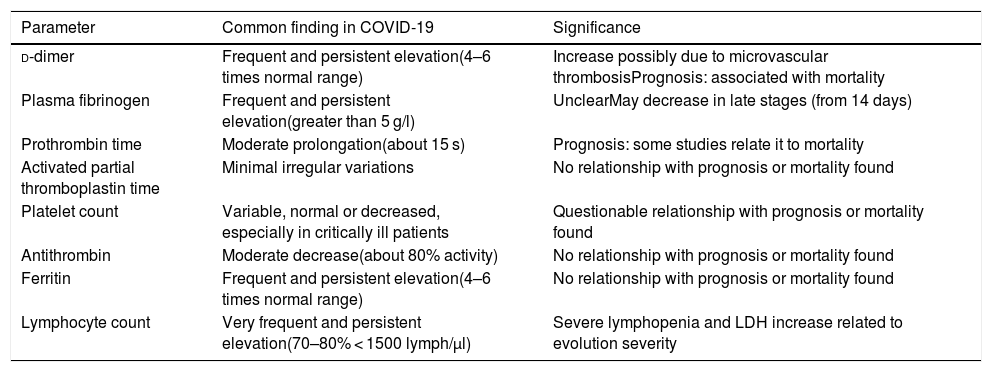
SARS-CoV-2 coronavirus infection, when it progresses to COVID-19, is frequently associated with characteristic alterations of certain haematological parameters, as shown in Table 1.1–7
Frequent haematological changes in patients with COVID-19.
| Parameter | Common finding in COVID-19 | Significance |
|---|---|---|
| d-dimer | Frequent and persistent elevation(4–6 times normal range) | Increase possibly due to microvascular thrombosisPrognosis: associated with mortality |
| Plasma fibrinogen | Frequent and persistent elevation(greater than 5 g/l) | UnclearMay decrease in late stages (from 14 days) |
| Prothrombin time | Moderate prolongation(about 15 s) | Prognosis: some studies relate it to mortality |
| Activated partial thromboplastin time | Minimal irregular variations | No relationship with prognosis or mortality found |
| Platelet count | Variable, normal or decreased, especially in critically ill patients | Questionable relationship with prognosis or mortality found |
| Antithrombin | Moderate decrease(about 80% activity) | No relationship with prognosis or mortality found |
| Ferritin | Frequent and persistent elevation(4–6 times normal range) | No relationship with prognosis or mortality found |
| Lymphocyte count | Very frequent and persistent elevation(70–80% < 1500 lymph/µl) | Severe lymphopenia and LDH increase related to evolution severity |
Several hypotheses have been put forward to explain this pattern of haematological behavior,8 including a hypercoagulable state, a reactivity typical of severe inflammation with endothelitis as a direct consequence of the action of the virus, and the respiratory distress-induced hypoxia frequently found in these cases.9,10 In any event, patients with severe COVID-19 appear to be at high risk of developing cardiovascular complications. Thrombotic events are particularly frequent — some studies have reported an overall incidence of venous thromboembolism of around 25%11 and an incidence of thrombotic complications in critically ill patients of 31%.12 Furthermore, an increase in microvascular thrombosis in the pulmonary circulation could play an important role in the course of respiratory failure.11,13
The presence of previous cardiovascular disease, on the other hand, is a risk factor for severe disease and mortality. In addition, when treating these patients clinicians must take into account that the drugs used to treat COVID-19 might interact with their background treatment, which may need to be adjusted accordingly.
In these circumstances, it is important to establish prophylactic or therapeutic anticoagulant therapy that may benefit these patients. The aim of this study is to put forward recommendations for the management of these patients. Taking into account the lack of available evidence, these recommendation should be reviewed and modified on the basis of clinical experience and the emergence of new evidence.
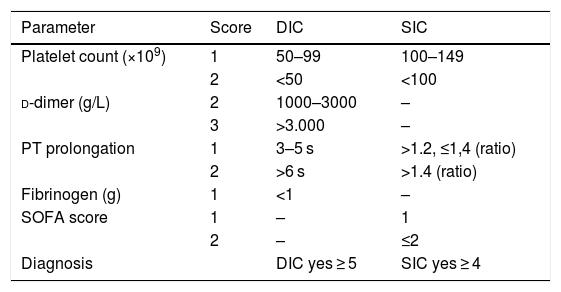
Thrombotic risk in patients with COVID-19An analysis of the characteristic pattern of increased d-dimer and fibrinogen appears to suggest an increase in procoagulant activity leading to an increase in fibrinolytic activity. In terms of pathophysiology, disseminated intravascular coagulation (DIC) can coexist with what has been called “sepsis-induced coagulopathy” (SIC)14–16 (Table 2). The literature is all but silent in this respect, and the presence of DIC has been described in only 8.7% of cases (16 of 183 patients) after applying the d-dimer cut-off points previously proposed for critically ill patients17; however, it was found in 71% of patients who died.2 The d-dimer increase has been associated not only with greater clinical severity (deterioration of respiratory failure), but also with an increase in microclots in the pulmonary vascularisature.18
Rating scale for the diagnosis of DIC and SIC (14–16), with defined cut points for critically ill patients (17).
