The Airway Management section of the Spanish Society of Anesthesiology, Resuscitation, and Pain Therapy (SEDAR), the Spanish Society of Emergency Medicine (SEMES), and the Spanish Society of Otorhinolaryngology and Head and Neck Surgery (SEORL-CCC) present the Guide for the comprehensive management of difficult airway in adult patients. Its principles are focused on the human factors, cognitive processes for decision-making in critical situations, and optimization in the progression of strategies application to preserve adequate alveolar oxygenation in order to enhance safety and the quality of care. The document provides evidence-based recommendations, theoretical-educational tools, and implementation tools, mainly cognitive aids, applicable to airway management in the fields of anesthesiology, critical care, emergencies, and prehospital medicine. For this purpose, an extensive literature search was conducted following PRISMA-R guidelines and was analyzed using the GRADE methodology. Recommendations were formulated according to the GRADE methodology. Recommendations for sections with low-quality evidence were based on expert opinion through consensus reached via a Delphi questionnaire.
La sección de Vía Aérea de la Sociedad Española De Anestesiología, Reanimación y Terapéutica del Dolor (SEDAR), la Sociedad Española de Medicina de Urgencias y Emergencias (SEMES) y la Sociedad Española de Otorrinolaringología y Cirugía de Cabeza y Cuello (SEORLCCC) presentan la Guía para el manejo integral de la vía aérea difícil en el paciente adulto. Sus principios están focalizados en el factor humano, los procesos cognitivos para la toma de decisiones en situaciones críticas y la optimización en la progresión de la aplicación de estrategias para preservar una adecuada oxigenación alveolar con el objeto de mejorar la seguridad y la calidad asistencial. El documento proporciona recomendaciones basadas en la evidencia científica actual, herramientas teórico/educativas y herramientas de implementación, fundamentalmente ayudas cognitivas, aplicables al tratamiento de la vía aérea en el campo de la anestesiología, cuidados críticos, urgencias y medicina prehospitalaria. Para ello se realizó una amplia búsqueda bibliográfica según las directrices PRISMA-R y se analizó utilizando la metodología GRADE. Las recomendaciones se formularon de acuerdo con esta metodología. Las recomendaciones de aquellas secciones con evidencia de baja calidad se basaron en la opinión de expertos mediante consenso alcanzado a través de un cuestionario Delphi.
Airway management is the cornerstone of multiple medical procedures.1 According to recent series, the incidence of difficult airway (DA) and failed tracheal intubation has fallen to 1.6 and 0.06 per 1000 cases, respectively,2 although both remain an important cause of morbidity and mortality.3,4
Many care-related complications are avoidable.5 Calculating the incidence of airway management-related complications from data available in national registries and closed claims plays a major role in detecting errors in clinical practice and implementing new strategies to overcome these failings.5–7 Although Spain does not have a register of airway management-related adverse events, approximate figures can be obtained by extrapolating data from other registries, particularly the UK 4th National Audit Project (NAP4),8,9 where the resulting 168 recommendations led to improvements in patient safety.10 Since then, various scientific societies have developed new guidelines, algorithms,11–16 and cognitive aids.17 Despite this, recent studies3,7,19–21 have shown that almost a decade later, many of the original failings persist,4,18 including inadequate evaluation and planning, failure to predict a difficult airway, failure to prepare airway rescue equipment, perseverance in a failed strategy, failure to oxygenate with a supraglottic airway when difficulties arise, and delay in establishing a surgical airway.5,18 This shows the extent to which human (HF) and ergonomics factors contribute to airway-related mismanagement,22,23 and highlights the importance of anticipating and preparing for complications, following guidelines, and stepping up efforts to introduce improvements.4,24
The strategies for addressing difficult airway management are conditioned by the environment, technological resources, and the experience of the involved professionals. Therefore, it is advisable to implement guidelines adapted to the national and institutional care settings,4,18,25 as recommended in the Helsinki Declaration on patient safety in anaesthesiology.26 Current decision-making tools are inadequate because they do not factor in the influence of HF and contextual issues, and promote interventions that may be ineffective and error-prone.23,27 Tracheal intubation (TI) is the primary objective in most algorithms,28 making them more suited to educational or theoretical training than real-world dynamic, stressful clinical situations23,24,29,30 where, according to some studies, they can even have a negative effect on decision making.31,32 Additionally, they exhibit irregular implementation and generally limited adherence.18,30,33 The reason for these findings has been attributed to their complex and inflexible designs, which are sometimes perceived as a barrier to workflow rather than as an aid in emergency situations.34 This raises the need for effective cognitive aids that simplify the transition from one technique to another and ensure35 airway management continuity.
This document provides professionals with a series of evidence-based recommendations and a set of reasonable, practical, decision-making tools to overcome airway management difficulties.
ObjectivesTo put forward the evidence-based recommendations for comprehensive management of the difficult airway in adult patients compiled by the Airway Management Division of the Spanish Society of Anaesthesiology, Reanimation and Pain Therapy (SEDAR), the Spanish Society of Emergency Medicine (SEMES) and the Spanish Society of Otolaryngology and Head and Neck Surgery (SEORL-CCC).
To provide clinicians with a set of rational, practical, tools consisting mainly of context-sensitive cognitive aids that are closely aligned with ergonomics, HFs, and cognitive processes in emergency airway situations. These tools can facilitate decision-making and optimize the implementation of sequential strategies to preserve adequate oxygenation throughout the procedure, reduce the incidence of complications, and improve patient safety and quality of care.
The principles described should not be considered mandatory standards, and given the diversity and contextual complexity, their application does not guarantee success in every situation. The recommendations are flexible, with the professional's clinical judgment always prevailing after an appropriate analysis of the risk-benefit balance in each specific case.
Validity and applicabilityThis guideline provides general recommendations based on the latest clinical evidence and can be applied in any situation and procedure requiring airway management, such as mask ventilation, supraglottic airway ventilation (SGAV) or TI, and by any clinician in charge of airway management.
Our understanding of airway management is constantly growing and new techniques are being developed, so these recommendations will be periodically reviewed and updated.
MethodologyThese guidelines were developed using AGREE II (Appraisal of Guidelines, Research and Evaluation II) criteria.36 The recommendations put forward in this document are supported by evidence obtained from a rapid systematic literature review performed using PRISMA Rapid review (PRISMA-R) criteria.
The topics to be addressed were chosen by the “Spanish Airway Management Group” - a task force made up of 27 members of SEDAR, SEMES and SEORL-CCC from across Spain with expertise in airway management and experience in training and research (anaesthesiologists and specialists in critical care and emergency medicine).
The databases MEDLINE, Embase, Scopus, Web of Science, PubMed, Science Citation Index, and The Cochrane Library were searched for records published between 1 June 2000 and 1 December 2022 using the terms “airway”, “airway management”, “difficult airway”, “tracheal intubation”, “guideline”, “algorithm”, “cognitive aid”, “checklist”, “awake tracheal intubation”, “fiberoptic intubation”, “videolaryngoscopy”, “supraglottic airway” “face mask”, “oxygenation”, “preoxygenation”, “apnoeic oxygenation”, “ventilation failure”, “rapid sequence induction”, “can't intubate can't ventilate”, “airway complications”, “emergency airway”, “front of neck access”, “cricothyrotomy”, “extubation”, “teaching”, “training”, “competence”. The search was limited to literature published in English and Spanish over the past 22 years, exclusively in adult patients. Search terms were used individually and in combination. Randomized controlled clinical trials, case reports, surveys, review articles, and editorials were included.
The GRADE (Grading of Recommendations Assessment, Development, and Evaluation) framework was used to analyse and summarise the data retrieved from the literature search.37 One reviewer (MAGR) screened for duplicates (abstracts and titles) using Rayyan software, following which 3 reviewers (JAS, TL, and AAG) independently performed a full text review to screen for suitability and documented their reasons for rejection. The references of the selected records were checked for relevant articles, and relevant studies published after the cut-off date were also taken into consideration. Evidence from the studies retrieved was uploaded to a summary of findings table and the quality was analysed.37 The resulting findings were formulated into recommendations and classified with their corresponding level of evidence.
Recommendations and justifications were initially drafted and critically reviewed by 4 authors, and were then forwarded to the task force for final review, formulation, classification and consensus during virtual meetings held in February and March 2023.
Recommendations supported by low-quality or practically non-existent evidence were included in a Delphi questionnaire (supplementary data), and those that achieved sufficient consensus were included in the guidelines.
The final text was sent to all group members and external consultants for review. Any further insights contributed at this stage were included in the final version.
The entire process was conducted without any support from industry or any external funding.
Supplementary data 1 shows the GRADE evidence scales.
DefinitionsThere is currently no universally accepted definition of a DA in the literature.38,39 The use of clear, concise, precise terminology is key to improving situational awareness and team communication and to developing cognitive processes and a common mindset that will produce coordinated actions, correct progression through an algorithm, prevent errors, and standardise criteria applied to research and documentation in the field of airway management.38,40–42Supplementary data 2 includes risk factors for the different entities.
Difficult airwayClinical situation in which an operator with conventional training has difficulty performing mask ventilation, SGAV, or TI, which may result in inadequate alveolar oxygenation.
Difficult mask (DMV) or supraglottic airway (DSGAV) ventilationSituation in which adequate ventilation cannot be delivered despite intense neuromuscular blockade (NMB) in the presence of one or more of the following issues: absence of exhaled carbon dioxide or absence of phases II and/or III of the capnography waveform, decreased oxygen saturation or inadequate saturation, absence or inadequacy of spirometric measurements of expired gas flow, incorrect seal, excessive leakage, or excessive resistance to the ingress or egress of gas. Signs of inadequate ventilation include but are not limited to: absence or inadequate movement of the chest, absence or inadequate breath sounds on auscultation, signs of severe obstruction, cyanosis, gastric dilation, and haemodynamic changes associated with hypoxaemia and hypercapnia. (e.g.: hypertension, tachycardia, arrhythmias).
Difficult laryngoscopyGiven the widespread use of videolaryngoscopy in different contexts, it is important makes it appropriate to differentiate between 43,44:
Difficult direct or conventional laryngoscopyA situation in which glottic structures cannot be visualised with the best possible laryngoscopic exposure and optimal conditions (patient position, correct blade, complete NMB, external laryngeal manipulation, or BURP), and is defined by a Cormack-Lehane (CL) grade 3 or 4.
Difficult videolaryngoscopy or indirect laryngoscopyA situation in which no percentage of glottic structures can be visualised with the best possible laryngoscopic exposure and optimal conditions (patient position, correct blade, complete NMB, external laryngeal manipulation, or BURP), and is defined by a POGO (Percentage Of Glottis Opening) 0%, equivalent to C-L grades 3 or 4 with direct laryngoscopy (DL).45
Difficult tracheal intubationIntubation requiring multiple attempts, additional operator(s), devices and/or adjuvants, or manoeuvres to advance the endotracheal tube through the trachea.
The level of difficulty can be quantified and documented using the Intubation Difficulty Scale (IDS) proposed by Adnet et al.,46 or the Fremantle Score,45,47 which includes the degree of laryngeal view, the ease of insertion of the endotracheal tube (ETT), the type of device used, and use of any adjuvants.
Failed tracheal intubationInability to inset an ETT despite several attempt with one or more devices and adjuvants.
Can’t intubate, can’t oxygenate (CICO)Impossible to achieve alveolar oxygenation through non-invasive oxygenation methods (TI, mask ventilation or SGAV) given the impossibility of maintaining a patent upper airway. Restoration of alveolar oxygenation requires invasive front of neck access (FONA).
Difficult front of neck access (DFONA)Difficulty identifying cervical anatomical structures (cricothyroid membrane, CTM) or securing a FONA.
Contextual Difficult AirwayA clinical situation in which a trained operator is not able to perform mask ventilation, or place an SGA or ETT due to complexities involving the patient, pathology, setting, operator, equipment, experience, and circumstances.
Failed attemptAn attempt within a specific airway management plan that does not result in success.
Failed PlanA plan that does not achieve success within 3 attempts.
Difficult tracheal extubationRemoval of an ETT in a patient with known or anticipated difficult airway.
Failed tracheal extubationLoss of airway patency and adequate ventilation after ETT removal.
Short apnoea tolerancePathophysiological state, usually caused by shunt, ventilation/perfusion mismatch or reduced functional residual capacity (FRC), that is manifested by hypoxaemia, little or no effectiveness of periprocedural oxygenation techniques, and/or short safe apnoea time (time from cessation of breathing or ventilation until peripheral arterial oxygen saturation falls to 90%).
Human and ergonomic factorsThe clinical setting is a complex and dynamic socio-technical system in which interactions among human operators, advanced technology, and organizational boundaries affect operational processes and, consequently, care outcomes.23 HFs encompass individual, team, environmental, and organisational factors that affect both decision-making and the overall performance of the system.34 HFs is the scientific discipline concerned with the understanding of interactions among humans and other elements of a system in order to optimise success (efficiency) and reduce errors (safety).48
HFs are as important as technical shortcomings in causing airway management complications.49 According to the NAP4, HFs were present in 40% of the major complications studied, with an average of four contributing HFs per case.50 Accidents usually involve “action errors”, such as omitting a critical task, and are fundamentally due to a lack of situational awareness.50 An emergency such as a CICO situation requires an immediate, coordinated, team response.23 However, pressure and information overload can lead to sensory dysfunction,30 and the stress response causes cognitive and behavioural changes that override systematic thinking and promote cognitive biases, which in turn increase the risk of errors.22,23,51,52 The existence of several different, largely impractical algorithms can paralyse the workflow, and factors such as fatigue, frequently the result of long shifts, will further exacerbate the situation.22 Errors, therefore, are not solely caused by poor individual skills, but by the very nature of cognitive processes and how they are handled in challenging situations.52 Evidence showing that 93% of difficult TIs are unanticipated53 shows the pressing need for effective tools that do not cause cognitive overload but instead facilitate complex processes such as planning, situational awareness, decision making, team coordination, and task management.48,54 The linear, algorithmic approach to crises is not suited to the flexible, intuitive cognitive processes that are activated to resolve stressful dynamic situations.29,55,56
For the reasons outlined, these guidelines provide clinicians with cognitive aids that can be used in any emergency airway situation, standardization of the difficult airway cart as an extension of this, a pre-procedure checklist, as well as ergonomic principles. Supplementary data 3 details the purpose of each element according to human factors principles.
