The Airway Management section of the Spanish Society of Anesthesiology, Resuscitation, and Pain Therapy (SEDAR), the Spanish Society of Emergency Medicine (SEMES), and the Spanish Society of Otorhinolaryngology and Head and Neck Surgery (SEORL-CCC) present the Guide for the comprehensive management of difficult airway in adult patients. Its principles are focused on the human factors, cognitive processes for decision-making in critical situations, and optimization in the progression of strategies application to preserve adequate alveolar oxygenation in order to enhance safety and the quality of care. The document provides evidence-based recommendations, theoretical-educational tools, and implementation tools, mainly cognitive aids, applicable to airway management in the fields of anesthesiology, critical care, emergencies, and prehospital medicine. For this purpose, an extensive literature search was conducted following PRISMA-R guidelines and was analyzed using the GRADE methodology. Recommendations were formulated according to the GRADE methodology. Recommendations for sections with low-quality evidence were based on expert opinion through consensus reached via a Delphi questionnaire.
La sección de Vía Aérea de la Sociedad Española de Anestesiología, Reanimación y Terapéutica del Dolor (SEDAR), la Sociedad Española de Medicina de Urgencias y Emergencias (SEMES) y la Sociedad Española de Otorrinolaringología y Cirugía de Cabeza y Cuello (SEORLCCC) presentan la Guía para el manejo integral de la vía aérea difícil en el paciente adulto. Sus principios están focalizados en el factor humano, los procesos cognitivos para la toma de decisiones en situaciones críticas y la optimización en la progresión de la aplicación de estrategias para preservar una adecuada oxigenación alveolar con el objeto de mejorar la seguridad y la calidad asistencial. El documento proporciona recomendaciones basadas en la evidencia científica actual, herramientas teórico-educativas y de implementación, fundamentalmente ayudas cognitivas, aplicables al tratamiento de la vía aérea (VA) en el campo de la anestesiología, cuidados críticos, urgencias y medicina prehospitalaria. Para ello, se realizó una amplia búsqueda bibliográfica según las directrices PRISMA-R y se analizó utilizando esta metodología. Las recomendaciones se formularon de acuerdo con esta metodología. Las recomendaciones de aquellas secciones con evidencia de baja calidad se basaron en la opinión de expertos mediante el consenso alcanzado a través de un cuestionario Delphi.