| Parameter | Score | DIC | SIC |
|---|---|---|---|
| Platelet count (×109) | 1 | 50–99 | 100–149 |
| 2 | <50 | <100 | |
| d-dimer (g/L) | 2 | 1000–3000 | – |
| 3 | >3.000 | – | |
| PT prolongation | 1 | 3–5 s | >1.2, ≤1,4 (ratio) |
| 2 | >6 s | >1.4 (ratio) | |
| Fibrinogen (g) | 1 | <1 | – |
| SOFA score | 1 | – | 1 |
| 2 | – | ≤2 | |
| Diagnosis | DIC yes ≥ 5 | SIC yes ≥ 4 |
Patients with severe disease related to SARS-CoV-2 infection, particularly those admitted to intensive care units (ICU), are usually bedridden, connected to mechanical ventilation, under sedation, and frequently receiving neuromuscular blockers. Furthermore, the hypoxia and inflammatory activity characteristic of this disease can lead to a hypercoagulable state involving multiple mediators, which has been documented in cases of sepsis.19 Therefore, it can be said that these patients are at particularly high risk of developing venous thromboembolism (VTE), and this complication must be evaluated in all cases.11,20 The results of some studies currently suggest a trend towards an increase in VTE among patients with COVID-19.12,21–24 Some studies published so far suggest that these cases can benefit from the administration of low molecular weight heparins (LMWH),2,12 albeit probably only in selected patients. Benefit from LMWH increases in parallel with an increase in D‐dimer levels, regardless of whether or not the patients met DIC criteria.25 In this study, the multivariate analysis concluded that the 3 independent risk factors for 28-day mortality were d-dimer increase, PT prolongation, and low platelet count. The authors also observed lower mortality in patients treated with LMWH or unfractionated heparin (UFH) at 28 days, in those with d-dimer levels more than 6 times the upper limit of normal, or with sepsis-induced coagulopathy (SIC scale ≥4).
There is no evidence to show the superiority of a particular LMWH dose, pending the results of studies currently underway.
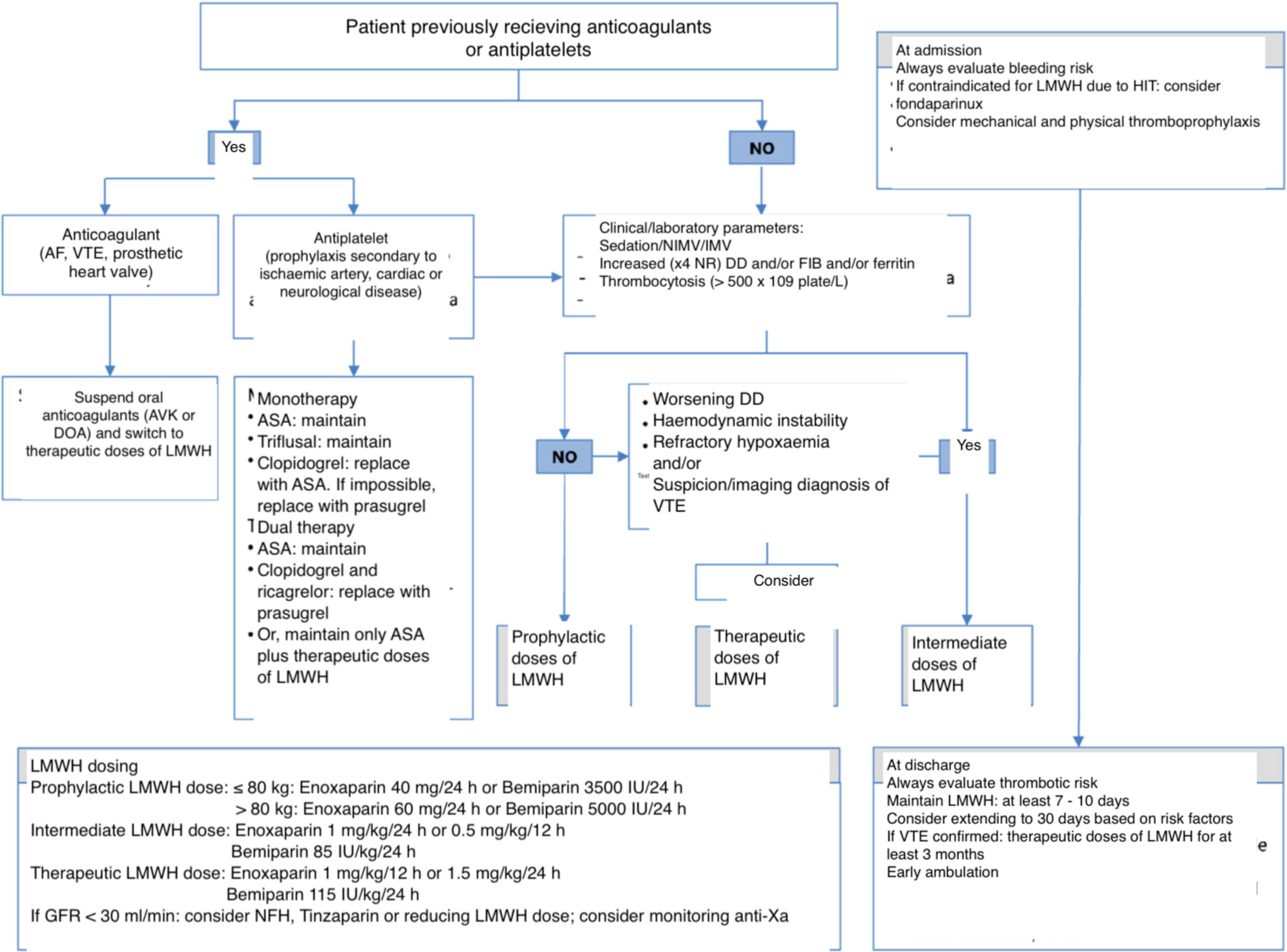
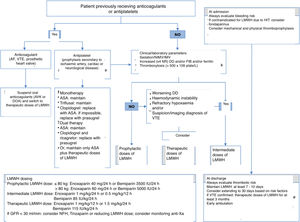
Recommendations (Fig. 1)- -