Cognitive aidsClinicians should use visual cognitive aids to deal with emergencies (expert opinion [EO] 97.1%).
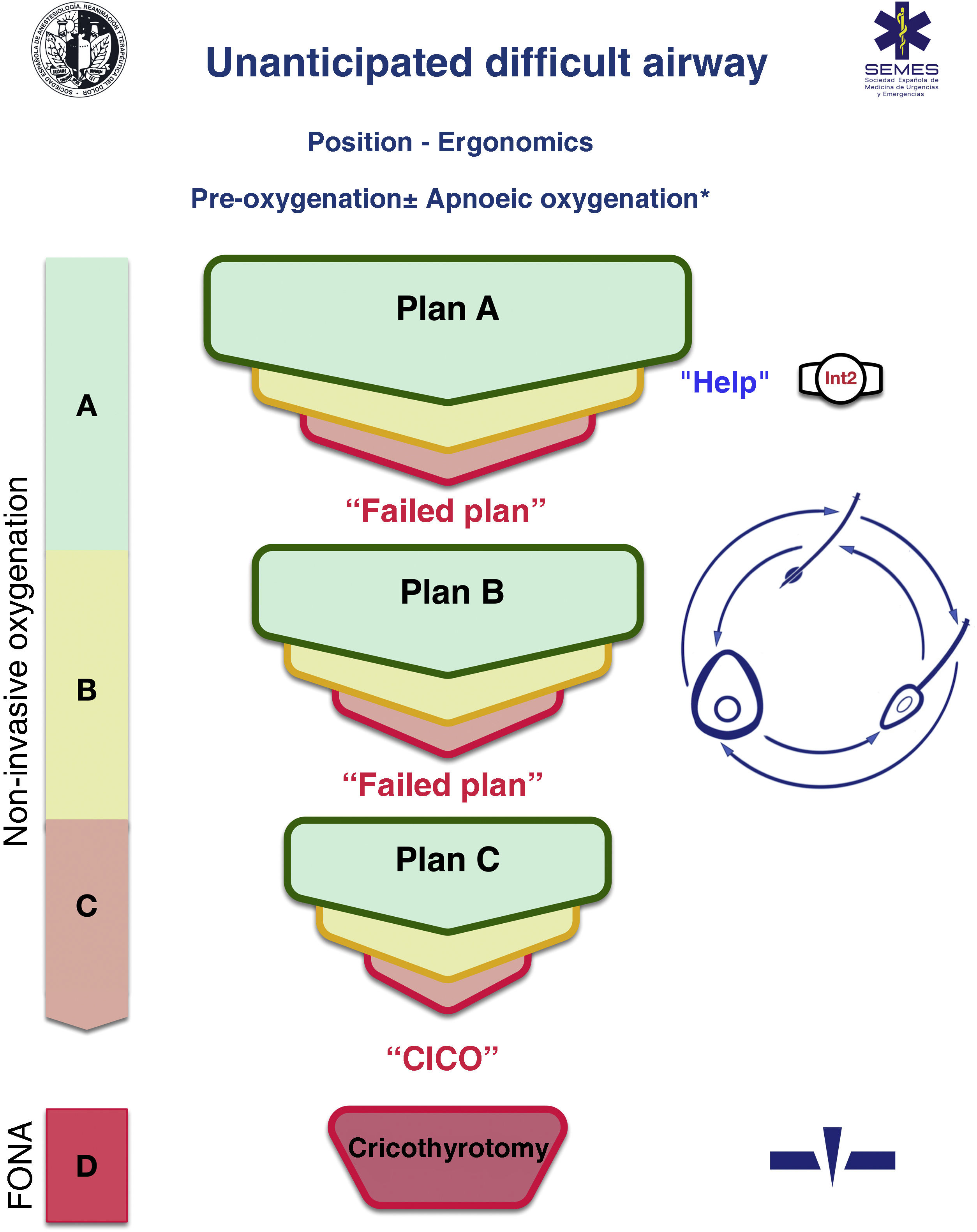
Cognitive aids are tools designed to maximise cognition (memory, perception, attention, concentration, language) and improve executive functions such as problem solving, planning, reasoning, and control.48Fig. 1 shows the SEDAR SEMES SEORL-CCC cognitive aid for dealing with unanticipated DA. The main aim is to reduce the instrumentation of the airway using the fewest possible attempts. This context-specific, HR-oriented, emergency decision-making tool consists of simple diagrams that guide practitioners through the sequence of evidence-based steps to achieve alveolar oxygenation in a patient with an unanticipated DA.
The design is based on Chrimes’ Vortex approach17 with the addition of universal traffic light symbolism.
Four different categories of techniques for preserving or restoring alveolar oxygenation are presented - 3 non-invasive techniques, namely, TI, mask ventilation, and SGAV, and 1 invasive approach: FONA, which is the last resort when all 3 non-invasive strategies have failed.
No more than 3 attempts should be made in each non-invasive airway management plan (EO 88.6%) The first attempt must be made under optimal conditions to maximize the chances of success.57–59 Each new attempt requires the use of a new device or new methods or adjuvants that optimise the previous attempt. If all attempts fail, “Failed Plan” must be verbally declared and a new plan initiated. If all 3 plans of non-invasive techniques fail, the “CICO situation” must be declared without delay and perform FONA, which is the last resort to safeguard alveolar oxygenation. To speed up the transition to FONA, the FONA kit should be opened after the first failed attempt at mask or SGAV.
Airway management can begin with any of the 3 non-invasive oxygenation strategies. The choice of first-line technique, as well as backup techniques, is context-sensitive (patient status, operator skill, location and availability of additional qualified help and equipment, and time of day). The first-line technique chosen is called “Plan A”. If the first attempt fails, the team must announce “Unanticipated Difficult Airway” and immediate ask for help. If all 3 “Plan A” attempts fail, the team must progress to “Plan B” which, if unsuccessful, must be followed by “Plan C” , using a circular scheme of non-invasive techniques in a clockwise or counter clockwise direction from the first-line technique. Alternating plans without exhausting the attempts for each is optional.
The warning signs that prompt the transition between techniques include poor or absent ventilation, time-sensitive desaturation and/or clinical signs of hypoxaemia, and the failure of a plan after three unsuccessful attempts.
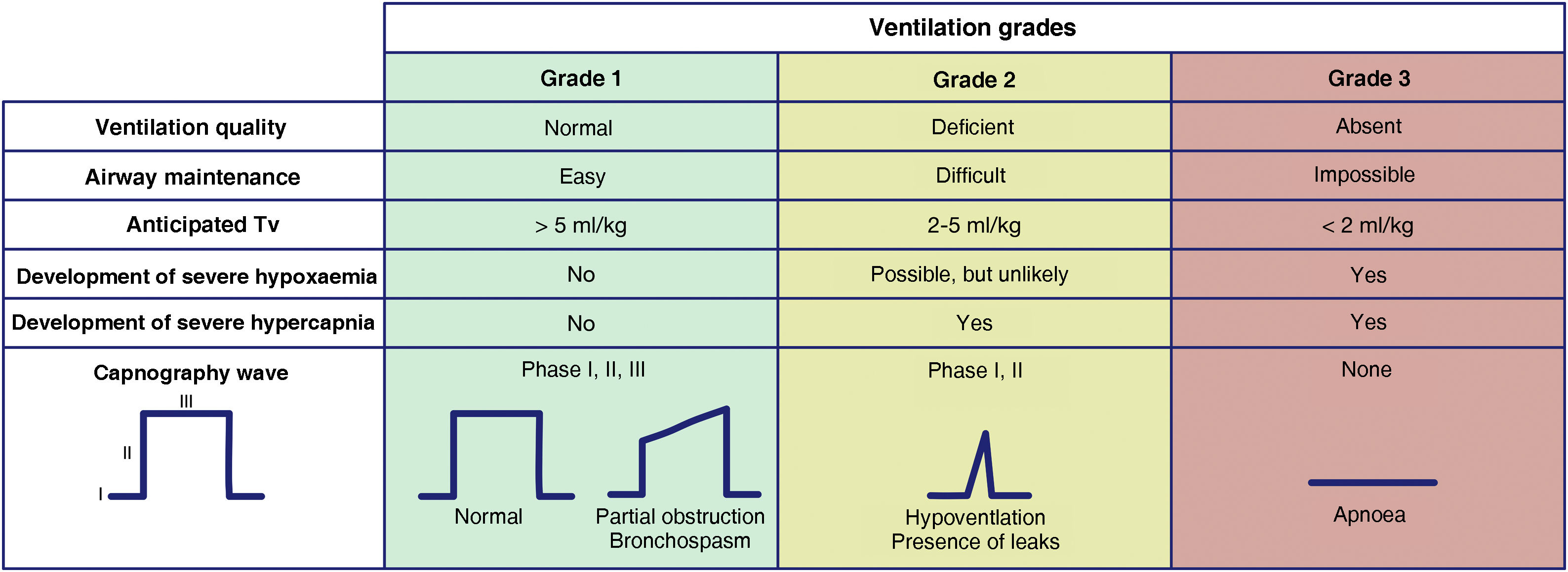
Waveform capnography is the gold standard for confirming alveolar ventilation, and must be available at all locations where airway management is performed to confirm successful of any of the 4 plans employed.60 We recommend evaluating ventilation using the classification proposed by the Japanese Society of Anaesthesiologists.61Fig. 2 is adapted from the Japanese guidelines. This system gives all team members an accurate, almost real-time mental model of the patient’s ventilation status, thereby ensuring timely transition between techniques or plans and avoiding fixation errors. Capnography waveforms can be used to predict severe hypoxaemia and hypercapnia in patients under spontaneous breathing (SB) or receiving face mask, SGA, ETT, or infraglottic cannula ventilation. Grade 2 or 3 ventilation indicates the need to switch technique or start a new, more effective plan to maintain oxygenation. We recommend declaring capnography as “absent” or “present” to aid the team’s situational awareness and generate coordinated actions.
Clinical signs such as chest movement or auscultation can be evaluated together, although they are less reliable. Tidal volume measurements can be more accurate and objective, although monitoring is not available in all locations.
Changes in peripheral oxygen saturation (SpO2) provide late feedback due to the relatively long “silent” period until desaturation.
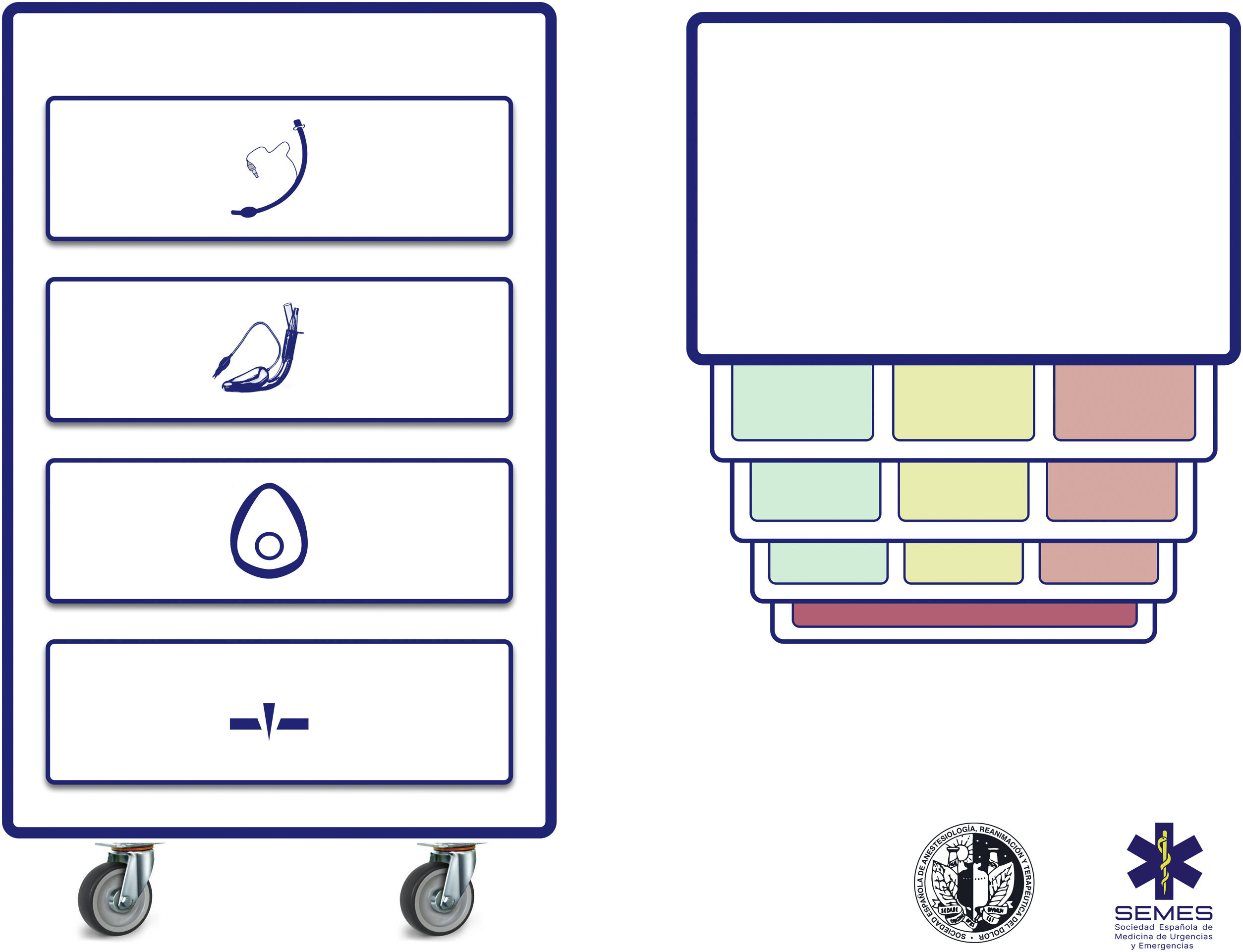
Difficult airway trolleyA standardized difficult airway trolley should be placed near any room where airway management is performed (EO 100%). Fig. 3 shows the airway trolley cart to complement the cognitive aid.