Awake airway management is the technique of choice in patients with known or anticipated difficult airway (EO 85.7%)1 since it (1) preserves airway patency and spontaneous ventilation, increases respiratory reserve, and protects against aspiration by preserving laryngeal reflexes1,2; (2) facilitates a gradual transition to positive pressure ventilation (PPV) and slow induction of general anaesthesia (GA) in patients at risk of haemodynamic collapse3,4; (3) facilitates tracheal intubation by preventing soft tissue collapse, dilating peritracheal structures, improving the glottic view by preventing the larynx from adopting a more anterior position, and allowing the operator to locate a distorted glottis by the presence of air bubbles; (4) can be performed in a sitting position in a collaborative patient, which also helps evaluate the patient’s neurological status; (5) keeps all treatment options open and allows for decision-making based on findings.1,3,5–8
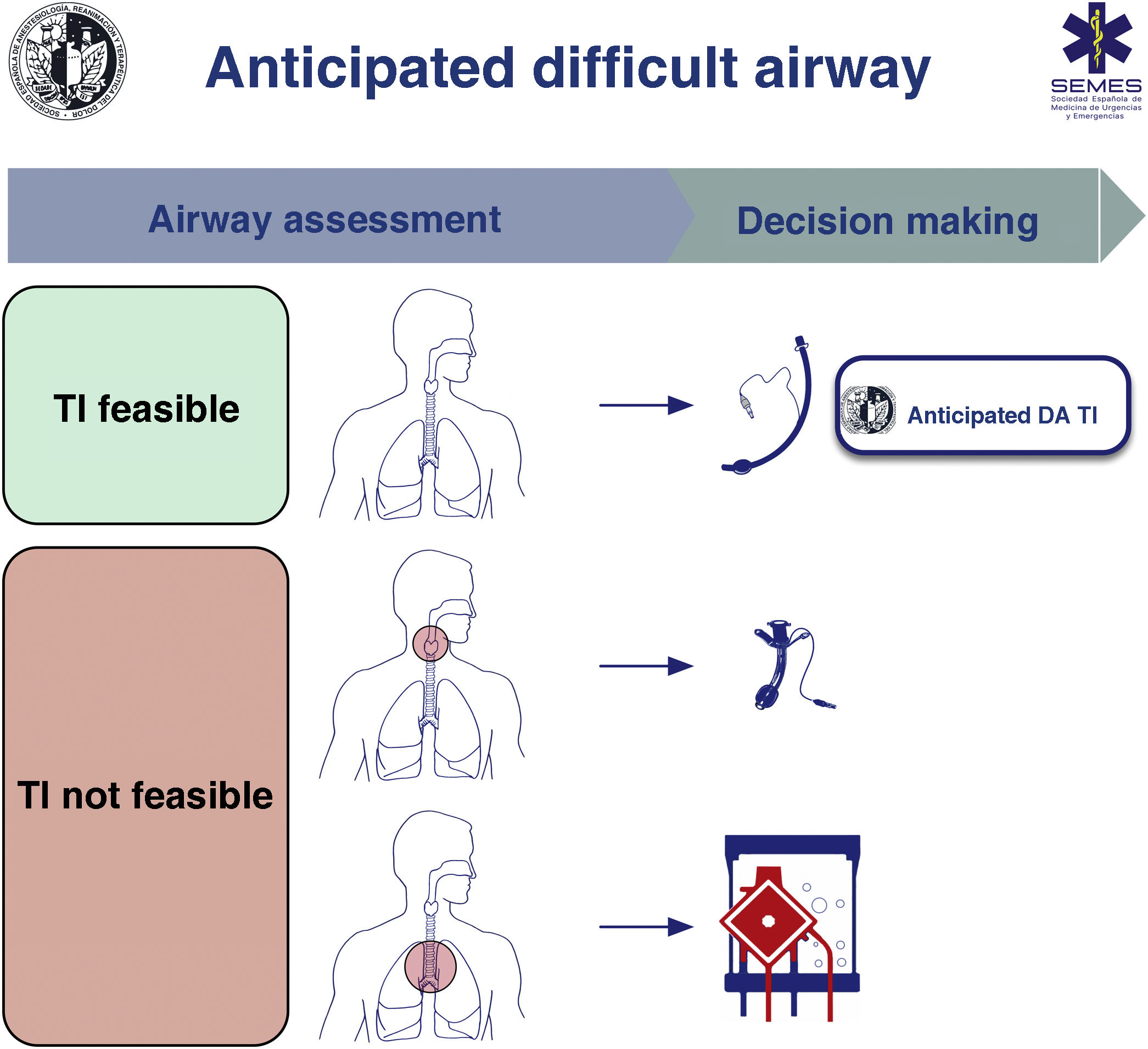
A known or anticipated DA requires formulation of team strategies with a comprehensive multidisciplinary discussion beforehand about sequential plans (primary and alternative) to achieve oxygenation and ventilation and prevent aspiration.1,7,9,10
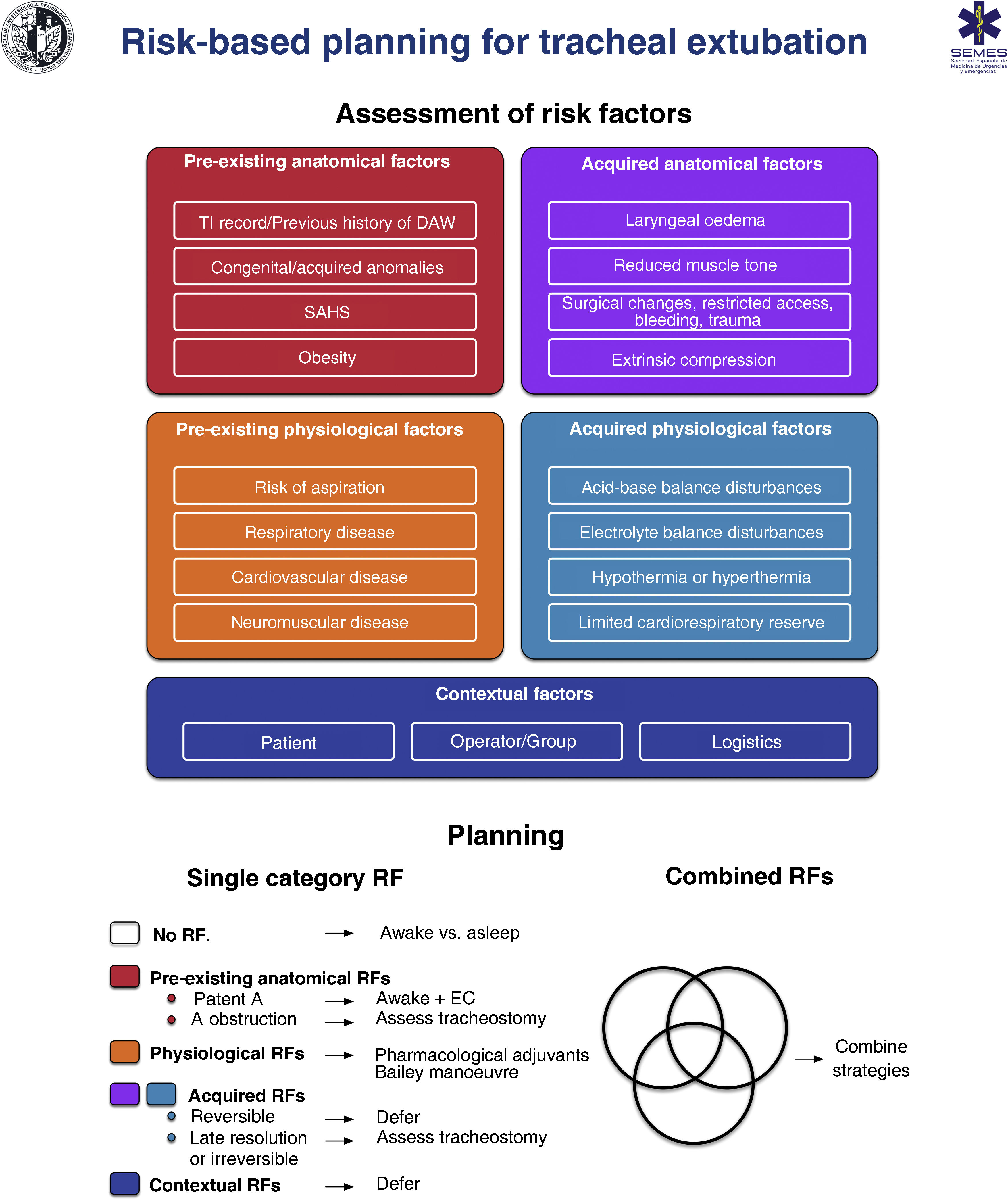
When the DA is secondary to an obstructive pathology, the team will need to evaluate the patient’s respiratory status and the cause, location, and degree of obstruction (greater or less than 50%) using clinical signs and symptoms, imaging tests, and flexible nasolaryngoscopy (FNL).1,7,10,11 The risks and benefits of each approach must be carefully considered and the strategy must be agreed by the entire medical-surgical team.1Fig. 1 shows a cognitive aid for decision-making in the event of an anticipated DA. Lower-tier plans act as rescue strategies in the event of failure of the upper-tier plan if selected as primary. In all cases, it is advisable to perform awake airway management. (1) Supraglottic lesions causing