In hospitalised patients with COVID-19 (Fig. 1), we recommend administering pharmacological thromboprophylaxis, preferably LMWH. We suggest adjusting the dose based on weight and kidney function.
- -
In patients with a history of heparin-induced thrombocytopaenia, we suggest as an alternative the administration of fondaparinux (2.5 mg/24 h subcutaneously) (off label).
- -
In seriously ill patients admitted to the ICU, when plasma levels of some risk markers are clearly elevated with respect to reference values (d-dimer, fibrinogen or ferritin: ×4 the normal range, or platelet count > 500 × 109/l), we suggest increasing the LMWH dose to an extended or intermediate dose regimen (100 IU/kg/24 h).
- -
Although insufficient data are currently available to recommend starting administration of therapeutic anticoagulation doses (150 IU/kg/24 h or 100 IU/kg/12 h) to all patients, we suggest considering this in patients presenting several of the aforementioned markers, or when there are data to indicate thrombosis but confirmatory diagnostic tests cannot be performed.
- -
We recommend considering the possibility of pulmonary thromboembolism in patients with sudden deterioration in oxygenation or sudden drop in blood pressure for no apparent cause.
- -
We recommend performing tests (chest CT-angiography, echocardiography, arterial or venous Doppler), whenever possible, to diagnose arterial or venous thrombosis when there is a significant, progressive or abrupt increase in d-dimer levels, or when clinical suspicion exists.
- -
We recommend starting anticoagulation at therapeutic doses, preferably with LMWH (100 IU/kg/12 h or 1.5 mg/kg/24 h) in patients with a confirmed diagnosis of thrombotic events.
- -
We recommend adjusting dosage and monitoring anti-Xa levels if the estimated glomerular filtration rate (GFR) increases (GFR < 30 ml/min/1.73 m2). In these cases, we recommend considering UFH or we suggest using equivalent doses of tinzaparin.
- -
We suggest monitoring anti-Xa levels in morbidly obese patients (body mass index > 30).
- -
We recommend performing serial d-dimer, fibrinogen and ferritin tests and platelet count and readministering DIC and SIC scales according to the protocol established in each hospital (at intervals of at least 24–72 h).