The NAP4 describes multiple incidents caused by the absence of essential material for airway management.8,9 The rapid availability and presentation of the necessary devices for executing various plans are critical contextual components.62 Airway devices are often stored in easily transportable trolleys,63 so standardising the content and layout of these trolleys will help teams adhere to algorithms, will promote situational awareness and sequential progression through the algorithm, and will reduce the risk of delayed decisions and cognitive overload.63
The proposed cart layout with integrated cognitive aid consists of 4 compartments represented by easily recognizable pictograms. Each of the first 3 compartments contains one of the three possible categories of non-invasive alveolar oxygenation techniques. Each compartment is in turn divided into 3 sub-compartments, each containing the different devices and fallback techniques for each category, as well as optimization strategies. These are colour-coded according to whether they are first-line (green), second-line (amber) or third-line (red), in the same way as the cognitive aid. Airway trolleys with integrated cognitive aids can maximise efficiency in difficult airway management.64 The prioritization of each alternative within a category can be standardized at each institution based on available devices. If planning for a specific anticipated airway management scenario advises altering the priority order of techniques within a category, this change should be made before starting the procedure, with the standard order restored after the case is completed. The fourth compartment is reserved for the FONA kit used in a CICO situation.
This standard layout of airway material allows nursing staff to more effectively fulfill their critical role as assistants in preparing alternative equipment while the operator is still implementing the preceding option, and to offer them immediately in case of failure. This facilitates anticipation, seamless transition between techniques, and prevents fixation on a given strategy.64
Ideally, difficult airway trolleys should be placed within 1 min’s access of all locations where airway management is performed.60,62 In addition to immediate access, all professionals must be correctly trained in the use of each device or technique.60,63 The contents of the trolley should be inspected at least once a week and also after each use following a checklist permanently attached to the cart.60
Pre-intubation checklistChecklists should be used to reduce human error, speed up tasks, and promote an airway management safety culture (EO 100%).
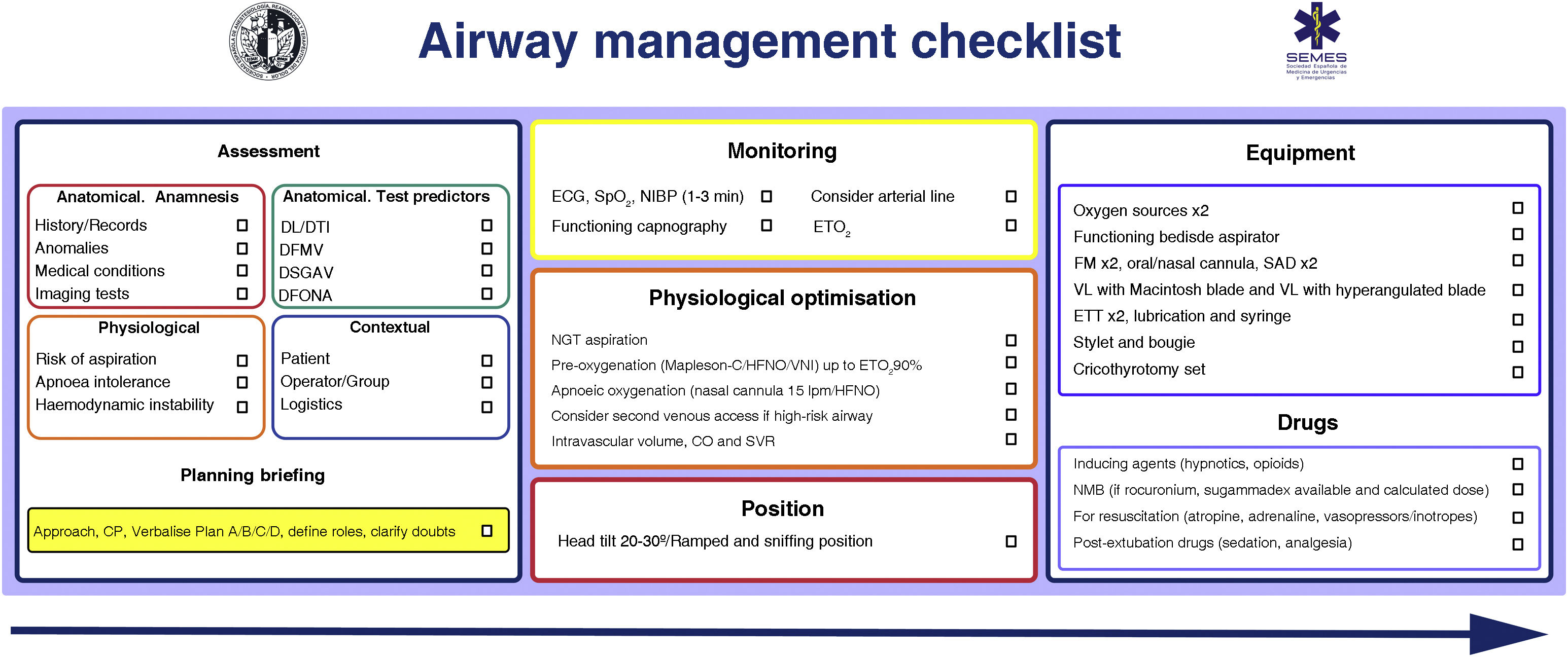
Patient safety is often a product of good communication, teamwork, and anticipation; checklists are the glue that binds all these factors.65,66 Checklists reduce the incidence of human error, shorten the time needed to perform tasks, and reinforce a culture of safety and “control”.22,29,48,67 They are particularly useful in demanding, high-workload situations in which clinicians are likely to develop “tunnel vision” (fixation errors) and omit crucial steps, but also help considerably in routine, repetitive tasks when lack of concentration can cause complacency and deviations from standard protocols.65 Systematic reviews analysing the use of checklists in the operating room have shown that they reduce complications and morbidity and mortality, but only when all team members are involved and compliance is high.68,69 Checklists also optimise anticipation and promote proactive discussion, teamwork, and effective communication,65 all of which can improve patient outcomes.70 Although the use of TI checklists does not appear to consistently improve some clinical outcomes,71,72 they have been shown to reduce the incidence of hypoxia.71 More evidence is needed to define their benefit,71 but they are widely recommended as essential cognitive aids to improve patient safety in airway management.65,73,74Fig. 4 shows the SEDAR SEMES SEORL-CCC pre-management airway read-do checklist.
SEDAR SEMES pre-management airway checklist.
CP: cricoid pressure; DFONA: difficult front of neck access; DL: difficult laryngoscopy; DMV: difficult mask ventilation; DSGAV: difficult supraglottic airway ventilation DTI: difficult tracheal intubation; ECG: electrocardiogram; EtO2: end-tidal oxygen concentration; ETT: endotracheal tube; FM: face mask; HFNO: high flow nasal oxygen therapy; NIBP: non-invasive blood pressure; NIV: non-invasive ventilation; NMB: neuromuscular blockade; SGA: supraglottic airway device; SpO2: peripheral oxygen saturation; VL: video laryngoscopy.
Hospitals should implement systems that promote ergonomics and communication (EO 91.4%).
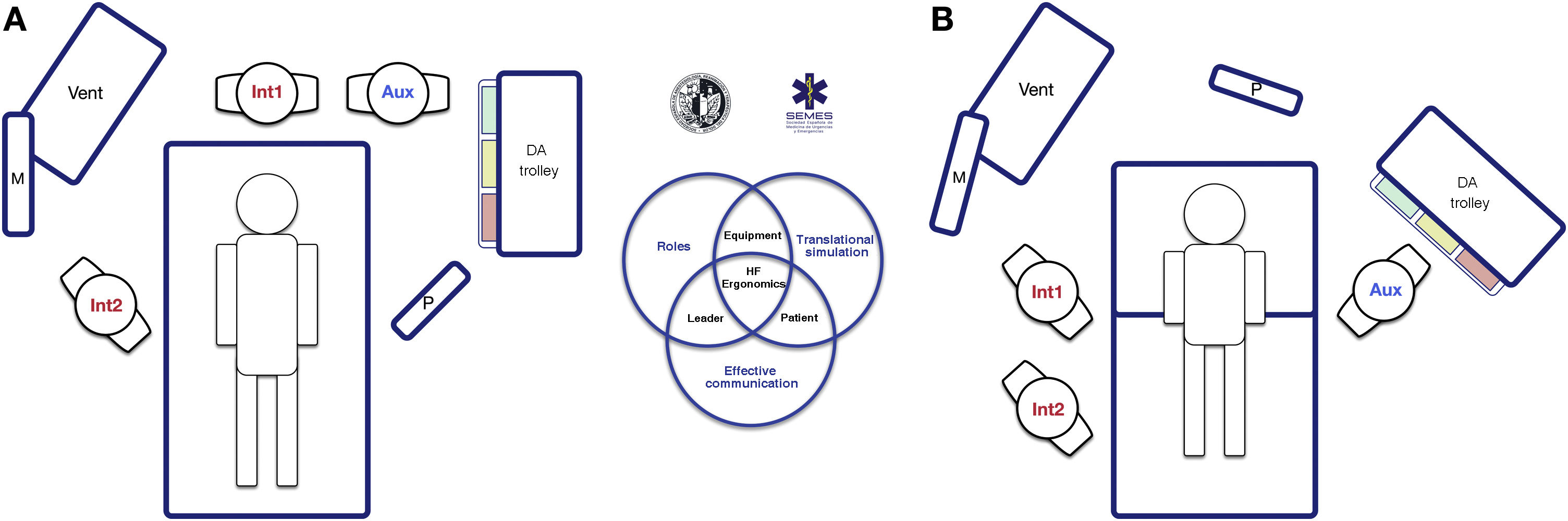
The socio-technical setting has a significant impact on the effectiveness, safety, and quality of care.75 Inappropriately designed systems have been associated with errors, distractions, and diminished efficiency.76 This is why pre-intervention planning of the workspace and the arrangement of human and material resources is key to promoting situational awareness, freedom of movement, and rapid response.77Fig. 5 shows 2 workspace layouts that optimize these factors.
Workplace ergonomics in Hospital Settings for tracheal intubation: (A) unanticipated difficult tracheal intubation after anaesthesia induction (supine position); and (B) known or anticipated difficult awake tracheal intubation (sitting position). Two roles are established in routine tracheal intubation: operator (Int1) and assistant (Aux). To ensure effective communication and teamwork, both must be in direct line of sight with each other, the patient monitoring system (M), the video screen (P) and the respirator (Vent). The team must immediately call for help if they encounter a patient with unanticipated difficult tracheal intubation (A), preferably from an expert in airway management who can assume the role of the second operator (Int2), and the airway trolley must be placed close to the assistant so he can provide the necessary devices to the operator. The leadership role can be interchangeable between operator and second operator. Patients with a known or anticipated difficult airway should ideally be placed in the sitting position (airway benefits from the effect of gravity) and a second operator must be present at the start of procedure. Layout B shows the suggested workplace ergonomic for FOI. The early assignment of team roles improves attention and effective communication among members and optimizes the outcomes of the intervention. As in simulation-based training, post-intubation debriefing and analysis of the case will improve the team’s performance in subsequent patients.
Teamwork improves outcomes and promotes a safety culture.78–80 Clinicians must function as a unit by effectively organising individual actions to achieve a common goal.81 The role of the leader is crucial for integrating these elements.25,80 The leader must explain to the team the procedure, expected events, and the operational plan, assign roles to streamline the workflow, and clearly and explicitly direct the entire procedure by creating shared mental models.22,80,82 Effective and dynamic communication is essential,22,83 and should be based on clarity, brevity, and empathy, and should enhance non-verbal communication,84 allow participation and feedback,80,85 and avoid noise and unnecessary information that would lead to distraction and errors.22,86
A critical event must be treated by a qualified operator with expertise in dealing with such situations. This will not necessarily be the most senior specialist, but rather the clinician with extensive knowledge of a certain advanced procedure. The expert must be notified as early as possible, and always after the first failure of the primary plan. When the qualified operator arrives and has been briefly informed of the situation and the techniques used so far, they must take decisive, immediate action to resolve the situation.
The availability and strategic location of airway equipment is one of the primary enablers of successful airway management. Devices with screens allow for sharing the progress of the procedure with the entire team, making them advisable for facilitating coordinated work and providing targeted support by anticipating the operator’s needs.87
Ergonomics are highly context-sensitive. The COVID-19 pandemic showed the importance of teamwork, communication, and the need to adapt guidelines to overcome new obstacles,88–90 such as personal protective equipment (PPE) or “intubation boxes”.91,92
The ARACHNID tool (Algorithms, Resilience or ability to recover from an adverse event, Cognitive aids, Checklists, Handover tools, Non-technical skills, Incident investigation, and Design of operating rooms and anaesthetic equipment) simplifies the organisation of all ergonomic elements.93
Pre-procedural assessment and planningGeneral evaluationWe recommend performing a pre-procedure evaluation in all patients who require airway management (EO 100%).