mild obstruction that can be overcome with an ETT allow for TI, usually fibreoptic intubation (FOI). (2) In the case of obstructive supraglottic lesions causing stenosis greater than 50% (or with inspiratory stridor at rest),7 non-obstructive supraglottic lesions that impede TI (or would cause unacceptable morbidity), and glottic or subglottic lesions, it is advisable to perform tracheotomy or cricothyrotomy as a primary approach.10 (3) Lower tracheal obstructive lesions not salvageable with an ETT or tracheal cannula require the application of extracorporeal membrane oxygenation (ECMO).10
Active oxygenation strategies should be implemented throughout the procedure. High-flow nasal oxygen therapy (HFNO), although requiring further validation in this context, may be the technique of choice. HFNO is recommended over conventional low-flow cannulas (EO 91.4%).
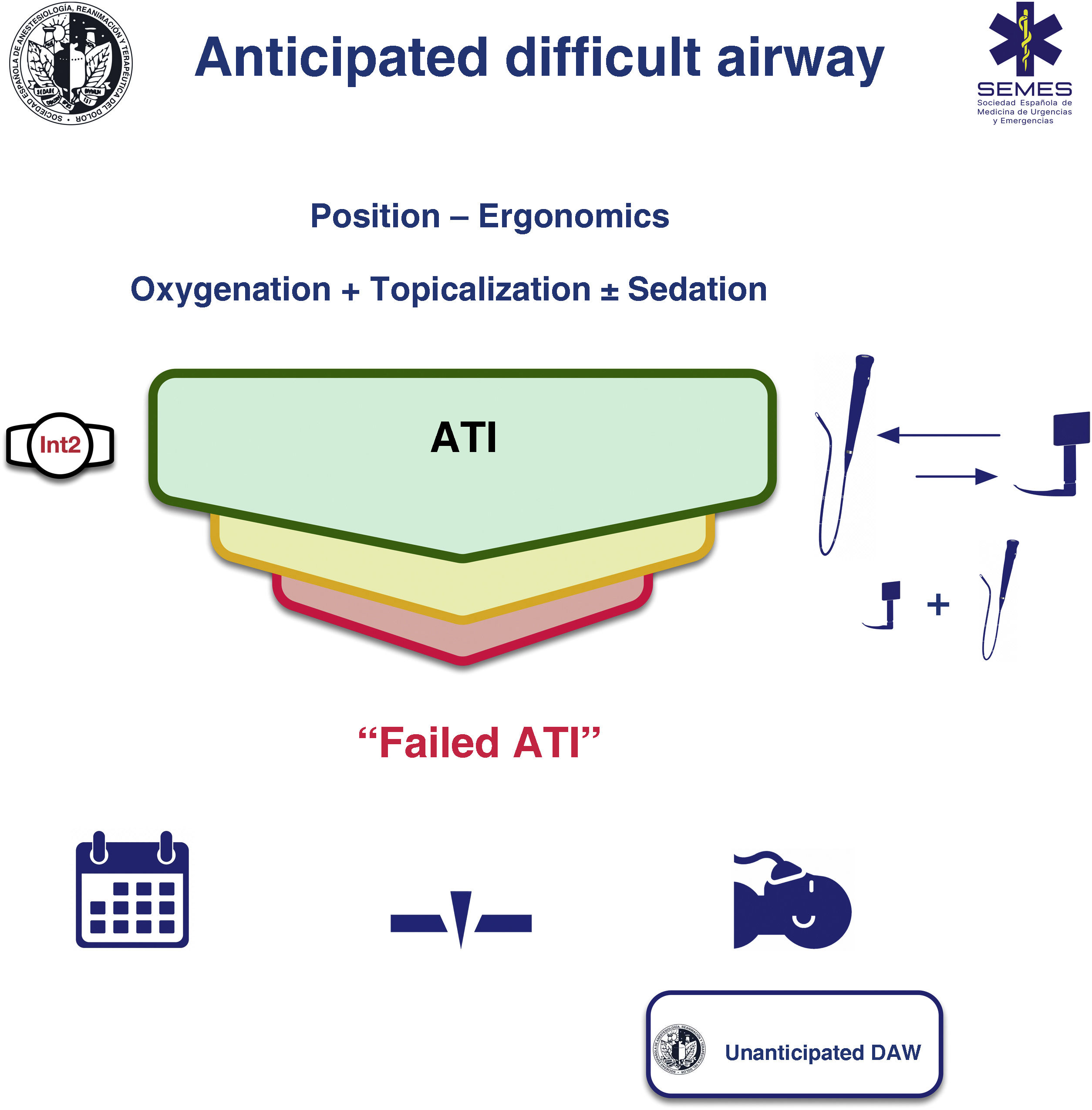
Awake tracheal intubationWhen the airway can be secured noninvasively, awake tracheal intubation (ATI) remains the gold standard technique3,12,13 due to its safety and reliability.14,15 The cornerstones of successful ATI are: continuous oxygenation, topicalization of the airway, sedation (optional), and selection, experience, and use of an appropriate TI device and technique. The ideal protocol in terms of efficacy and safety is unknown, so the most appropriate one should be chosen based on the clinical context and the individual characteristics of each patient, as well as the experience and preferences of the operator.16–18Fig. 2 shows the SEDAR SEMES SEORL-CCC cognitive aid for TI of the anticipated DA.
Continuous oxygenation improves safety by preventing or minimizing hypoxaemia.12,19 Conventional methods may be insufficient to avoid desaturation.20 HFNO increases tolerance of an airway obstruction, hypoventilation, and longer apnoea time,20–23 and despite the scant evidence, it is becoming the method of choice.13,24
NIV through an endoscopy face mask could be useful when intubating critically ill patients with severe hypoxaemia (EO: 82.9%).25
TopicalizationApplying topical anaesthesia to the airway is a key step in successful ATI.1 Lidocaine (2%–4%), which has a favourable cardiovascular and systemic toxicity risk profile, is the most widely used local anaesthetic.12,19,26–28 The maximum dose should not exceed 9 mg/kg,13,29 and only the minimum necessary dose should be used.