- -
We recommend not suspending antithrombotic prophylaxis due to laboratory abnormalities in coagulation unless there is active bleeding or a decrease in the platelet count (<30 × 109/l).
- -
In patients at high risk of haemorrhage, mechanical thromboprophylaxis should be prioritised (intermittent pneumatic compression). In these circumstances, we suggest considering viscoelastic tests for additional haemostasis monitoring.
- -
In patients at high risk of thrombosis, we suggest associating mechanical (intermittent pneumatic compression) and pharmacological prophylaxis.
Figure 1.Outline of thromboprophylaxis and management of anticoagulant and antiplatelet drugs in patients with COVID-19 infection.
ASA: acetylsalicylic acid; AF: atrial fibrillation; AVK: antivitamin K; DD: d-dimer; DOA: direct oral anticoagulant; FIB: fibrinogen; GFR: glomerular filtration rate; HIT: heparin-induced thrombocytopaenia; IMV: invasive mechanical ventilation; LMWH: low molecular weight heparin; NR: normal range; NIMV: non-invasive mechanical ventilation; UFH: unfractionated heparin; VTE: venous thromboembolism.
(0.43MB).
Pregnant women have a 4–5 times higher risk of presenting VTE than non-pregnant women due to hormonal and mechanical changes (because the increased size of the pregnant uterus impairs venous return in the lower limbs and pelvis) and decreased mobility secondary to weight gain. Therefore, the risk of thrombosis during pregnancy and the puerperium is usually evaluated, and prophylactic treatment with LMWH is established accordingly.
There are very few data on SARS-CoV-2 infection during pregnancy. However, the possible effect of COVID-19 in pregnant women can be inferred from data on infection by other similar coronaviruses (severe acute respiratory syndrome [SARS-CoV] or middle east respiratory syndrome [MERS-CoV]).
Reports suggest that SARS-CoV-2 infection is associated with a hypercoagulable state, and although no studies have provided sufficient evidence to establish firm recommendations in infected pregnant patients, it has been suggested that these should be based on thrombotic risk factors and lab data that are known predictors of poor evolution. However, for obvious reasons, it is not possible to consider d-dimer in the context of the peripartum, so in this context only fibrinogen (given that fibrinogen levels are usually higher during pregnancy and postpartum, the cut point of thrombotic risk could be 7 g/l instead of 5 g/l), plasma ferritin and platelet count can be considered predictors of a hypercoagulable state
The recommendations for thromboprophylaxis in pregnant women with COVID-19 have been based on a review of recommendations on thrombophilia in pregnant women,26–28 given that antithrombotic therapy is not mentioned in specific studies on the management of pregnant patients.29–31
At the end of pregnancy, both the route and time of delivery should be evaluated individually by a multidisciplinary team.28 According to the multidisciplinary technical document “Management of pregnant patients and infants with COVID-19”,31 if delivery can be performed by caesarean section, neuraxial anaesthesia (spinal, epidural or combined) should be the technique of choice, provided it is not contraindicated.
Thromboprophylaxis in pregnant patients with COVID-19- -
We recommend evaluating thrombotic risk factors during pregnancy and postpartum.
- -
We recommend administering LMWH at weight-adjusted prophylactic doses in all patients with confirmed SARS-CoV-2 infection and no thrombotic risk factors, including asymptomatic patients.
- -
We suggest considering the administration of weight-adjusted LMWH at extended or intermediate doses in all pregnant women with confirmed SARS-CoV-2 infection and thrombotic risk markers (increased plasma ferritin, fibrinogen > 7 g/l or thrombocytosis), based on the estimated degree of risk.
- -
In patients requiring hospital admission, we recommend following the recommendations on thromboprophylaxis for any patient admitted for COVID-19. Treatment should be adjusted in each case according to the risk or the method of delivery (multidisciplinary decision).
- -
If caesarean section is indicated, we recommend using a neuraxial anaesthetic technique. If an epidural is administered, we recommend removing the catheter after caesarean delivery to facilitate anticoagulation treatment and ensure that all patients are monitored carefully to detect early signs indicative of spinal compression.
- -
We recommend restarting antithrombotic prophylaxis with LMWH after delivery, administering the first dose at 6–8 h (depending on the risk of bleeding and the difficulty involved in performing neuraxial puncture).
- -
After delivery, we suggest adjusting the LMWH dose on the basis of the thrombotic risk markers indicated above.
- -
We recommend monitoring thrombotic risk markers (plasma ferritin, fibrinogen, and platelet count). If plasma fibrinogen level are greater than 7 g/l or thrombocytosis is observed (platelets > 500 × 109/l), we suggest considering increasing LMWH to therapeutic doses (100 IU/kg/12 h or 150 IU/kg/24 h).