Anticipation and planning are the cornerstones of crisis management, and pre-procedure airway assessment is a standard clinical practice.39 Current tests for predicting a difficult airway have limited, inconsistent diagnostic value,39,94–100 since the vast majority are designed solely to predict difficult DL99,101 and all have low sensitivity and a low negative predictive value; therefore, none are entirely suitable.39,96 The bite test has the highest sensitivity (0.67 [95% CI (0.45−0.83]) to predict difficult DL, while the modified Mallampati score (0.51[(0.40−0.61]) is most suitable for predicting difficult TI.39,96,98 The combination of Mallampati score and thyromental distance is the most accurate predictor of difficult TI.94 Most studies have focused on individual tests, although a combination of tests is usually used in clinical practice 97. Multivariate models could have greater predictive capacity (EO 97.1%),44,102–108 but very few, with the exception of the Wilson test, have been studied.98 The MACOCHA test,106 which combines anatomy, physiology, and operator characteristics, is the only evaluation validated for critically ill patients. Although airway assessment is recommended as a standard of care, even in emergencies,51,96,110 93% of difficult TI are unanticipated53 and cause up to 17% of airway-related adverse events.109 Airway assessment is important for several reasons: (1) it allows to stratify the risk and plan accordingly,39 rapidly and effectively transition between airway techniques, and spare resources 96,99,110; and (2) it can be considered a cognitive forcing strategy that allows clinicians to prepare for a possible unanticipated airway, and thus promotes a safety culture.97,99,110 Studies in airway management-related morbidity have shown the dangers of omitting the airway assessment or ignoring its findings.7–9 In medical malpractice claims, failure to provide a documented airway assessment is considered to fall below the standard of care.3
Pre-procedural airway assessment should be multifactorial, structured, and aimed at detecting anatomical, physiological, and contextual difficult airway (EO 97.1%).25,97,111
A pre-procedure medical history and physical examination are recommended, if feasible.51 A complete history begins with reviewing previous TIs and identifying factors that may alter the anatomy of the neck or airway, such as radiation therapy, surgery, or previous medical conditions.112 The diagnosis of SAHS is a predictor of difficult mask ventilation (1C) and difficult TI (1B). A history of difficult TI is the risk factor with the greatest predictive value for difficult TI.98,113 Reviewing any imaging studies (CT, MRI) is advised, as these can provide valuable information on the level and severity of any stenosis or obstruction identified.51,97,114 In patients with known or suspected obstructive glottic or supraglottic pathology, decision-making will be facilitated by preoperative flexible nasopharyngoscopy (FNP) or fibreoptic nasendoscopy examination of the airway by an ENT specialist.115–117
The airway examination can begin by detecting predictors of difficulty or failure for the primary plan and subsequently for the three alternative plans (EO 97.1%).97,118,119 Some experts recommend only evaluating the possibility of difficult FONA in patients with DA.51,97,120,121 In this case, the CTM must be identified by palpation120,122 and ultrasound51,121 as a preventive measure. Ultrasound identification ensures a higher cricothyrotomy success rate and fewer complications.123
Ultrasound has shown promise as a tool for rapidly identifying a difficult airway.124 It has a level of accuracy comparable to CT and radiography, and is far more effective than the modified Mallampati test.125,126 It is particularly useful in determining risk of aspiration121,127–130 and difficult airway in unconscious or uncooperative patients.99
The existence of a physiologically difficult airway (PDA)1,111,131,132 due to the presence of pathophysiological changes that increase the risk of complications during TI, such as short apnoea tolerance, haemodynamic instability, severe metabolic acidosis or full stomach, and the presence of contextual difficult airway due to lack of patient cooperation, emergency situations, unskilled or inexperienced operator, or the absence of additional qualified help or the most appropriate device must be taken into account when planning the airway management approach.1,25,97
The end result of the assessment should be a clearly defined airway management plan that should be discussed and shared with the entire team before starting the procedure. The plan must include a fallback strategy for unanticipated DA in all patients, even in the absence of predictors of difficulty.97
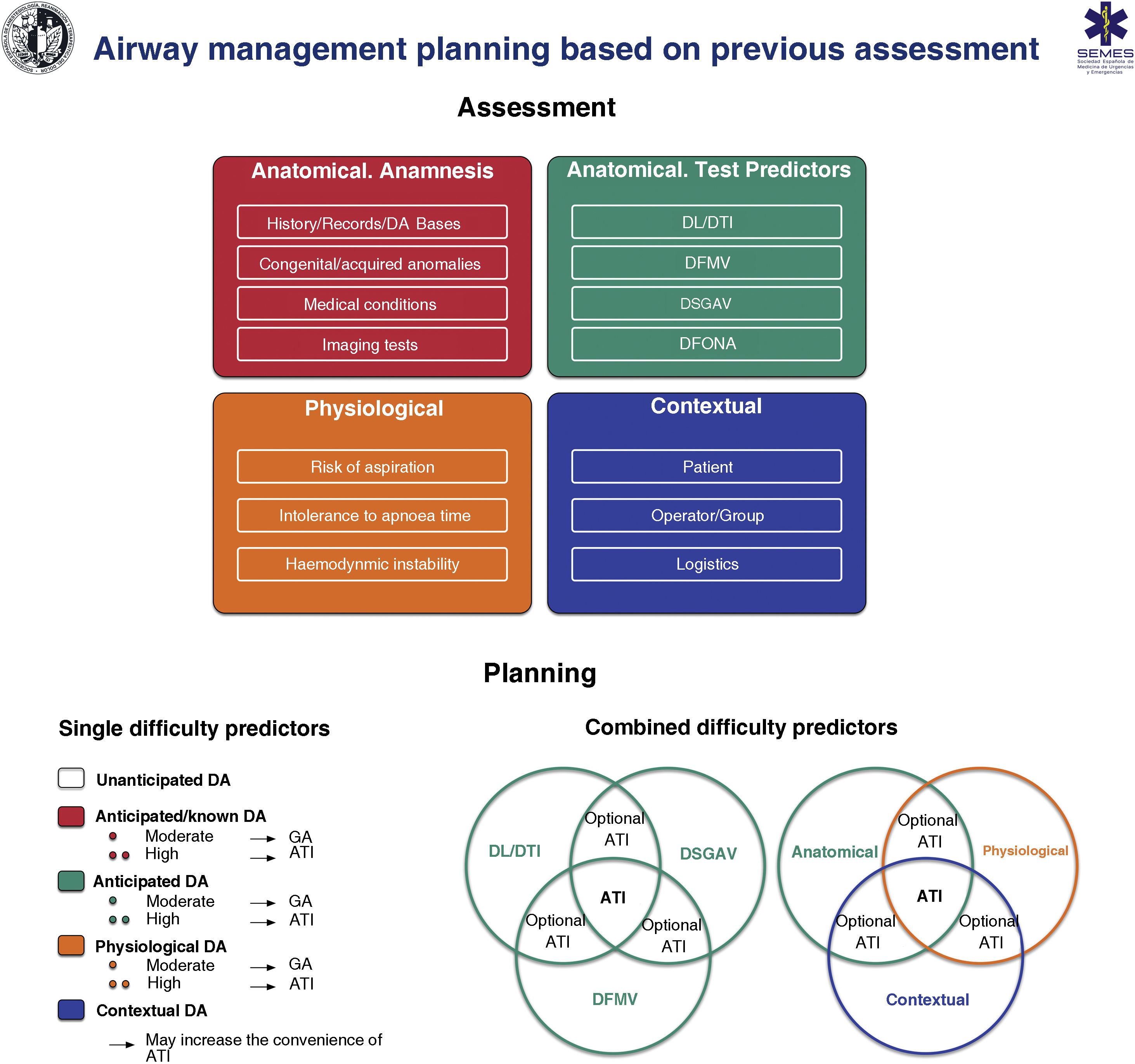
Fig. 6 shows an implementation tool for airway assessment and management.
Implementation tool for airway evaluation and management planning. ATI: awake tracheal intubation; DA: difficult airway; DFONA: difficult front of neck access; DL: difficult laryngoscopy; DMV: difficult mask ventilation; DSGAV: difficult supraglottic airway ventilation; DTI: Difficult tracheal intubation; GA: induction of general anaesthesia.
Venn diagram adapted from Law JA, Heidegger T: Structured Planning of Airway Management, Core Topics in Airway Management, 3 edition. Edited by Cook T, Kristensen MS. Cambridge, Cambridge University Press, 2020, pp 38-49.
Decision-making must be individualized to the patient, operator, contextual considerations, and time of day (EO 97.1%).119,133
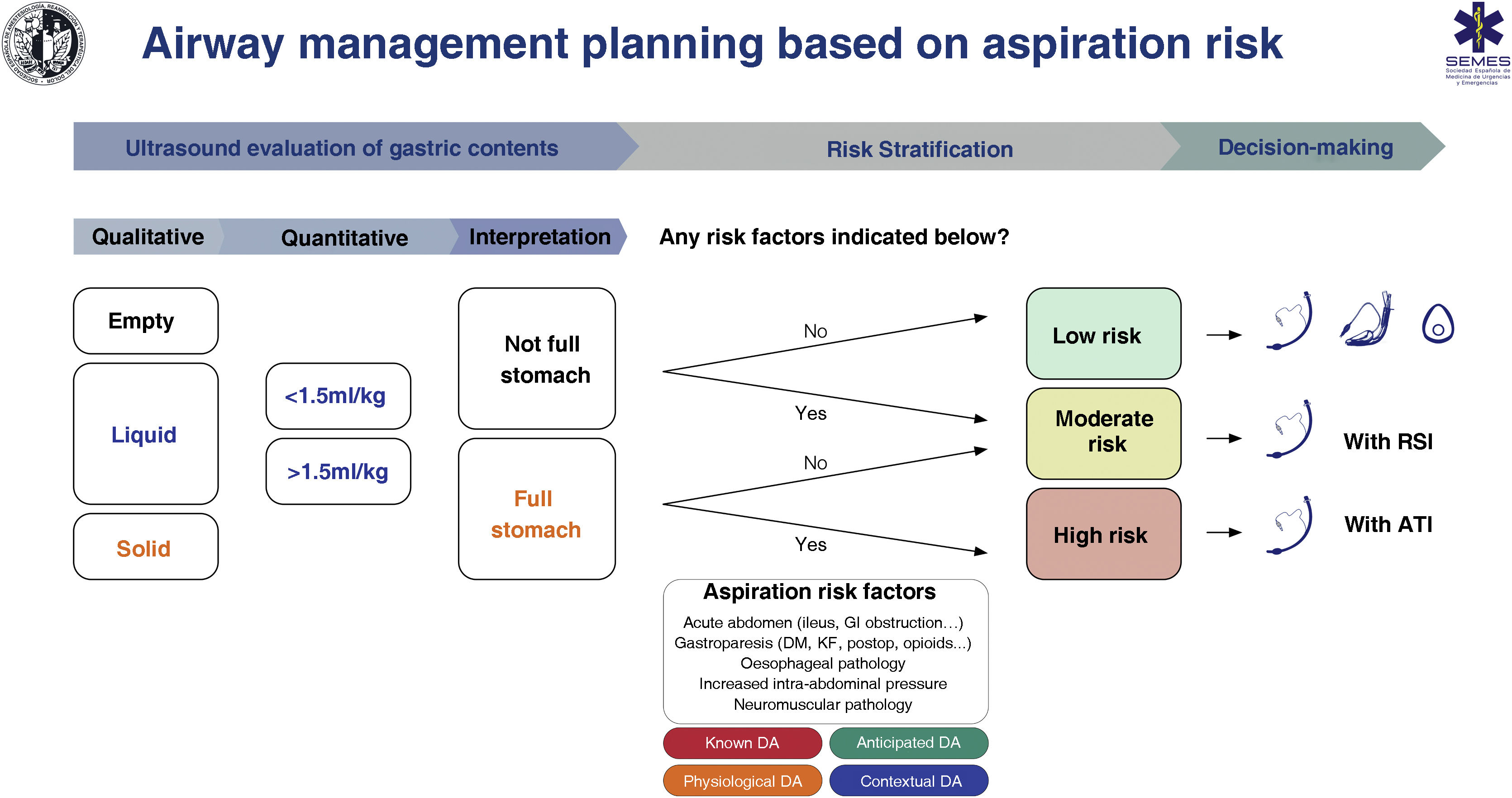
RecommendationAspiration risk assessmentAspiration is responsible for up to 50% of airway-related deaths,134,135 and prevention of this complication must be a priority. Poor risk assessment and planning are the root cause of aspiration.8 Most cases can be prevented by cognitive aids and adherence to guidelines.136 A full stomach is the main risk factor,135,137 and can be avoided by following preoperative fasting guidelines (EO 97.1%) 138; however, these cannot be relied on in certain situations, such as 134,137,139–141: (1) non-compliance with fasting instructions or uncertain prandial status (e.g., emergency surgery, language barrier, cognitive dysfunction); (2) pathologies that delay gastric emptying (for example, diabetes mellitus, advanced liver or kidney dysfunction, Parkinson's disease, critical illness, sympathetic activation, pain, chronic opioid administration); and (3) increased intra-abdominal pressure (morbid obesity, predominantly truncal, ascites, masses, obstruction). Fasting guidelines, therefore, should be complemented by an objective examination to increase the safety margin.141,142 Gastric ultrasound is a simple, non-invasive, point-of-care tool with high sensitivity (1.0) and specificity (0.975) for determining the risk of aspiration by identifying the nature and volume of gastric contents.139,143–145
Despite limited evidence of its cost-effectiveness, studies have shown that gastric ultrasound has led to changes in decision-making.135,146 No special precautions are required in patients with an empty stomach and no risk factors; however, a full stomach with or without additional risk factors is an indication for TI to protect the airway (EO 88.6%). The patient’s clinical context and other specific risk factors for aspiration must be taken into account during decision making.119,147 We recommend performing gastric ultrasound to evaluate the risk of aspiration in risk situations (1C)
Fig. 7 shows a cognitive aid for airway management based on aspiration risk.