The “spray as you go” (SAYGO) technique with an epidural catheter or an atomizer, and regional nerve blocks (glossopharyngeal, superior laryngeal, or transtracheal injection) are the most employed methods for topicalization of the respiratory mucosa.2,12,27 These techniques are often used in combination.28 There is no evidence of the superiority of any particular technique,16 although nerve blocks are more invasive, require multiple bilateral injections, demand experience, and are associated with a higher incidence of complications.2,13,16 Transtracheal injection (4ml of 4% lidocaine) is perhaps the most useful invasive method as it: (1) anaesthetises the infraglottic larynx, upper trachea, and even the supraglottic structures30; (2) has a success rate of over 95%12; and (3) is seldom associated with complications (1:10,000), although they can be important.28,31
The SAYGO technique can minimize the risk of aspiration, because laryngeal reflexes are maintained until just before inserting the ETT.2 Premedication with an antisialogogue is recommended to maximise the local anaesthetic effect and improve the field of vision (EO 80%). The antisialogogue of choice is glycopyrrolate (3 μg/kg), because it is fast-acting, has no effect on the central nervous system, and has a mild vagolytic action.2,12,32 When administered 15−20 min before reduces the dilution and esophageal elimination of the local anesthetic by secretions.1
Regardless of the method used, topicalization should include the oral cavity, nasal cavity if nasotracheal intubation is planned, oropharynx, periglottic area, larynx and trachea.1,30 Otherwise, the insertion of the device and the ETT can provoke reflex responses from the airway, such as coughing or laryngospasm, as well as a cardiovascular response mediated by the sympathetic nervous system.33