- -
We suggest maintaining prophylaxis for at least 7–10 days after delivery, and considering prolonging it until the aforementioned thrombotic risk markers have normalised.
- -
We suggest considering prolonging administration of LMWH at prophylactic doses for up to 6 weeks postpartum in pregnant women with thrombotic risk factors have developed SARS-CoV-2 infection with severity criteria in the third trimester.
- -
In patients admitted to the ICU after delivery, we recommend following the treatment guidelines established for other patients.
- -
If pharmacological prophylaxis is contraindicated, we recommend using mechanical methods (intermittent pneumatic compression).
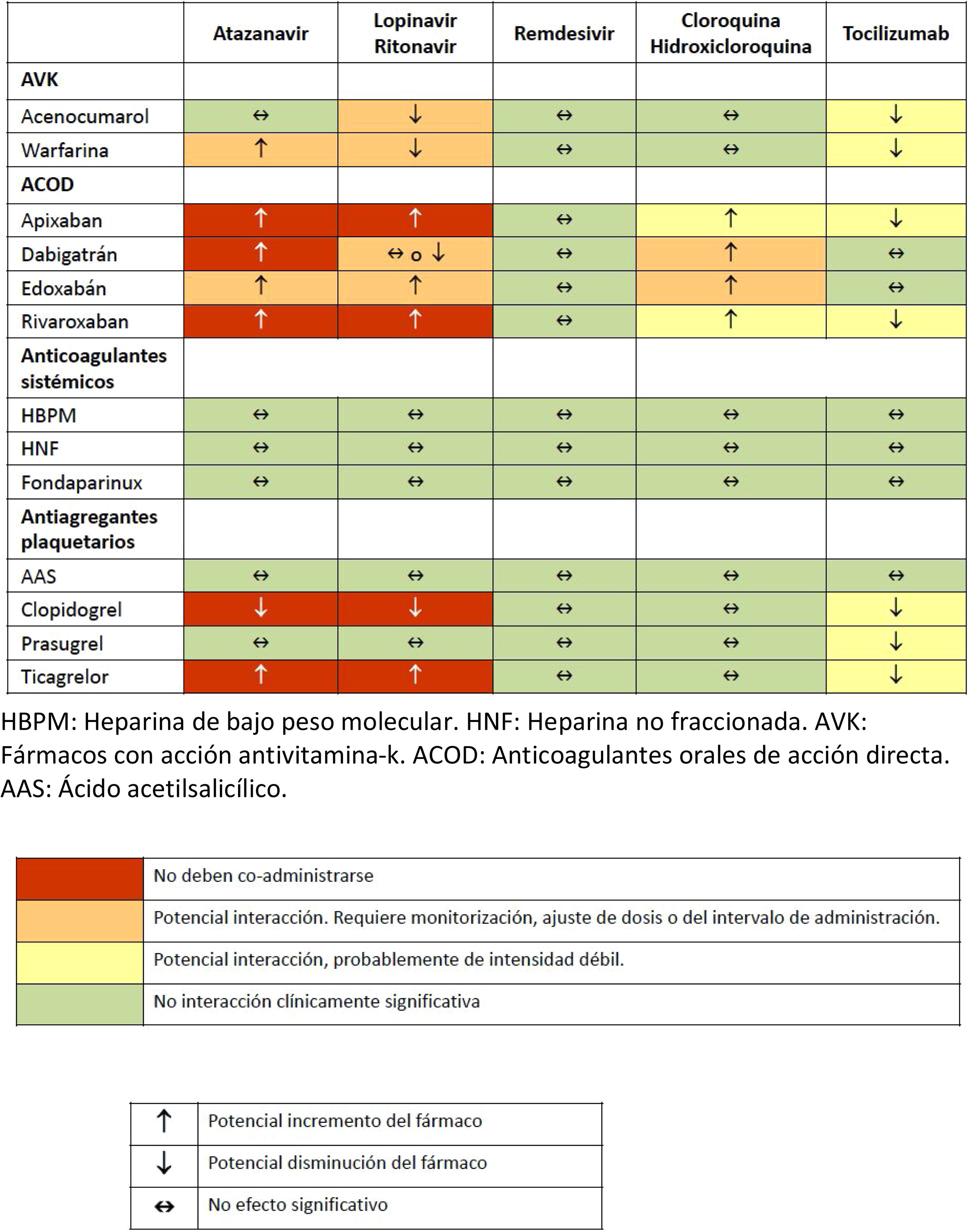
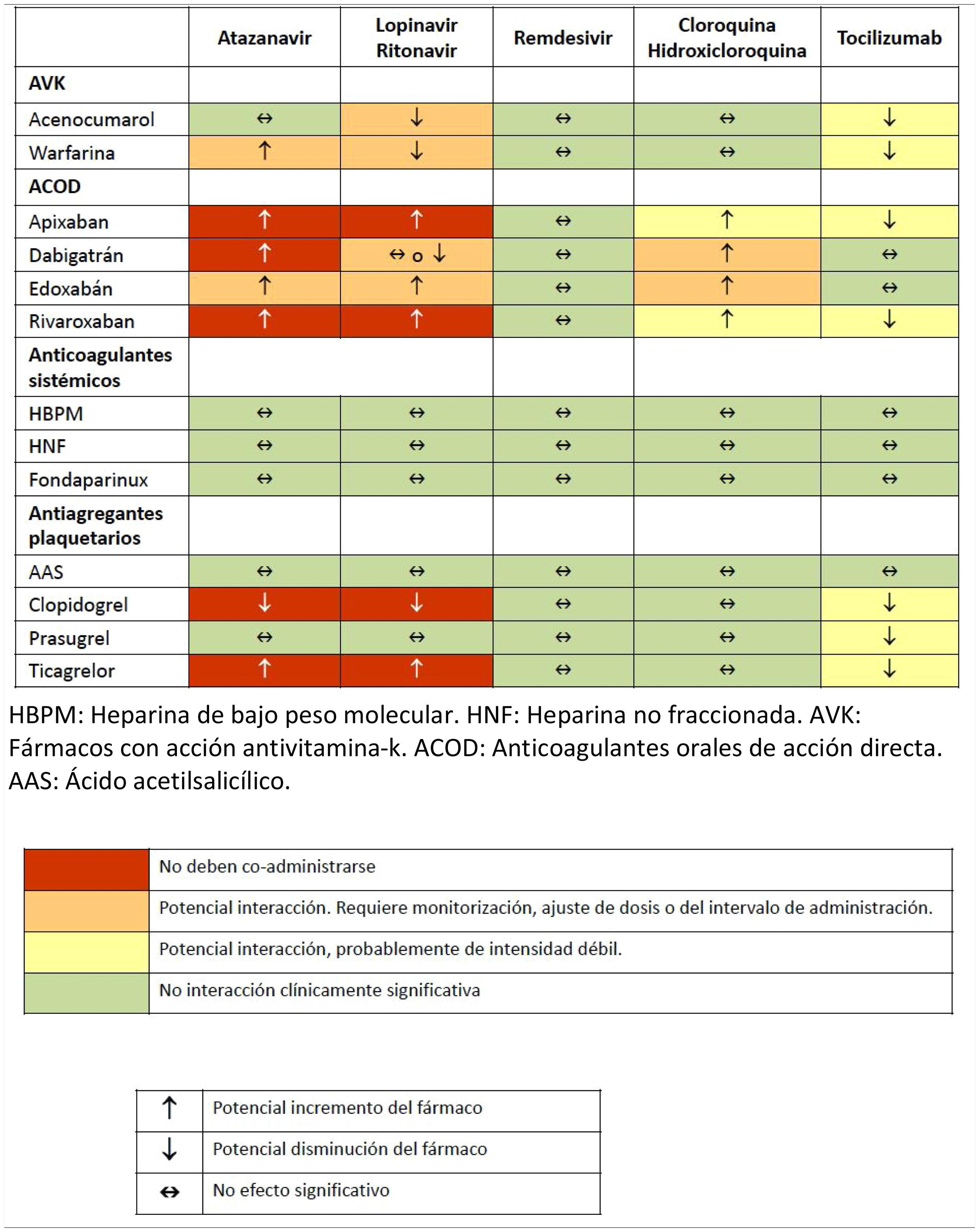
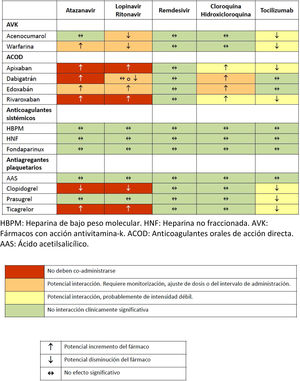
Various pharmacological interactions between anticoagulant or antiplatelet drugs and drugs routinely administered to treat COVID-19 have been described. Table 3 shows the most significant interactions, although more detailed information can be found on the website of reference.32
Drug interactions between anticoagulants and antiplatelet agents and drugs commonly used in the treatment of patients with severe COVID-19 infection.