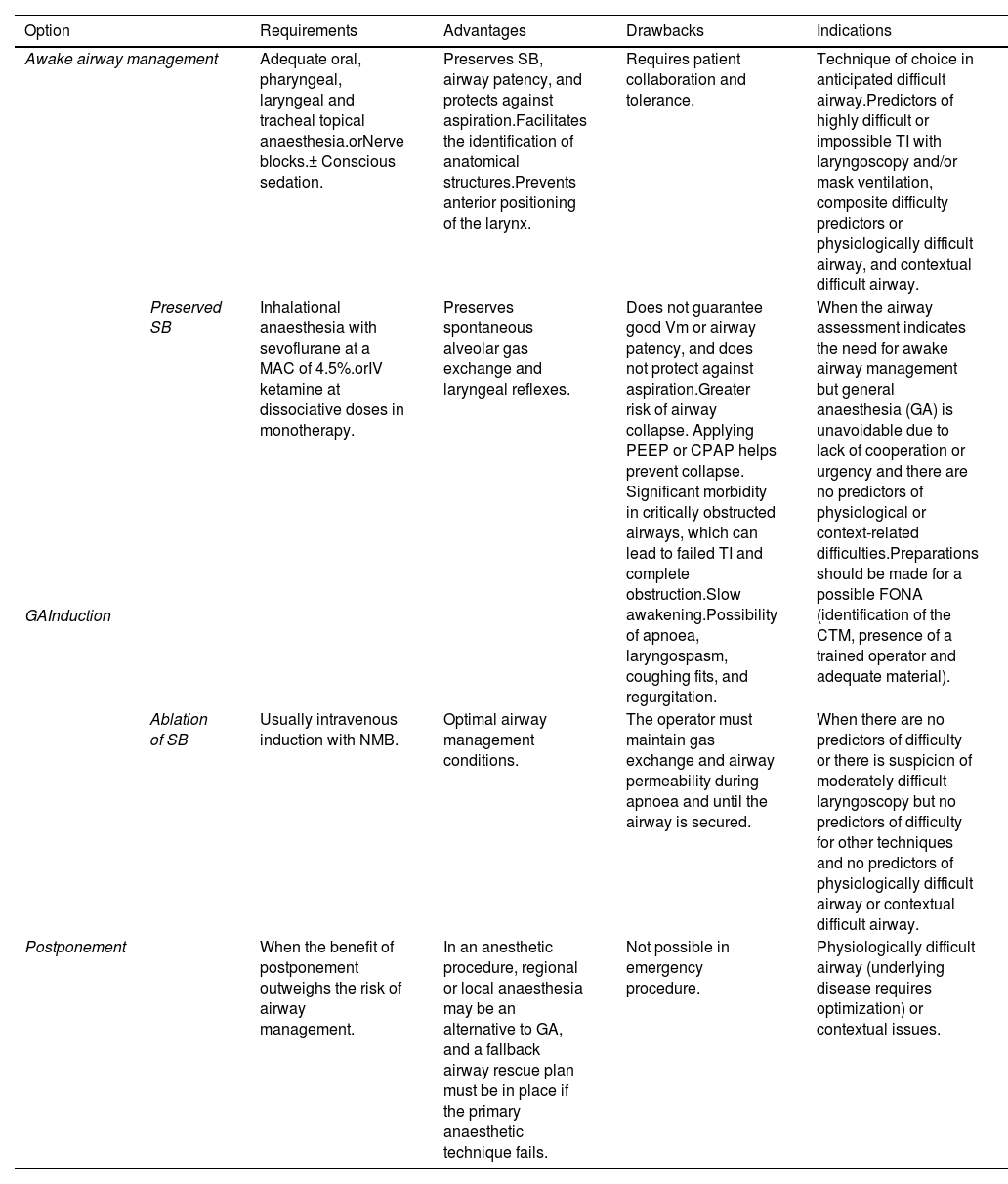
RecommendationDifficult airway management: basic optionsAirway management is associated with certain risks.6,148,149 Most techniques involve ablating SB and protecting against aspiration.134,137 Laryngeal injuries are common after simple airway management, in healthy low-risk patients, and after scheduled procedures.3,19,150,151 Therefore, it is important to perform a pre-intubation risk-benefit analysis to determine the suitability of airway management (EO 97.1%). Once the indication has been confirmed, clinicians must decide on the best approach to achieve the basic aims of airway management: maintaining alveolar oxygenation, ensuring airway patency, and minimizing the risk of aspiration. Patient preference and operator skill should be factored into this decision. Options include 110:
| Option | Requirements | Advantages | Drawbacks | Indications | |
|---|---|---|---|---|---|
| Awake airway management | Adequate oral, pharyngeal, laryngeal and tracheal topical anaesthesia.orNerve blocks.± Conscious sedation. | Preserves SB, airway patency, and protects against aspiration.Facilitates the identification of anatomical structures.Prevents anterior positioning of the larynx. | Requires patient collaboration and tolerance. | Technique of choice in anticipated difficult airway.Predictors of highly difficult or impossible TI with laryngoscopy and/or mask ventilation, composite difficulty predictors or physiologically difficult airway, and contextual difficult airway. | |
| GAInduction | Preserved SB | Inhalational anaesthesia with sevoflurane at a MAC of 4.5%.orIV ketamine at dissociative doses in monotherapy. | Preserves spontaneous alveolar gas exchange and laryngeal reflexes. | Does not guarantee good Vm or airway patency, and does not protect against aspiration.Greater risk of airway collapse. Applying PEEP or CPAP helps prevent collapse. Significant morbidity in critically obstructed airways, which can lead to failed TI and complete obstruction.Slow awakening.Possibility of apnoea, laryngospasm, coughing fits, and regurgitation. | When the airway assessment indicates the need for awake airway management but general anaesthesia (GA) is unavoidable due to lack of cooperation or urgency and there are no predictors of physiological or context-related difficulties.Preparations should be made for a possible FONA (identification of the CTM, presence of a trained operator and adequate material). |
| Ablation of SB | Usually intravenous induction with NMB. | Optimal airway management conditions. | The operator must maintain gas exchange and airway permeability during apnoea and until the airway is secured. | When there are no predictors of difficulty or there is suspicion of moderately difficult laryngoscopy but no predictors of difficulty for other techniques and no predictors of physiologically difficult airway or contextual difficult airway. | |
| Postponement | When the benefit of postponement outweighs the risk of airway management. | In an anesthetic procedure, regional or local anaesthesia may be an alternative to GA, and a fallback airway rescue plan must be in place if the primary anaesthetic technique fails. | Not possible in emergency procedure. | Physiologically difficult airway (underlying disease requires optimization) or contextual issues. | |
Awake airway management is recommended when TI is likely to be highly difficult or impossible, or there are composite predictors of difficulty, physiological alterations, and negative contextual issues (EO 82.9%).
General anaesthesia with preserved SB should be induced whenever awake tracheal intubation is advisable but general anaesthesia is unavoidable due to lack of cooperation or urgency and there are no predictors of physiological or context-related difficulties or obstructive airway pathology (EO 91.4%).
When there are predictors of physiological or context-related difficulties, postponement can be considered if it outweighs the risk of proceeding with airway management, or alternative anaesthesia strategies can be considered (EO 85.7%).
PreparationInformed consentInformed consent is an essential requirement of the lex artis ad hoc. It is usually given in writing for invasive procedures and, in general any procedure that involves risk, including airway management. However, procedures such as TI are included in other procedures such as general anaesthesia or the informed consent for critical care protocol,152,153 so specific informed consent is not required. Nevertheless, all the topics discussed and the process of obtaining informed consent must be documented, particularly in the case of non-routine procedures, such as awake airway management.154
In cases where the need for informed consent is waived,153 this circumstance must be clearly justified in the medical history and the patient’s family or representative must be notified of the decision.155 A short discussion is often possible.
MonitoringAirway management procedures must be monitored using the same standards as those used in anaesthesia.156,157
Capnography waveform must be available wherever airway management is performed, and must be used to confirm successful ventilation with any of the 4 airway plans used (EO 97.1%),158 to promptly detect the displacement of an artificial airway, and to identify hyper or hypoventilation.1,6,9,159 Capnography should also be used during awake airway management under moderate or deep sedation.
Monitoring end-tidal oxygen concentration (EtO2) is the gold standard for evaluating the effectiveness of preoxygenation.160
If a neuromuscular blockade has been administered, depth of blockade must be monitored to ensure optimal conditions for TI and to determine whether a reversal agent is required.161,162
When performing inhalational induction of anaesthesia, it is also advisable to monitor the end-tidal concentration of the volatile anesthetic agents administered.
In hemodynamically unstable patients, advanced invasive haemodynamic monitoring may be necessary to achieve pre-procedure goal-directed optimization.111,163
PositionEnsuring optimal positioning before any intervention optimises the patient’s anatomical and physiological conditions,164 facilitates laryngoscopy and TI, and improves upper airway patency, preoxygenation, apnoeic oxygenation, and mask ventilation.165,166 It also improves access to the airway such as the CTM, and improves respiratory mechanics. In obese patients, the ramp position or a 30° head-up tilt is recommended to improve TI conditions (1C). Ramping prolongs the safe apnoea time in this population (1B).
The sitting or semi-sitting (Fowler) position or the head elevated 25−30°, or the 30° reverse Trendelenburg position if haemodymically feasible, is advisable in patients at high risk of desaturation or aspiration,1,159,167,168 since it increases FRC, reduces the formation of atelectasis,169,170 reduces the risk of aspiration,159 and could be associated with better laryngeal exposure,171 better first-attempt TI success rates,172 and fewer complications.173 The sitting or semi-sitting position is both anatomically and physiologically ideal for awake tracheal intubation.174,175
To facilitate TI, the external auditory canal should be aligned with the suprasternal fourchette in the horizontal axis.1,176 In the case of obese patients, this is facilitated by ramping, in which the upper part of the torso and head are elevated using a wedge or pillow.40,177 The “sniffing” position (lower cervical flexion and upper cervical extension) is optimal for DL.1,178,179 Both positions are equally suitable for TI,180,181 although ramping may improve laryngeal exposure in the surgical population.181 Placing the head in hyperextension could be the most appropriate position for awake fibreoptic intubation (FOI), as it improves glottic vision.182
RecommendationPeriprocedure oxygenationGiven the potential difficulty in managing the airway, periprocedural oxygenation should be universally implemented.183 This increase the pulmonary reserve of oxygen primarily through FRC, and extend apnoea time without the risk desaturation.184,185 The ideal oxygenation technique will depend on the physiology, level of cooperation, and clinical status of the patient.184
PreoxygenationPreoxygenation, which extends the safe apnoea time (time from cessation of breathing or ventilation until peripheral arterial oxygen saturation falls to 90%), is a standard of care186 and should be performed in all patients, particularly those with predictors of difficult airway management, patients at high risk of hypoxaemia, or when manual ventilation is contraindicated.187 Preoxygenation is one of the cornerstones of rapid sequence induction (RSI).184
The goal is to achieve EtO2 > 90% before starting anaesthesia induction.184
Conventional preoxygenation consists of delivering 100% oxygen via a face mask for 3 min of tidal volume (Vt) breathing or, in the case of emergency TI, 8 vital capacity (8VC) breaths over 1 min.160,188 The oxygen flow rate must be sufficient to eliminate rebreathing; 5 l/min for 3−5 min for Vt and 10 l/min for 1 min for 8CV.188 Leakage from the face mask and rebreathing of exhaled gases reduces effectiveness because it does not allow an FiO2 of 1.0 to be achieved. A normal capnography waveform (grade 1 ventilation), clear measurement of inspiratory CO2 and EtCO2 values, and normal reservoir bag movement are indicative of a good seal.184 If a leak is confirmed, a nasal cannula with a flow exceeding 10 L/min should be added.189,190
Apnoeic oxygenationNasal Oxygen During Eforts Securing a Tube (NO DESAT), pharyngeal insufflation of oxygen, and high-flow nasal oxygen (HFNO) therapy at 40–70 l/m186 can prolong apnoea time up to 100 min, but do not prevent progressive respiratory acidosis due to hypercapnia.160,186,191,192 Standard nasal cannulas at 10−15 l/min are a well-tolerated, low-cost, low-risk method of apnoeic oxygenation.193
Apnoeic oxygenation has been shown to be useful in reducing desaturation in emergency TI.160,194–198
Apnoeic oxygenation should be performed with high-flow nasal cannulas (NO DESAT/HFNO) (1C).
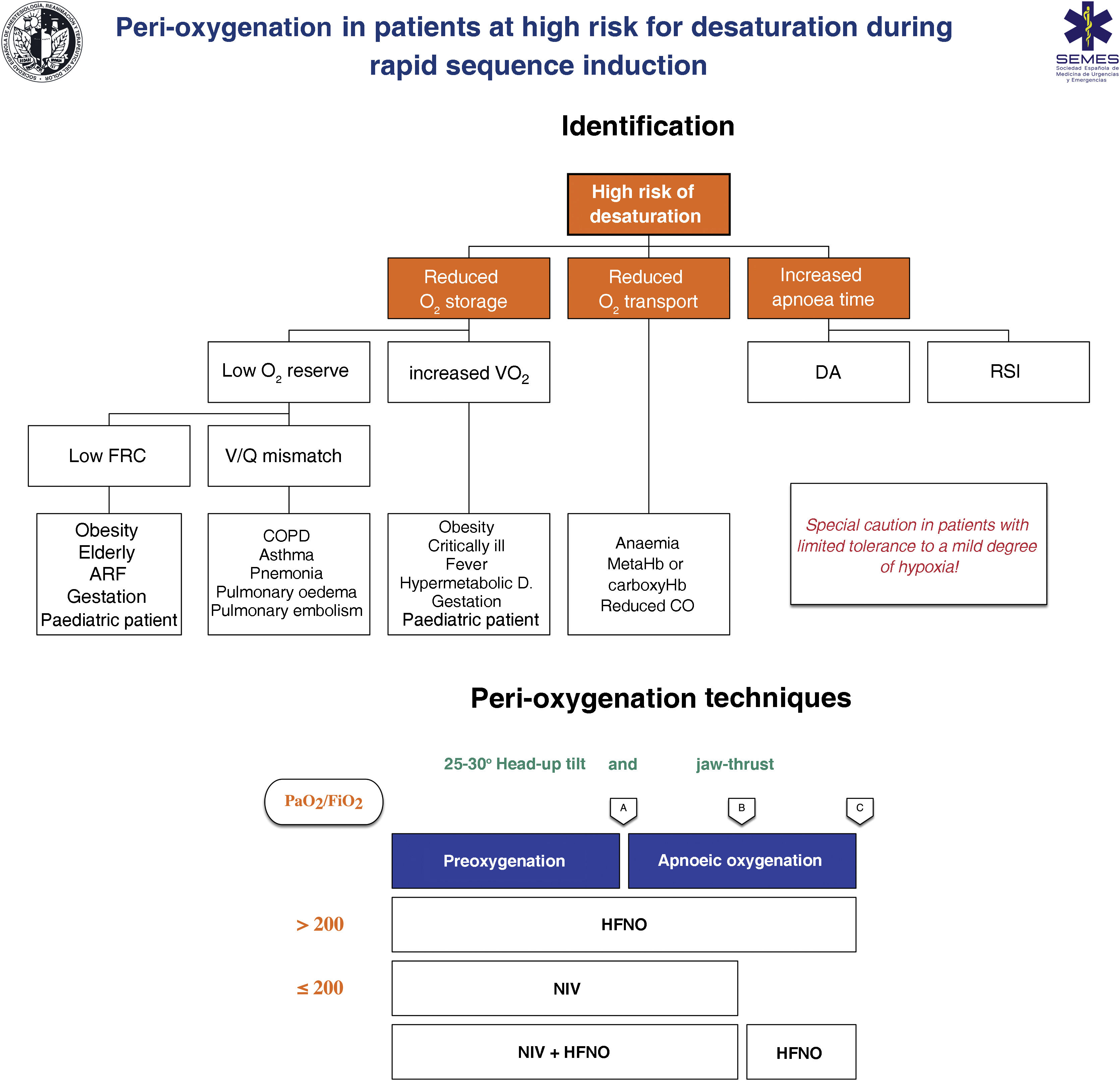
RecommendationTechniques for patients at high risk or with poor tolerance of hypoxaemiaThe effectiveness of conventional techniques is limited in patients at high risk of hypoxaemia (due to shunt, V/Q mismatch, low FRC, or increased oxygen consumption) and with low tolerance of hypoxaemia (e.g. cerebrovascular disease, epilepsy, or coronary artery disease).199 Trying to compensate for this by increasing the preoxygenation time can even worsen hypoxaemia, probably due to resorption atelectasis.200 RSI is associated with desaturations in 10%–30% of cases. Clinicians should ask themselves the following questions before starting preoxygenation201: Are there likely to be difficulties with ventilation and/or TI? How quickly will the patient desaturate? What is the minimum safe oxygen desaturation level? Fig. 8 show the main risk factors for desaturation and the periprocedure oxygenation techniques recommended in high-risk patients.