SedationSedation is an optional complement to adequate topical anaesthesia in ATI16 (EO 88.6%), as awake tracheal intubation with prior psychological preparation can be performed safely and effectively without sedation.19,28,34 It should never compensate for inadequate topicalization. Although very high levels of anxiety can increase the physiological response to stress and reduce tolerance, oversedation can cause loss of cooperation, respiratory depression, hypoxia, hypercapnia, airway obstruction, aspiration, and cardiovascular instability,19,35,36 so sedation should only be administered after a detailed risk-benefit analysis. The goals of sedation are (EO 94.3%)1,19,35: (1) effective anxiolysis and amnesia while maintaining patient cooperation (“conscious sedation”, sedation level 2–3 on the Ramsay scale)28,37; (2) analgesia to suppress the cough and gag reflexes and reduce the haemodynamic response while preserving airway patency, spontaneous ventilation, and avoiding aspiration. Sedation must be carefully monitored to avoid oversedation, so ideally a second operator should be exclusively responsible for its administration and monitoring.13,19
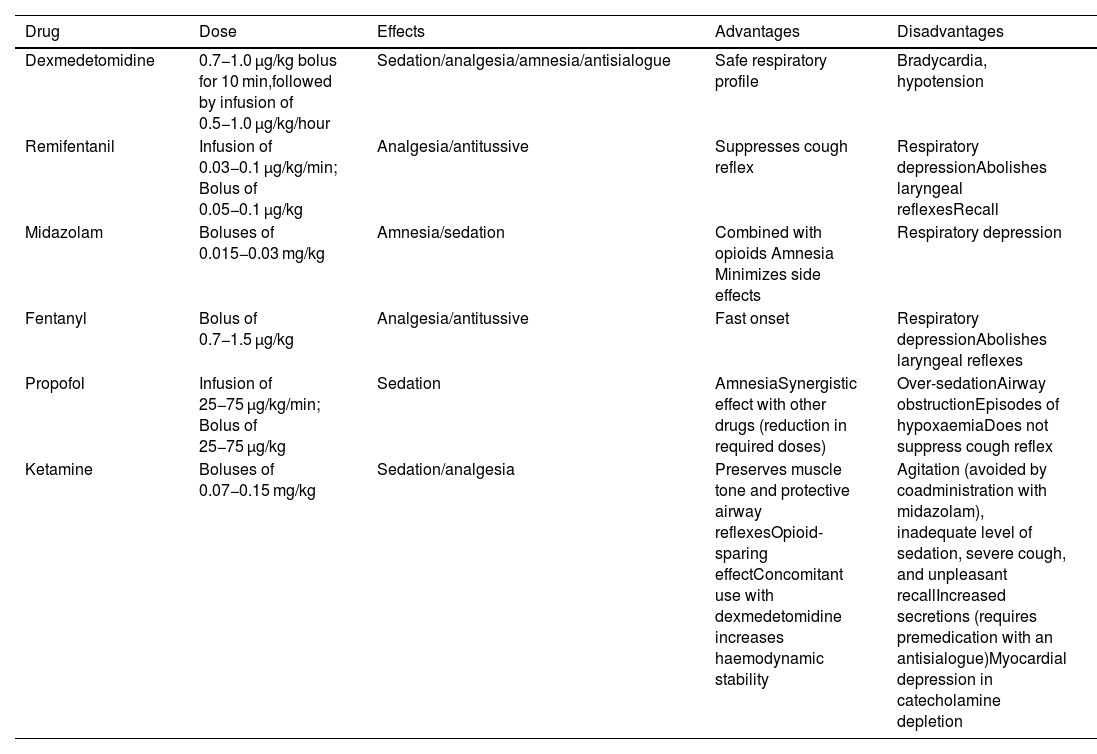
All sedation regimens used have shown a satisfactory level of efficacy and safety16; however, dexmedetomidine, with its anxiolytic, sedative, analgesic, and sympatholytic action, may be the safest and most effective option because it is less likely to cause apnoea and desaturation, is well tolerated, and is associated with better intubation conditions and less recall compared to other drugs16,38–40; however, it can produce episodes of severe bradycardia and hypotension.35 Its ability to maintain respiratory function integrity, even with deep levels of sedation, makes it a good choice for patients at risk of airway obstruction and/or respiratory failure.35 Opioids, particularly remifentanil, attenuate the cough and gag reflexes, although they may increase the risk of chest rigidity and laryngospasm.6,13,19 They are also associated with a high incidence of recall when used alone, and are therefore usually combined with a benzodiazepine such as midazolam.19,28 Monotherapy is usually more predictable and reliable, although the availability of specific opioid and benzodiazepine antagonists improves their safety profile. Remifentanil is a good option when topical anaesthesia has been ruled out.35,41 Propofol is associated with a low incidence of recall, although it can increase the risk of over sedation, airway obstruction, and coughing.13,35,42Table 1 shows the main drugs used for sedation.