ASA: acetylsalicylic acid; AVK: drugs with antivitamin K action; DAOA: direct acting oral anticoagulants; DAOC: direct acting oral anticoagulants; LMWH: low molecular weight heparin; UFH: unfractionated heparin.
Source: Interactions with experimental COVID-19 therapies.32
The indications for chronic anticoagulation therapy are well known, and include atrial fibrillation, stroke, recent history of VTE, or mechanical heart valve.33 Patients with COVID-19 infection who are being treated with an oral anticoagulant, either antivitamin K or direct oral anticoagulant, should continue with the therapy regardless of the course of the disease, provided it is not associated with bleeding, and a switch to the parenteral route should be considered.34 Nevertheless, given the large number of interactions between orally administered anticoagulant drugs and drugs used to treat COVID-19 and the changes in their bioavailability in critically ill patients, the anticoagulation regimen should be based on the administration of parenteral drugs. Although both UFH and LMWH can be used, LMWH is preferred because the regimen is easy to manage and anticoagulation monitoring is only required in patients presenting kidney failure.
Recommendations (Table 4)- -
We recommend maintaining Table 4 anticoagulants in patients requiring this therapy, according to their indications.
- -
For anticoagulation maintenance, we do not recommend using drugs with antivitamin K action or direct oral anticoagulants, either due to their interactions or to the difficulty involved in managing or monitoring anticoagulant activity.
- -
We recommend using LMWH at therapeutic doses (10,000 IU/24 h, 150 IU/kg/24 h or 100 IU/kg/12 h) for anticoagulation in these patients. It is impossible to recommend an optimal dosing protocol; instead, the protocol most widely used should be chosen in each case.
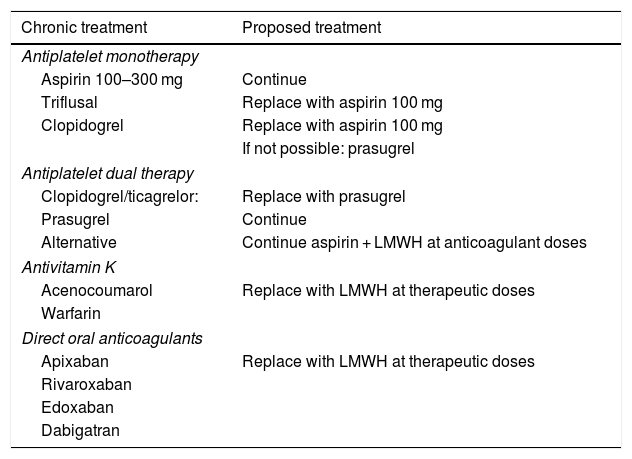
Table 4.Management of antiplatelet drugs and oral anticoagulants.
Chronic treatment Proposed treatment Antiplatelet monotherapy Aspirin 100–300 mg Continue Triflusal Replace with aspirin 100 mg Clopidogrel Replace with aspirin 100 mg If not possible: prasugrel Antiplatelet dual therapy Clopidogrel/ticagrelor: Replace with prasugrel Prasugrel Continue Alternative Continue aspirin + LMWH at anticoagulant doses Antivitamin K Acenocoumarol Replace with LMWH at therapeutic doses Warfarin Direct oral anticoagulants Apixaban Replace with LMWH at therapeutic doses Rivaroxaban Edoxaban Dabigatran
Antiplatelet therapy with acetylsalicylic acid (ASA) should be continued in patients previously receiving it or who develop a disease requiring this therapy. No important interactions between ASA and drugs commonly used in these patients have been described.
However, due to interactions with lopinavir/ritonavir,32 clopidogrel (decreased conversion of the drug to its active metabolite, and therefore decreased pharmacological effect) and ticagrelor (increased plasma concentration) are not recommended. In patients requiring dual therapy (ASA plus a P2Y12 receptor inhibitor), prasugrel, which has fewer interactions, is recommended.
Recommendations (Table 4)- -
ASA: we recommend continuing the ASA regimen started before onset of COVID-19 if indicated for secondary prevention.
- -
Clopidogrel: not recommended due to the potential decrease in its effect when interacting with lopinavir/ritonavir. Consider replacing with prasugrel if necessary.
- -
Ticagrelor: not recommended due to the potential increase in its effect when interacting with lopinavir/ritonavir. If it is administered as monotherapy and there is no contraindication, consider replacing it with ASA or prasugrel.