Theoretical/Educational Tool for detecting patients at high risk of desaturation and recommended preoxygenation and apnoeic oxygenation techniques during rapid sequence induction. A: anaesthesia induction; ARF: acute respiratory failure; B: laryngoscopy; C: tracheal intubation; CO: cardiac output; COPD: chronic obstructive pulmonary disease; FRC: functional residual capacity; HFNO: high flow nasal oxygen therapy; NIV: non-invasive ventilation; RSI: rapid sequence induction; VO2: oxygen consumption; V˙/Q˙ mismatch: ventilation/perfusion mismatch. The diagram shows the two methods used to increase pulmonary oxygen stores: preoxygenation and apnoeic oxygenation. Preoxygenation refers to oxygen delivery before anaesthesia induction, while apnoeic oxygenation refers to oxygen delivery after ablation of spontaneous ventilation.
Adapted from Gómez-Ríos MA, Úbeda-Iglesias A, Esquinas AM. Anesthesiology Pre-intubation and upper airways procedure. Respiratory care in non invasive mechanical ventilatory support. principles and practice. Esquinas AM, AlAhmari MD. Nova Science Publishers. New York. 2021.
The greater the risk of desaturation, the greater the number of combined techniques required.202 The use of pre-apnoea aids, such as upright head position, jaw thrust, PEEP, and apnoeic oxygenation will optimise the O2 safety reserve.160,167 HFNO, NIV, or a combination of both are more effective than conventional methods203 as they reduce shunt and improve the V/Q mismatch by alveolar recruitment. HFNO should be considered the first-line preoxygenation technique for patients with mild hypoxaemia (PaO2/FiO2 > 200 mmHg) (1C). NIV is the technique of choice in those with severe hypoxaemia (PaO2/FiO2 ≤ 200 mmHg) (EO 87.15%)204–209 as it generates greater PEEP and allows pressure support to be applied to increase FRC.210,211
High flow nasal oxygen (HFNO) therapyPreoxygenation with HFNO has given inconclusive results.212–214 A recent meta-analysis showed that in adults with hypoxaemia, HFNO reduced the risk of TI-related complications compared with conventional oxygen therapy.215 This suggests that HFNO is superior to conventional methods,216–220 but inferior to NIV,215,221 although it is a good alternative when the latter is not well tolerated.168
For preoxygenation, patients should perform tidal volume breaths through their nose with their mouth closed tightly for 3 min while receiving an initial O2 of 30 l/min and FiO2 100%. The cannula must be well adjusted to the nostrils to avoid contamination. After induction, the flow should be increased to 70 l/min and maintained until TI. Airway patency should be maintained by jaw thrust.160,189
HFNO provides effective apnoeic oxygenation during laryngoscopy, and is probably the main mechanism for reducing desaturation.194,222,223
HFNO obstructs EtO2 monitoring,192 can worsen TI conditions,224 and potentially causes gastric insufflation.225 Recent studies have shown that the latter is unlikely,226,227 although it is uncertain whether these data can be extrapolated to patients with a full stomach.225
RecommendationNIV is particularly beneficial in patients with low FRC,212,228 and it maximizes preoxygenation in obese and/or critically ill patients.160,186,202 The beneficial effect on PaO2 persists 30 min after TI due to alveolar recruitment and increased lung volume.229 NIV should be prioritised over conventional oxygen therapy for anaesthesia induction in obese patients (1B).
Studies have shown that CPAP (5–10 cmH2O) with ventilation assistance (Vt 7–10 ml/kg) provides better oxygenation in clinical practice.230 NIV must be interrupted during laryngoscopy 228; therefore, it may be superior to HFNO during the spontaneous breathing phase,207 and HFNO may be more beneficial during apnoeic oxygenation.204,205,210 Preoxygenation with NIV plus HFNO and apnoeic oxygenation with HFNO, which significantly reduces the risk of desaturation, should be a priority in critically ill patients (EO 85.7%).214,231,232
Analgosedation with dexmedetomidine or ketamine-induced disassociation (10−20 mg IV boluses) may be considered in order to facilitate preoxygenation (delayed sequence intubation) in patients with delirium or interface intolerance.111,168,211,233
NIV should be considered both before and after general anaesthesia (GA) in obese patients.199,234
Pressures >20 cmH2O can cause gastric distention, so this risk must be weighed against the potential benefits in patients at risk of aspiration. A different preoxygenation method is advisable in patients with facial fractures, in those that have undergone laryngeal, oesophageal or gastric surgery, and in those with haemodynamic instability, pulmonary arterial hypertension, pulmonary embolism, or right ventricular failure.209
RecommendationPhysiologically difficult airwayThe considerations in this section refer to patients with previously defined physiologically difficult airway (PDA) or critically ill patients.235 Urgent TI is a high-risk procedure.1,131,167,235–237 It increases the likelihood of DA 20-fold compared to scheduled TI,238 and the likelihood of adverse events causing death or brain damage by approximately 30- to 60-fold.10,239 Underlying pathophysiological disorders, such as hypoxaemia and haemodynamic instability, are responsible for peri-intubation decompensations due to myocardial depression secondary to hypoxia or low perfusion241–243 leading to cardiovascular collapse in up to 30% of critically ill patients.240,241 This means that up to 50% of critically ill patients may suffer an intubation-related major adverse event.243 This risk is exacerbated when more than 1 TI attempt is required.111,243,244 Difficult TI is an independent predictor of death. The incidence of complications increases 5-fold after a second TI attempt,245,246 so achieving TI on the first attempt is particularly important in critically ill patients.58,111,243,247,248
Peri-intubation desaturation is the greatest risk factor for cardiorespiratory arrest. It occurs in 19%–70% of critically ill patients,168 and is the main reason to achieve intubation on the first attempt.168 Desaturation is mainly prevented by preoxygenation and apnoeic oxygenation,248,249 so this should be performed on all patients in an upright position.168,235
Haemodynamic instability is an independent predictor of mortality after TI.235,250,251 Up to 46% of patients with a PDA present peri-intubation hypotension,168,252,253 which leads to longer ICU stays, target organ damage, and higher in-hospital mortality.241,252,254 Pre-intubation risk factors include MAP ≤ 65 mm Hg and a shock index (SI, heart rate/systolic blood pressure) >0.7.168,235 In critically ill patients, the risk of cardiovascular collapse during TI is increased due to hypovolaemia, altered systemic vascular resistance, anaesthesia-induced vasodilation and myocardial depression, hypoxia and/or hypercapnia-induced sympathetic stimulation, and reduced venous return due to conversion to positive pressure ventilation (PPV).167,240,242,255
Physiological factors are, therefore, as dangerous as technical difficulties, and they must be factored in to the airway approach and optimised before TI whenever feasible.1,256 There is scant evidence in the literature on pre-intubation physiology optimisation strategies,167,236,243 so it is prudent to tailor the interventions to the needs of each patient.236 If time permits, a point-of-care ultrasound examination may help identify a particular patient’s needs.257Table 1 shows the main predictors of PDA and the methods proposed to reduce peri-intubation complications.1,111,131,159,167,168,236,248,258
Main predictors of physiologically difficult airway and methods to reduce the corresponding peri-intubation complications.
| Hypoxaemia | Mild
|
| Hypotension | “Fluid responders”
|
| Severe metabolic acidosis | Hypoventilation/apnoea: loss of respiratory compensation →↓↓↓pH→ haemodynamic deterioration.Avoid TI. If unavoidable:
|
| Severe pulmonary hypertension | ↑ hypoxaemia or ↑ hypercapnia → ↑ pulmonary vasoconstriction, ↑PVR and ↑RV afterload (the same as PPV).
|
| Right ventricular failure |
|
| Full stomach |
|
CI: continuous infusion; CO: cardiac output; ECMO: extracorporeal membrane oxygenation; FRC: functional residual capacity; HFNO: high flow nasal oxygen therapy; ITP: intrathoracic pressure; LV: left ventricle; MAP: mean arterial pressure; MP: mean pressure; MV: mechanical ventilation; NIV: non-invasive ventilation; PAP: pulmonary arterial pressure; PAPm: mean pulmonary arterial pressure; PEEP: positive end-expiratory pressure; PL: peripheral line; PPV: positive pressure ventilation; PS: pressure support; PVA: peripheral venous access; PVR: pulmonary vascular resistance; RV: right ventricle; SBP: systolic blood pressure; SI: shock index; TI: tracheal intubation; Vm: minute volume; Vt: tidal volume; VR: venous return; V˙/Q˙ mismatch: ventilation/perfusion mismatch.
Pre-intubation administration of fluid boluses has minimal benefit.240,259 In patients without cardiogenic pulmonary oedema, however, administration of 500 ml of isotonic crystalloids as part of a TI care bundle that included preoxygenation with NIV and early start of noradrenaline if diastolic blood pressure falls to <35 mmHg after TI has been associated with a 50% relative reduction in cardiovascular collapse and severe hypoxaemia,260 suggesting that this approach could prevent peri-intubation hypotension. However, routine preinduction administration of a crystalloid bolus in patients not receiving PPV may not be justified, as it has only shown benefit in a subgroup of patients who received NIV for preoxygenation or mask ventilation between induction and laryngoscopy, and could be harmful in patients that do not respond to fluids.240 The implementation of a TI protocol could reduce these complications.260–262
Although its effectiveness in avoiding peri-intubation hypotension has not yet been proven,248,255 some authors suggest that preventive administration or early start of vasopressors could be beneficial,211 and that an expert operator should be placed in charge of TI while another member of the team oversees leads the management of the patient’s haemodynamic status.1,168,243 Norepinephrine infusion is the first-line vasoactive therapy.168,235 Initial administration through peripheral venous cannulas is safe,263,264 indicating that the initiation of vasopressors does not require central venous access.235
Rapid sequence inductionTI is the gold standard for securing the airway, and RSI is the recommended technique when there is a considerable risk of aspiration in an airway without predictors of difficulty (EO 97.1%).265,266 The aim of RSI, which requires gastric decompression, prior preparation, adequate positioning, peri-intubation oxygenation, anaesthesia induction and cricoid pressure in selected cases,223,267–269 is to: (1) shorten the time from loss of protective reflexes to tracheal sealing with ETT cuff inflation; (2) optimise conditions for successful TI on the first attempt by ensuring adequate depth of anaesthesia and NMB to avoid coughing, vomiting, or increased intra-abdominal pressure 265; and (3) minimize RSI-related risks, mainly hypoxia, hypotension and difficult TI. RSI must be clearly indicated,22,268 given the scant evidence to support its use266,268,270–273 and the potential harm it can cause.266,274,275 The key is to identify patients at risk of aspiration (Fig. 7). In case of doubt, or if a gastric ultrasound is not feasible, it is advisable to assume the highest risk.268 RSI, with or without the Sellick manoeuvre, is also recommended in all emergency TIs (EO 84.4%), given the likelihood of poor gastric emptying and the high risk of aspiration in the frail, critically ill patients.223,268,276
An RSI checklist should be used to improve patient safety (EO 97.1%). Using a checklist (Fig. 4) can reduce the risk of complications71,277–279 by minimizing the cognitive load and the risk of human errors, and can improve safety by standardising the technique.223,235,266,280
Patients with high risk of aspiration should be premedicated with a nonparticulate antacid (e.g., sodium citrate) immediately before induction or an H2 receptor antagonist or a proton pump inhibitor 40−60 min before induction to increase pH and reduce the volume of gastric contents (EO 82.9%).265,281
Use of a nasogastric tube should be individualized (EO 88.6%) as there is no solid scientific basis for routine application.265,282 It is usually used inserted if the residual gastric volume is expected to exceed 200−300 ml based on ultrasound assessment or clinical estimation.265,268 Pre-operative gastric emptying with a Salem double-lumen tube is mandatory in patients with ileus or intestinal obstruction.265,283,284 Gastric emptying should start as soon as possible on the surgical ward and continued during the pre-induction and post-extubation periods.267,284 The tube should be left in place for continuous suction during RSI.265,284,285
Preparation for RSI includes evaluating potential anatomical, physiological, or contextual challenges, developing a primary and rescue plan with clear instructions, and assembling the personnel, equipment, and medication necessary to perform emergency TI.223,266,286 High-efficiency, large-calibre, multi-hole suction instruments, such as a Yankauer or DuCanto catheter, must be readily available to treat possible regurgitation (EO 100%).223,287
A 20–30° head-up position is recommended (sitting or semi-sitting position or reverse Trendelenburg) to prevent passive regurgitation. Should this occur, the patient should be placed in the Trendelenburg position with the head turned to one side to aspirate fluid from the pharynx and trachea before starting PPV (EO 94.3%).267,288
Optimal preoxygenation, apnoeic oxygenation, and individualized haemodynamic optimization are mandatory before anaesthesia induction.223,286 The type of hypnotic agent used during induction has been identified as the sole independent factor associated with cardiovascular instability and/or collapse,289 and must therefore be chosen carefully.255 The agent, dose, and rate of administration must be individualized (EO 91.4%) to the patient’s comorbidities and haemodynamic status, and urgency needed in securing the airway.223,266 Propofol (2−3 mg/kg) provides the best TI conditions, and is therefore first choice in haemodynamically stable euvolemic patients.265,274,276 In unstable patients, however, it can increase haemodynamic complications and the risk of a fatal outcome.243 It has also been identified as an independent risk factor for peri-intubation haemodynamic collapse,289 suggesting that it should be avoided in critically ill patients at risk of haemodynamic instability,255 in which case etomidate (0.2−0.3 mg/kg) and ketamine (1−2 mg/kg iv) can be used instead.275,286 Ketamine, which induces mild myocardial depression, may cause haemodynamic collapse in patients with depleted sympathetic reserve (e.g., severe hypovolaemic shock),290 and should therefore be avoided in patients with acute myocardial ischaemia.223,291 Etomidate may be associated with a lower risk of postinduction hypotension compared to ketamine.290 In agitated and non-cooperative patients, delayed sequence induction, which consists of ketamine in 0.25−0.5 mg/kg boluses to achieve a dissociative state, followed by preoxygenation and subsequent administration of a neuromuscular relaxant, may be considered.233,292–294
Although RSI has not hitherto included opioids, the use of alfentanil (15−40 μg/kg), remifentanil (1 μg/kg), and fentanyl (2−5 μg/kg) is now common practice, since they reduce the dose of hypnotic agent required, promote haemodynamic stability by attenuating the cardiovascular response to laryngoscopy, and improve intubation conditions265,271,283,285 without causing excessive hypotension and bradycardia.275,283,295
The administration of a neuromuscular blocking agent is the cornerstone of RSI,286 as it improves TI conditions, suppresses coughing and laryngospasm, reduces complications, and optimizes chest wall compliance.296,297 Neuromuscular blockade should be performed to improve TI conditions and reduce the incidence of intubation-related adverse events in the general population (1B).