Main drugs used for sedation during awake tracheal intubation.
| Drug | Dose | Effects | Advantages | Disadvantages |
|---|---|---|---|---|
| Dexmedetomidine | 0.7−1.0 μg/kg bolus for 10 min,followed by infusion of 0.5−1.0 μg/kg/hour | Sedation/analgesia/amnesia/antisialogue | Safe respiratory profile | Bradycardia, hypotension |
| Remifentanil | Infusion of 0.03−0.1 μg/kg/min; Bolus of 0.05−0.1 μg/kg | Analgesia/antitussive | Suppresses cough reflex | Respiratory depressionAbolishes laryngeal reflexesRecall |
| Midazolam | Boluses of 0.015−0.03 mg/kg | Amnesia/sedation | Combined with opioids Amnesia Minimizes side effects | Respiratory depression |
| Fentanyl | Bolus of 0.7−1.5 μg/kg | Analgesia/antitussive | Fast onset | Respiratory depressionAbolishes laryngeal reflexes |
| Propofol | Infusion of 25−75 μg/kg/min; Bolus of 25−75 μg/kg | Sedation | AmnesiaSynergistic effect with other drugs (reduction in required doses) | Over-sedationAirway obstructionEpisodes of hypoxaemiaDoes not suppress cough reflex |
| Ketamine | Boluses of 0.07−0.15 mg/kg | Sedation/analgesia | Preserves muscle tone and protective airway reflexesOpioid-sparing effectConcomitant use with dexmedetomidine increases haemodynamic stability | Agitation (avoided by coadministration with midazolam), inadequate level of sedation, severe cough, and unpleasant recallIncreased secretions (requires premedication with an antisialogue)Myocardial depression in catecholamine depletion |
Doses extracted from Gil K, et al.6
FOI is classically considered the method of choice for ATI,2,17,37,43 due to its versatility and unique ability to combine with other devices in any treatment plan,44 as well as its efficacy and safety.16,45 However, FOI has a steep learning curve and skills must be maintained with regular practice; it is also fallible and not available in all settings.37,46
VL-guided ATI may be faster than FOI – a factor that could reduce the risk of aspiration - and the skills are easier to learn and maintain.46,47 It has a similar success rate and safety profile as FOI, and equally high patient and operator satisfaction rates,17,43,47,48 making it a valid alternative for a first-line technique.13,17,46–49 Videolaryngoscopes with a hyperangulated blade, with or without a guide channel, are indicated for ATI. In certain circumstances, VL offers additional advantages over FOI.17,37,43 It can accommodate an ETT of any diameter37 and allows the operator to change the ETT without removing the videolaryngoscope,47 and unlike FOI, which is performed blind, it allows the operator to observe the passage of the ETT through the vocal cords, thus reducing the risk of ETT impingement and the resulting trauma.37,50,51 However, unlike FOI, VL-guided ATI is not feasible in patients with a mouth opening of less than 18−20 mm or a space-occupying lesion in the oral cavity,37 and in patients with cervical spine instability without manual stabilization it may cause greater cervical spine movement,52 although the outcomes appear to be similar.53,54 VL, therefore, cannot completely replace FOI.37,43
There is insufficient evidence to recommend any particular technique, so the choice should be context-oriented.43,48 Both approaches are equivalent and complementary43; a failed attempt with one can be rescued with the other, and they can be used in combination - mainly in highly complex cases.2,17,37
Single-use flexible FBs are as safe as reusable scopes,55,56 although there is evidence to suggest that they minimise the risk of cross-contamination infection and are more resource-saving and cost-efficient.57,58
ProcedureConditions of the 4 components of ATI must be optimal from the first attempt in order to maximize the likelihood of success and minimize the number of attempts. If the first-line technique (FOI or VL) fails, the alternative technique should be used (EO 80%). A third attempt may benefit from a multimodal approach, namely VL + FOI (EO 100%), which combines the advantages of both devices.59 This combination can improve the first attempt success rate, reduce TI time, TI-related morbidity,60,61 and could be the approach of choice in patients with severely distorted airways.
The combination of an intubating SGA and FOI can be a useful rescue technique to maintain oxygenation and airway patency and be used as a conduit to facilitate TI (EO 100%). SGA acts as a conduit for FOI, and protects the periglottic structures from secretions or blood.62–64 It also facilitates the localization of the glottis and reduces the difficulty of railroading the ETT over the FB,51 while the FB aids correct SGA placement. This technique can be further simplified by using the latest video laryngeal masks.65,66
No more than 3 attempts at TI should be made (EO 88.6%), because repeated attempts increase the risk of oedema, laryngeal bleeding, and total airway obstruction.51 This is particularly important in the case of preexisting airway obstruction, which can rapidly progress to a CICO situation.9,67 Each failed attempt should be followed by an evaluation of each component of ATI to determine if optimization is feasible.