- -
Prasugrel: recommended instead of clopidogrel or ticagrelor in patients requiring a P2Y12 receptor inhibitor antiplatelet agent.
- -
Dual therapy: in patients requiring dual therapy (ASA) + P2Y12 inhibitor), we suggest maintaining ASA in combination with LMWH at therapeutic doses as an alternative to AAS + prasugrel.
SARS-CoV-2 infection has not so far been associated with a significant incidence of bleeding complications, despite the reported alterations in standard clinical coagulation tests.
PT alterations can generally be classed as minor, since prolongation, if present, has little clinical significance. It is interesting to note that aPTT is within normal ranges in the vast majority of critically ill patients with COVID-19. This suggests that haemostasis is not affected and there is no bleeding tendency; therefore, empirical treatment should not be given on the basis of coagulation tests.
A moderate decrease in plasma antithrombin concentration to around 80% of activity has been observed.2 Given that this decrease does not generally imply a clinically significant alteration, we do not recommend the systematic use of replacement therapy.
The fibrinolysis observed in this clinical setting, which is caused by an almost systematic increase in plasma d-dimer levels, as mentioned above, appears to derive from the lysis of microthrombi in the context of an inflammatory reaction involving multiple cellular and biochemical mediators. In the absence of experience with antifibrinolytics in COVID-19, it would be prudent to discourage the use of any such therapy in COVID-19 patient presenting bleeding.
There is no experience in the use of viscoelastic tests for the management of bleeding complications in seriously ill patients with COVID-19, therefore no recommendations can be established in this scenario. However, in cases of severe acute bleeding, parameters used in patients with severe acute bleeding in other clinical settings can be used to aid in decision-making.
On the basis of the foregoing and on recently published guidelines, we propose the following recommendations.20,35
Recommendations- -
We recommend not administering anticoagulants to correct the results of coagulation tests in the absence of significant bleeding.
- -
If bleeding is present, we suggest administering plasma as the first option to treat bleeding (10−15 ml/kg), provided the PT-ratio is greater than 1.5.
- -
We recommend not administering prothrombin complex concentrate as the first treatment option for bleeding in these patients due to the lack of experience and lack of safety data.
- -
In patients with acute bleeding, we recommend maintaining plasma fibrinogen levels ≥1.5 g/l, preferably administering fibrinogen concentrate or cryoprecipitate instead of fresh plasma.
- -
In patients with acute bleeding, we recommend maintaining a platelet count of at least 50 × 109/l, which is usually sufficient to achieve haemostasis, and we recommend performing platelet transfusion to achieve this level.
- -
We do not recommend administering antithrombin in the context of SARS-CoV-2 infection.
- -
We do not recommend administering tranexamic acid in this context, except in the case of severe bleeding and confirmation of hyperfibrinolysis by viscoelastic tests.
- -
In patients presenting severe acute bleeding, we suggest basing the administration of haemostasis agents on the results obtained from viscoelastic tests, when available.
- -
The use of factor VIIaa is not recommended in patients with COVID-19.
Severe SARS-CoV-2 infection most frequently affects patients with various comorbidities, particularly cardiovascular disease. This means that a considerable percentage of these patients will be receiving antiplatelet or anticoagulant drugs, and these therapies must be properly managed to ensure a good outcome. In addition, changes in haemostasis observed in patients with COVID-19 may imply an increased risk of developing thrombosis, so early administration of LMWH should be part of routine treatment.
Finally, any bleeding complications appearing in these patients should be treated with extreme caution, given their prothrombotic status and the multifactorial aetiology of such complications.
Julián Álvarez (anaesthesiologist), M. Ángeles Ballesteros (intensivist), Misericordia Basora (anaesthesiologist), Ricard Ferrer (intensivist), Inocencia Fornet (anaesthesiologist), Emilia Guasch (anaesthesiologist), Manuel Herrera-Gutiérrez (intensivist), M. Cruz Martín (intensivist), Pablo Monedero (anaesthesiologist), Pilar Paniagua (anaesthesiologist), Azucena Pajares (anaesthesiologist), Juan Carlos Ruiz-Rodríguez (intensivist), Gabriel Tirado (intensivist).
The names of the components of the Reading and Review Committee are listed in Appendix 1.
Please cite this article as: Llau JV, Ferrandis R, Sierra P, Hidalgo F, Cassinello C, Gómez-Luque A, et al. Recomendaciones de consenso SEDAR-SEMICYUC sobre el manejo de las alteraciones de la hemostasia en los pacientes graves con infección por COVID-19. Rev Esp Anestesiol Reanim. 2020;67:391–399.