Rocuronium 1.0–1.2 mg/kg is comparable to succinylcholine 1.0–1.5 mg/kg in RSI,269,298–300 has a safer clinical profile, provides longer blockade,266 and, using sugammadex (16 mg/kg), can be reversed more quickly than succinylcholine 301; the rescue dose must be precalculated and immediately available for emergency reversal.266,302 Succinylcholine can cause malignant hyperthermia, hyperkalaemia, and the muscle fasciculations caused increase intragastric pressure and shorten apnoea time.303,304 Overall, rocuronium is a better choice.235,303–305 The combination rocuronium + sugammadex is not inferior to succinylcholine in RSI (1B). Priming or precurarization are not recommended due to their questionable effectiveness and safety, given the risk of loss of protective reflexes.265,306
The use of the cricoid pressure is controversial.265,268,295 It has not been shown to prevent aspiration,307–309 it is biomechanically impossible to maintain the recommended pressure,310 and it decreases lower oesophageal sphincter tone.311 It can also contribute to airway obstruction,270 hamper laryngoscopy, TI,309 mask ventilation312 and insertion, ventilation, and TI through an SGA,313 obstruct the glottic view on FOI,314 and prolong TI time.309,315 For all these reasons, the routine use of cricoid pressure cannot be recommended (EO 81.3%) 223,260,286,316,317; it must be planned individually and applied when mask ventilation will be needed during apnoea,286 since it prevents gastric insufflation.318 When indicated, cricoid pressure must be: (1) performed correctly: 1 kg (10 N) of pressure until loss of consciousness, followed by 3 kg (30 N) until the ETT cuff has been inflated 265,317; and (2) released if it hampers laryngoscopy, TI, or ventilation, before inserting a SGA, and in case of vomiting.
Apnoeic oxygenation is associated with a lower incidence of desaturation and a higher first-attempt TI success rate.196,319–321 A “modified RSI”, after weighing up the individual risks vs. benefits, can be used in patients at high risk of hypoxia who are not candidates for awake tracheal intubation (EO 85.7%).322 This consists of two-handed mask ventilation or mechanical ventilation with low inspiratory pressure (<15 cmH2O with no cricoid pressure or <20 cmH2O with cricoid pressure).132,159,223,266,268,318,323,324 This practice, excluding high-risk aspiration patients, has been associated with a significantly lower incidence of desaturation without increasing the risk of aspiration.325,326
For TI, clinicians should choose the type of laryngoscope and blade that will maximise the likelihood of first-attempt success. There is no evidence to support any particular device; the choice will depend on the patient’s clinical status and operator preference. 266 VL with a stylet could be the best option.211,327–330
RecommendationWe recommend performing neuromuscular blockade to improve TI conditions and reduce the incidence of intubation-related adverse events in the general population.
Strong recommendation; moderate level of evidence (⊕⊕⊕⊝)
The combination rocuronium + sugammadex is not inferior to succinylcholine in RSI
Strong recommendation; moderate level of evidence (⊕⊕⊕⊝)
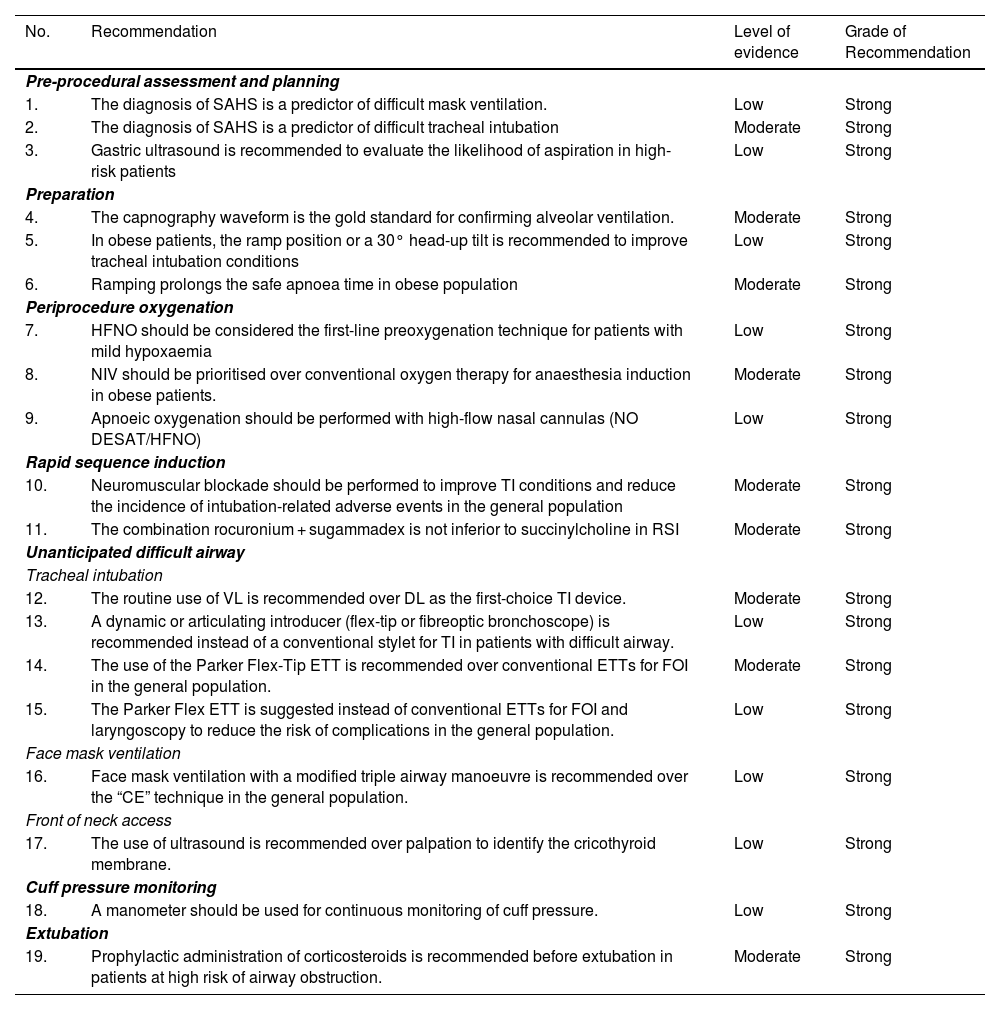
Search strategies and GRADE tables are shown in Appendix. Supplementary data.
| No. | Recommendation | Level of evidence | Grade of Recommendation |
|---|---|---|---|
| Pre-procedural assessment and planning | |||
| 1. | The diagnosis of SAHS is a predictor of difficult mask ventilation. | Low | Strong |
| 2. | The diagnosis of SAHS is a predictor of difficult tracheal intubation | Moderate | Strong |
| 3. | Gastric ultrasound is recommended to evaluate the likelihood of aspiration in high-risk patients | Low | Strong |
| Preparation | |||
| 4. | The capnography waveform is the gold standard for confirming alveolar ventilation. | Moderate | Strong |
| 5. | In obese patients, the ramp position or a 30° head-up tilt is recommended to improve tracheal intubation conditions | Low | Strong |
| 6. | Ramping prolongs the safe apnoea time in obese population | Moderate | Strong |
| Periprocedure oxygenation | |||
| 7. | HFNO should be considered the first-line preoxygenation technique for patients with mild hypoxaemia | Low | Strong |
| 8. | NIV should be prioritised over conventional oxygen therapy for anaesthesia induction in obese patients. | Moderate | Strong |
| 9. | Apnoeic oxygenation should be performed with high-flow nasal cannulas (NO DESAT/HFNO) | Low | Strong |
| Rapid sequence induction | |||
| 10. | Neuromuscular blockade should be performed to improve TI conditions and reduce the incidence of intubation-related adverse events in the general population | Moderate | Strong |
| 11. | The combination rocuronium + sugammadex is not inferior to succinylcholine in RSI | Moderate | Strong |
| Unanticipated difficult airway | |||
| Tracheal intubation | |||
| 12. | The routine use of VL is recommended over DL as the first-choice TI device. | Moderate | Strong |
| 13. | A dynamic or articulating introducer (flex-tip or fibreoptic bronchoscope) is recommended instead of a conventional stylet for TI in patients with difficult airway. | Low | Strong |
| 14. | The use of the Parker Flex-Tip ETT is recommended over conventional ETTs for FOI in the general population. | Moderate | Strong |
| 15. | The Parker Flex ETT is suggested instead of conventional ETTs for FOI and laryngoscopy to reduce the risk of complications in the general population. | Low | Strong |
| Face mask ventilation | |||
| 16. | Face mask ventilation with a modified triple airway manoeuvre is recommended over the “CE” technique in the general population. | Low | Strong |
| Front of neck access | |||
| 17. | The use of ultrasound is recommended over palpation to identify the cricothyroid membrane. | Low | Strong |
| Cuff pressure monitoring | |||
| 18. | A manometer should be used for continuous monitoring of cuff pressure. | Low | Strong |
| Extubation | |||
| 19. | Prophylactic administration of corticosteroids is recommended before extubation in patients at high risk of airway obstruction. | Moderate | Strong |
AW: airway; DL: direct laryngoscopy; ETT: endotracheal tube; FOB: fiberoptic bronchoscopy; HFNO: high flow nasal oxygen therapy; NIV: non-invasive ventilation; NOT DESETT: Nasal oxygen therapy during efforts to secure an ETT; RSI: Rapid sequence induction; SAHS: Sleep apnoea-hypopnoea syndrome; TI: tracheal intubation; VL: videolaryngoscopy.