Factors such as surgical access, anatomical characteristics, extubation plan, and operator preference determine the choice of TI technique.68
The main cause of TI failure19,51 and glottic or subglottic injury50,69 is difficulty railroading the ETT over the FB, stylet or exchanger and through the vocal cords due to impingement on periglottic structures, mainly the right arytenoid cartilage.2,50 Silicone ETTs with a Murphy eye, such as the LMA Fastrach™ ETT (Teleflex Medical, Dublin, Ireland),70 those with a central orifice and backward-facing bevel such as the Parker Flex-Tip™ ETT (Parker Medical, Highlands Ranch, CO 80126, USA),71–74 LMA Fastrach™ ETT75 and BlockBuster ETT (Tuoren Medical Instrument co, Ltd, Changyuan city, China),69 and those made of flexible material with or without a slight anterior curvature, such as the LMA Fastrach™ wire-reinforced ETT69,76 reduce the incidence of this complication.50,69,72,76,77 The Parker Flex-Tip ETT is recommended over conventional ETTs for FOI in the general population (1B). Inserting the tube with the bevel facing backwards, or rotating the tube 90º counter-clockwise after insertion facilitates advancement.78–80 Other useful manoeuvres are neck flexion and releasing mandibular traction or cricoid pressure.51,80 There should be less difference between the external diameter of the FB and the internal diameter of the ETT in order to facilitate FOI (EO 85.7%),2,51,81 or an intubation catheter (e.g., Aintree catheter [AIC; Cook Critical Care, Bloomington, IN, USA]) can be used between the fiberscope and the ETT. Similarly, using the specific ETT for each device will facilitate VL-guided TI.82 The Parker Flex-Tip ETT should be considered instead of conventional ETTs to reduce the risk of FOI- and laryngoscopy-related complications in the general population (1C).
Narrow-bore ETTs give better laryngeal vision during laryngoscopy and facilitate TI by reducing the risk of impingement on periglottic structures.51,83,84 Large-bore ETTs are associated with greater morbidity.83,85–90 ETTs with up to 6.0 mm internal diameter allow the passage of intubation devices, suction devices, and narrow-bore FBs. PPV can be performed without increasing the risk of ventilator-induced lung injury or air trapping,91,92 even when high minute volumes (Vm) are required. The use of narrow-bore ETTs does not increase the risk of injury from aspiration or high cuff pressure, and might even provide a better seal than large bore ETTs.93 Narrow-bore ETTs may not be safe in all cases, such as in patients with abundant secretions or impaired airflow. Therefore, a smaller than usual ETT is recommended when performing FB and VL (EO 85.7%), provided a context-oriented risk-benefit analysis has been performed.83,84,90,94
After TI has been confirmed visually (visualisation of passage of the ETT through the glottis on VL and identification of the carina and advancement of the ETT up 2 or 3 tracheal rings above the carina on FB), GA should be induced after inflating the cuff and confirming TI with capnography (EO 94.3%).2
Fallback techniques and approaches should be planned in advance and implemented without delay after failure of the primary technique (EO 100%).12,67 If a failed ATI is declared, the team has 3 options (1) postpone the procedure, if feasible; (2) perform awake FONA or emergency FONA in the case of airway obstruction and loss of control of the airway; (3) induce GA with complete NMB and follow the algorithm for unanticipated DA. This is a high risk option. Deep sedation or GA induced with ketamine under spontaneous ventilation could be a more favorable step than establishing apnea95 (Fig. 2).
A FONA plan must be prepared before attempting ATI if it is likely to fail and result in complete airway obstruction (EO 88.6%)96 (the “double setup”, see below): the CTM should be marked on the neck to guide the incision,3 and the neck and FONA kit must be prepared with the surgical team present.9 Multidisciplinary management and coordination with an ENT specialist is essential.1,97
RecommendationThe use of the Parker Flex-Tip ETT is recommended over conventional ETTs for FOI in the general population.
Strong recommendation; moderate level of evidence (⊕⊕⊕⊝)
The Parker Flex-Tip ETT is suggested instead of conventional ETTs for FOI and laryngoscopy to reduce the risk of complications in the general population.
Strong recommendation; low level of evidence (⊕⊕⊝⊝)
Awake tracheostomy under local anaesthesia should be performed in patients with pre-existing critical airway compromise (EO 82.9%). If the upper airway is severely distorted or obstructed due to a tumour, haematoma, severe oedema, bilateral vocal cord paralysis, or haemorrhage, awake FONA under local anaesthesia could be the safest option to secure the airway as the primary plan67,98–102 because: (1) instrumentation of the upper airway can cause bleeding or oedema, can aggravate occlusion, and even cause distal tumour seeding7,9; (2) topical anaesthesia may exacerbate a pre-existing occlusion1,103 or trigger laryngospasm1,9; (3) FB can cause a “cork in a bottle” effect7 and complete airway collapse. Multidisciplinary DA teams work faster and have a higher first-attempt success rate.104
The recommended technique is tracheostomy performed by a trained operator.105 Sedation should be avoided if possible. HFNO appears to be effective in extending safe apnoea time,106 although full precautions should be taken and no electrical instruments should be used during the tracheal opening.107 The procedure requires patient collaboration, since the supine position and neck extension are often poorly tolerated.106 Anaesthesia can be induced once ventilation has been confirmed with capnography.108 The full moon view of the tracheal lumen on FB confirms that the cannula is correctly positioned. A crescent moon image indicates the need to reposition or change the cannula.