Expert statement derived from the results of the Delphi questionnaire| No. | Statement | % in favour [for; neutral; against] |
|---|---|---|
| Human factors | ||
| 1. | No more than 3 attempts should be made in each non-invasive airway management plan | 88.6 [31; 2; 2] |
| 2. | The first attempt must be made under optimal conditions | 100 [35; 0; 0] |
| 3. | The most appropriate first-line technique should be the one that gives the greatest likelihood of achieving success on the first attempt. | 94.3 [33; 1; 1] |
| 4. | Clinicians should use visual cognitive aids to deal with emergencies | 97.1 [34; 1; 0] |
| 5. | A standardized difficult airway trolley should be placed near any room where airway management is performed | 100 [35; 0; 0] |
| 6. | Checklists should be used to reduce human error, speed up tasks, and promote an airway management safety culture | 100 [35; 0; 0] |
| 7. | Systems that promote ergonomics and communication models should be implemented | 91.4 [32; 3; 0] |
| Pre-procedural assessment and planning | ||
| 8. | A pre-procedure evaluation should be performed in all patients who require airway management | 100 [35; 0; 0] |
| 9. | Pre-procedural airway assessment should be multifactorial, structured, and aimed at detecting anatomical, physiological, and contextual difficult airway | 97.1 [34; 1; 0] |
| 10. | The airway assessment can begin by detecting predictors of difficulty or failure for the primary plan and subsequently for the three alternative plans | 97.1 [34; 1; 0] |
| 11. | Multivariate models could have greater predictive capacity | 97.1 [34; 1; 0] |
| 12. | Decision-making must be individualized to the patient, operator, context, and time of day | 97.1 [34; 1; 0] |
| 13. | Intake of food and liquids should be restricted according to preoperative fasting guidelines | 97.1 [34; 1; 0] |
| 14. | A full stomach is an indication for TI to protect the airway | 88.6 [31; 2; 2] |
| Preparation | ||
| 15. | Waveform capnography must be available at all locations where airway management is performed to confirm successful ventilation with any of the 4 plans used | 97.1 [34; 1; 0] |
| Difficult airway management: basic options | ||
| 16. | A pre-intubation risk-benefit analysis must be performed to determine the suitability of the airway management technique | 97.1 [34; 1; 0] |
| 17. | Awake airway management is recommended when TI is likely to be highly difficult or impossible, or when there are composite predictors of difficulty, physiological alterations, and negative contextual issues | 82.9 [29; 5; 1] |
| 18. | General anaesthesia with preserved spontaneous breathing should be induced whenever awake tracheal intubation is advisable but general anaesthesia is unavoidable due to lack of cooperation or the urgency of the situation, and when there are no predictors of physiological or context-related difficulties or obstructive airway pathology | 91.4 [32; 2; 1] |
| 19. | In patients with predictors of physiological or context- difficulties, postponement can be considered if it outweighs the risk of proceeding with airway management, or alternative anaesthesia strategies can be considered | 85.7 [30; 5; 0] |
| Known or anticipated difficult airway | ||
| 20. | Awake tracheal intubation is the technique of choice in patients with known or anticipated difficult airway | 85.7 [30; 4; 1] |
| 21. | High-flow nasal oxygen therapy is recommended over conventional low-flow cannulas | 91.4 [32; 3; 0] |
| 22. | NIV through an endoscopy face mask could be useful when intubating critically ill patients with hypoxaemia | 82.9 [29; 6; 0] |
| 23. | Premedication with an antisialogogue, preferably glycopyrrolate, is recommended to optimize the local anaesthetic effectiveness and improve the field of vision. | 80 [28; 5; 2] |
| 24. | Sedation is an optional complement to adequate topical anaesthesia in awake tracheal intubation | 88.6 [31; 2; 2] |
| 25. | The goals of conscious sedation in awake tracheal intubation are effective amnesia, patient satisfaction, and analgesia to reduce coughing, gagging, and haemodynamic reflexes while preserving airway patency, spontaneous breathing, and protective laryngeal reflexes. | 94.3 [33; 2; 0] |
| 26. | If the first-line technique (FB or VL) fails, the alternative technique should be used. | 80 [28; 6; 1] |
| 27. | A third attempt may benefit from a multimodal approach (VL + FB) | 100 [35; 0; 0] |
| 28. | The combination of intubating SGA and FOI can be a useful rescue technique to maintain oxygenation and airway patency and be used as a conduit to facilitate TI | 100 [35; 0; 0] |
| 29. | A smaller calibre ETT than usual is recommended when performing FB and VL. | 85.7 [30; 4; 1] |
| 30. | There should be less difference between the external diameter of the FB and the internal diameter of the ETT in order to facilitate FOI. | 85.7 [30; 3; 2] |
| 31. | Standard PVC ETTs are not recommended for FOI as they are more likely to impinge on the glottic structures. | 71.9 [23; 4; 5] |
| 32. | After visual confirmation of tracheal intubation, general anaesthesia should be induced after inflating the cuff and confirming intubation with capnography. | 94.3 [33; 2; 0] |
| 33. | Fallback techniques and approaches should be planned in advance and implemented without delay after failure of the primary technique. | 100 [35; 0; 0] |
| 34. | A FONA plan must be prepared before attempting awake tracheal intubation if it is likely to fail and result in complete airway obstruction. | 88.6 [31; 4; 0] |
| 35. | Awake tracheostomy under local anaesthesia should be performed in patients with pre-existing critical airway compromise. | 82.9 [29; 6; 0] |
| 36. | Awake cricothyrotomy would be the most appropriate technique in the event of emergency critical airway compromise. | 91.4 [32; 3; 0] |
| 37. | Awake ECMO under local anaesthesia may be the safest option when all 4 conventional plans are likely to be impossible, to fail, or to be ineffective and the patient is at risk of complete airway obstruction. | 90.6 [29; 1; 2] |
| Unanticipated difficult airway | ||
| Periprocedure oxygenation | ||
| 38. | HFNO should be considered the first-line preoxygenation technique for patients with mild hypoxaemia (PaO2/FiO2 > 200 mmHg), while NIV is the technique of choice in those with severe hypoxaemia (PaO2/FiO2 ≤ 200 mmHg). | 87.5 [28; 3; 1] |
| 39. | Preoxygenation with NIV + HFNO and apnoeic oxygenation with HFNO should be a priority in critically ill patients undergoing TI. | 85.7 [30; 4; 1] |
| Rapid sequence induction | ||
| 40. | RSI is the recommended technique when there is a considerable risk of aspiration in an airway without predictors of difficulty | 97.1 [34; 1; 0] |
| 41. | RSI, with or without the Sellick manoeuvre, should be performed in in all emergency TIs | 84.4 [27; 1; 4] |
| 42. | An RSI checklist should be used to improve patient safety | 97.1 [34; 1; 0] |
| 43. | Patients with high risk of aspiration should be premedicated with a nonparticulate antacid immediately before induction or an H2 receptor antagonist or a proton pump inhibitor 40−60 min before induction to increase pH and reduce the volume of gastric contents | 82.9 [29; 5; 1] |
| 44. | Use of a nasogastric tube should be individualized | 88.6 [31; 4; 0] |
| 45. | High-efficiency, large-calibre, multi-hole suction instruments must be readily available to treat possible regurgitation | 100 [35; 0; 0] |
| 46. | A 20–30° head-up position is recommended to prevent passive regurgitation. Should this occur, the patient should be placed in the Trendelenburg position with the head turned to one side to aspirate fluid from the pharynx and trachea before starting PPV | 94.3 [33; 2; 0] |
| 47. | The choice, dose, and rate of administration of hypnotic agent must be individualized | 91.4 [32; 3; 0] |
| 48. | In agitated, non-cooperative patients, delayed sequence induction can be considered to achieve adequate preoxygenation. | 71.9 [23; 3; 6] |
| 49. | The routine use of cricoid pressure cannot be recommended | 81.3 [26; 2; 4] |
| 50. | A “modified RSI” can be used in patients at high risk of hypoxia who are not candidates for awake tracheal intubation | 85.7 [30; 5; 0] |
| Tracheal intubation | ||
| 51. | Videolaryngoscopes with standard Macintosh blade (which allow for both direct and indirect laryngoscopy) are appropriate for tracheal intubation in patients without predictors of difficulty, while those with hyperangulated blade (with or without a guiding channel) are indicated in patients with known or anticipated difficult airway. | 94.3 [33; 1; 1] |
| 52. | The availability of a tracheal tube introducer is recommended at all locations where airway management is performed. | 97.1 [34; 1; 0] |
| 53. | A flat capnography trace (grade 3 ventilation) indicates failed TI until proven otherwise. | 80 [28; 6; 1] |
| 54. | Capnography waveform monitoring during maintenance of mechanical ventilation is highly recommended in all settings | 100 [35; 0; 0] |
| Ventilation with a supraglottic airway | ||
| 55. | An SGA should be inserted without delay to preserve alveolar oxygenation in the event of difficult or failed TI | 85.7 [30; 3; 2] |
| 56. | Immediate availability of a 2GSGA is recommended, along with the necessary proficiency for its use in all locations where airway management is conducted. | 100 [35; 0; 0] |
| 57. | If cricoid pressure has been applied, it must be released during placement of an SGA | 80 [28; 5; 2] |
| 58. | A 90-degree rotation, jaw thrust, and the use of DL or VL (of choice) with the “insert-detect-correct-as-you-go” technique enhance the efficacy and safety of SGAs by facilitating insertion, increasing the success rate on the first attempt, and reducing pharyngeal trauma. | 82.9 [29; 4; 2] |
| 59. | FOI can be performed through the SGA if the situation is stable, NMB is adequate, and if the operator is skilled in the technique | 97.1 [34; 1; 0] |
| Mask ventilation | ||
| 60. | The optimal mask ventilation technique should be used as first-line the triple airway manoeuvre of head extension, jaw thrust and mouth opening, placement of a nasopharyngeal or oropharyngeal cannula and the two-handed “VE” technique in a patient with optimal positioning under intense NMB. | 80 [28; 3; 4] |
| 61. | The declaration of failed mask ventilation necessitates an immediate transition to SGAV. | 85.7 [30; 2; 3] |
| Front of neck access | ||
| 62. | The failure of the 3 non-invasive plans (primary and rescue), regardless of the SpO2 value, requires the verbalization of CICO and subsequent execution of FONA. | 90.6 [29; 0; 3] |
| 63. | Cricothyrotomy is the technique of choice in a CICO situation | 91.4 [32; 2; 1] |
| 64. | The scalpel-introducer-tube cricothyrotomy technique is recommended | 91.4 [32; 2; 1] |
| 65. | The performance of FONA should be feasible at any location where airway management is conducted. | 100 [35; 0; 0] |
| 66. | Emergency cricothyrotomy should be converted to ETT or tracheostomy, as there is insufficient evidence to support its use as long-term treatment | 85.7 [30; 3; 2] |
| 67. | Failure of a cricothyrotomy to secure the airway makes it advisable to perform a tracheostomy by an experienced operator. | 94.3 [33; 1; 1] |
| 68. | All clinicians involved in airway management must acquire and maintain the skills needed to perform a surgical or percutaneous cricothyrotomy using the Seldinger technique. | 100 [35; 0; 0] |
| Cuff pressure monitoring | ||
| 69. | Cuff pressure should be established with the minimum pressure needed to guarantee a safe, effective seal. Cuff pressure should remain between 20−30 cm H2O in ETTs and tracheostomy and cricothyrotomy cannulas, and <60 cm H2O for SGAs | 94.3 [33; 1; 1] |
| Extubation | ||
| 70. | Reintubation should always be considered potentially difficult due to the addition complexity involved | 97.1 [34; 1; 0] |
| 71. | The cuff leak test, preferably using a quantitative evaluation technique, ultrasound evaluation and laryngeal visualization with VL or fibreoptic bronchoscope can facilitate decision-making. | 97.1 [34; 1; 0] |
| 72. | Awake extubation and the use of advanced techniques is the most suitable approach in patients with difficult airway | 94.3 [33; 2; 0] |
| 73. | Waveform capnography should be available in recovery units and should be used in high-risk patients | 97.1 [34; 1; 0] |
| Documentation | ||
| 74. | A history of failure in previous procedures is the most accurate predictor of failure in subsequent treatments. | 97.1 [34; 1; 0] |
| Organisational aspects of airway management | ||
| 75. | Each institution should appoint an “Airway Lead” | 100 [35; 0; 0] |
| Teaching and training | ||
| 76. | Skill acquisition should be gradual, involving a cognitive phase, simulation, and clinical training with problem-solving until the learning curve is completed, with evaluation and feedback from the instructor at each stage. | 100 [35; 0; 0] |
| 77. | Continuing education and regular training are required for the development of new skills or techniques and the maintenance of competencies, preferably on an annual basis. | 97.1 [34; 1; 0] |
ATI: awake tracheal intubation; DA: difficult airway; DL: direct laryngoscopy; ECMO: extracorporeal membrane oxygenation; ETT: endotracheal tube; EtCO2: end-tidal carbon dioxide concentration; EtO2: end-tidal oxygen concentration; FOB: fiberoptic bronchoscopy; FiO2: inspiratory fraction of oxygen; FMV: Face mask ventilatio; FONA: front-of-neck access; 2GSGA: second generation supraglottic device; HFNO: high flow nasal oxygen therapy; IC: informed consent; NIV: non-invasive ventilation; NMB: neuromuscular blockade; PaO2: arterial partial pressure of oxygen; RSI: rapid sequence induction; SGA: supraglottic device; SGAV: supraglottic airway ventilation; SpO2: peripheral oxygen saturation; TI: tracheal intubation; VL: videolaryngoscopy.
Authors’ contribution- •
Manuel Á. Gómez-Ríos: drafting of the manuscript, preparation of all cognitive aids, graphic material, tables and appendices, literature review, critical reading, levels of evidence, final revision of the document.
- •
José Alfonso Sastre: Draft of RSI sections, SGA, and checklist, risk factor tables, information document models, literature review, critical reading, levels of evidence, final revision of the document.
- •
Xavier Onrubia-Fuertes: FONA contribution, unanticipated difficult tracheal intubation, literature review, final revision of the document.
- •
Teresa López: Draft of SGA and ECMO sections, literature review, critical reading, levels of evidence, final revision of the document.
- •
Alfredo Abad-Gurumeta: literature review, critical reading, levels of evidence, final revision of the document.
- •
Rubén Casans-Francés: literature review, critical reading, final revision of the document.
- •
David Gómez-Ríos: literature review, critical reading, final revision of the document.
- •
José Carlos Garzón: literature review, critical reading, final revision of the document.
- •
Vicente Martínez-Pons: Algorithm for unanticipated difficult tracheal intubation, literature review, final revision of the document.
- •
Marta Casalderrey-Rivas: literature review, critical reading, final revision of the document.
- •
Miguel Ángel Fernández-Vaquero: Algorithm for unanticipated difficult tracheal intubation, literature review, and critical reading aimed at AW predictors and assessment, final revision of the document.
- •
Eugenio Martínez-Hurtado: Algorithm for unanticipated difficult tracheal intubation, final revision of the document.
- •
Ricardo Martín-Larrauri: Algorithm for unanticipated difficult tracheal intubation, final revision of the document.
- •
Laura Reviriego-Agudo: Algorithm for unanticipated difficult tracheal intubation, literature review, final revision of the document.
- •
Uxía Gutierrez-Couto: literature search strategies.
- •
Javier García-Fernández: critical reading, final revision of the document.
- •
Alfredo Serrano Moraza: prehospital setting section, literature review, critical reading, final revision of the document.
- •
Luis Jesús Rodríguez Martín: prehospital setting section, final revision of the document.
- •
Carmen Camacho Leis: prehospital setting, final revision of the document.
- •
Salvador Espinosa Ramírez: prehospital setting, final revision of the document.
- •
José Manuel Fandiño Orgeira: critical reading, final revision of the document.
- •
Manuel José Vázquez Lima: critical reading, final revision of the document.
- •
Miguel Mayo-Yáñez: FONA contribution, final revision of the document.
- •
Pablo Parente-Arias: FONA contribution, final revision of the document.
- •
Jon Alexander Sistiaga-Suárez: critical reading, final revision of the document.
- •
Manuel Bernal-Sprekelsen: critical reading, final revision of the document.
- •
Pedro Charco-Mora: coordination, algorithm for unanticipated difficult tracheal intubation, draft ergonomic options, draft teaching and training, literature review, critical reading, final revision of the document.
MAGR received lecture honoraria from Medtronic.
XOF received honoraria for lecture and practical wordshop on neuromuscular blockade from Merck Sharp & Dohme.
RCF received honoraria for lectures from FreseniusKabi.
AAG received honoraria for lectures from Merck Sharp & Dohme and 3 M Edwars.
Delphi expert panelManuel Á. Gómez-Ríos, José Alfonso Sastre, Xavier Onrubia-Fuertes, Teresa López, Alfredo Abad-Gurumeta, José Carlos Garzón, Vicente Martínez-Pons, Marta Casalderrey-Rivas, Miguel Ángel Fernández-Vaquero, Eugenio Martínez-Hurtado, Ricardo Martín-Larrauri, Laura Reviriego-Agudo, Javier García-Fernández, Pedro Charco-Mora, Raquel García Álvarez, Alfredo Panadero Sánchez, Alejandra Prieto Gundín, María Luisa Santos Marqués, David Domínguez García, Irma María Barrio, Uxío García-Aldao, Aixa Espinosa, Carmen M. Holgado Pascual, Jesús Carazo Cordobés, Cristobal Añez Simón, Natividad Quesada Gimeno, Marina Gómez-Morán Quintana, Silvia Bermejo, Pilar Cabrerizo Torrente, Francisca Llobell, Roque J. Company Teuler, Teresa del Castillo Fernández de Betoño, Carlos González Perrino and Paola Hurtado.
External reviewersJaideep Pandit, Luis Gaitini, Tomasz Gaszyński and Pavel Michalek.
CollaboratorsYou can consult the list of collaborators in Appendix. Supplementary data.