Awake cricothyrotomy secures the airway more rapidly, and is therefore indicated in patients presenting critical airway compromise (EO 91.4%).109
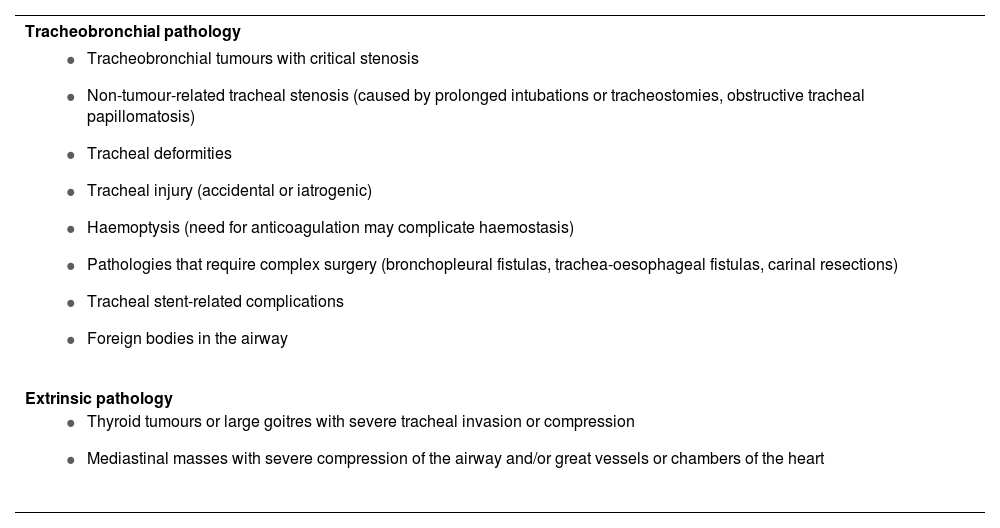
Extracorporeal membrane oxygenationAwake ECMO under local anaesthesia may be the safest option when all 4 conventional plans are likely to be impossible, to fail, or to be ineffective and the patient is at risk of complete airway obstruction (EO 90.6%). Recent advances in technology have made it possible to include ECMO as a means of securing adequate gas exchange in these situations,110,111 particularly when difficulty is due to tracheobronchial disease or extrinsic compression that causes critical central airway obstruction or makes FONA unfeasible.67,110 In these cases, elective awake ECMO under local anaesthesia may be the safest option.67,112,113 Once the situation has been brought under control, the airway is secured to prevent aspiration. Table 2 shows the main indications for ECMO.
Main indications for extracorporeal membrane oxygenation (ECMO).
| Tracheobronchial pathology |
|---|
|
| Extrinsic pathology |
|
ECMO, however, is costly and can lead to complications,111,114–116 so the decision to use it must be taken by a multidisciplinary team.116 If the indication is uncertain, the ECMO system can be put on standby with the vessels cannulated and a perfusionist present before proceeding with airway management.116
Veno-venous ECMO is associated with fewer complications, does not require therapeutic levels of anticoagulation, requires only a single double-lumen cannula, and could therefore be the technique of choice in these situations.110,111 In haemodynamically unstable patients requiring cardiopulmonary support due to obstructive pathology such as a large mediastinal mass, veno-arterial ECMO or even a conventional extracorporeal circulation circuit may be needed.110
In extreme cases, such as massive haemoptysis or central foreign bodies, ECMO may be the last resort.64,117 Establishing ECMO, however, can be complicated and time-consuming, so it is currently not indicated to rescue a CICO situation after induction of GA, even though several authors have described its use in this context.
Unanticipated difficult airwayPeriprocedure oxygenationDiscussed in the corresponding section
Airway managementTI is associated with more complications than other non-invasive plans, so it should be carefully weighed up and only performed when indicated.118
The primary goal in airway management should be to minimize the number of attempts in order to avoid a CICO situation and the need for FONA. Planning and optimization are essential.119
Given the unreliability of predictors,120,121 planning should be geared towards a potentially DA.122 Decision-making should be “context-oriented” rather than focused on specific devices and techniques.8,123
The first attempt must be made under optimal conditions (EO 100%) in order to maximize the likelihood of success (“make the first attempt the best attempt”).119,122,124–126 Additional attempts are only justified when there is room for improvement and a new approach will optimise the foregoing technique or substantially improve the probability of success (for example, changing the size or type of device, using a new adjunct, or changing the operator, as required).119,127
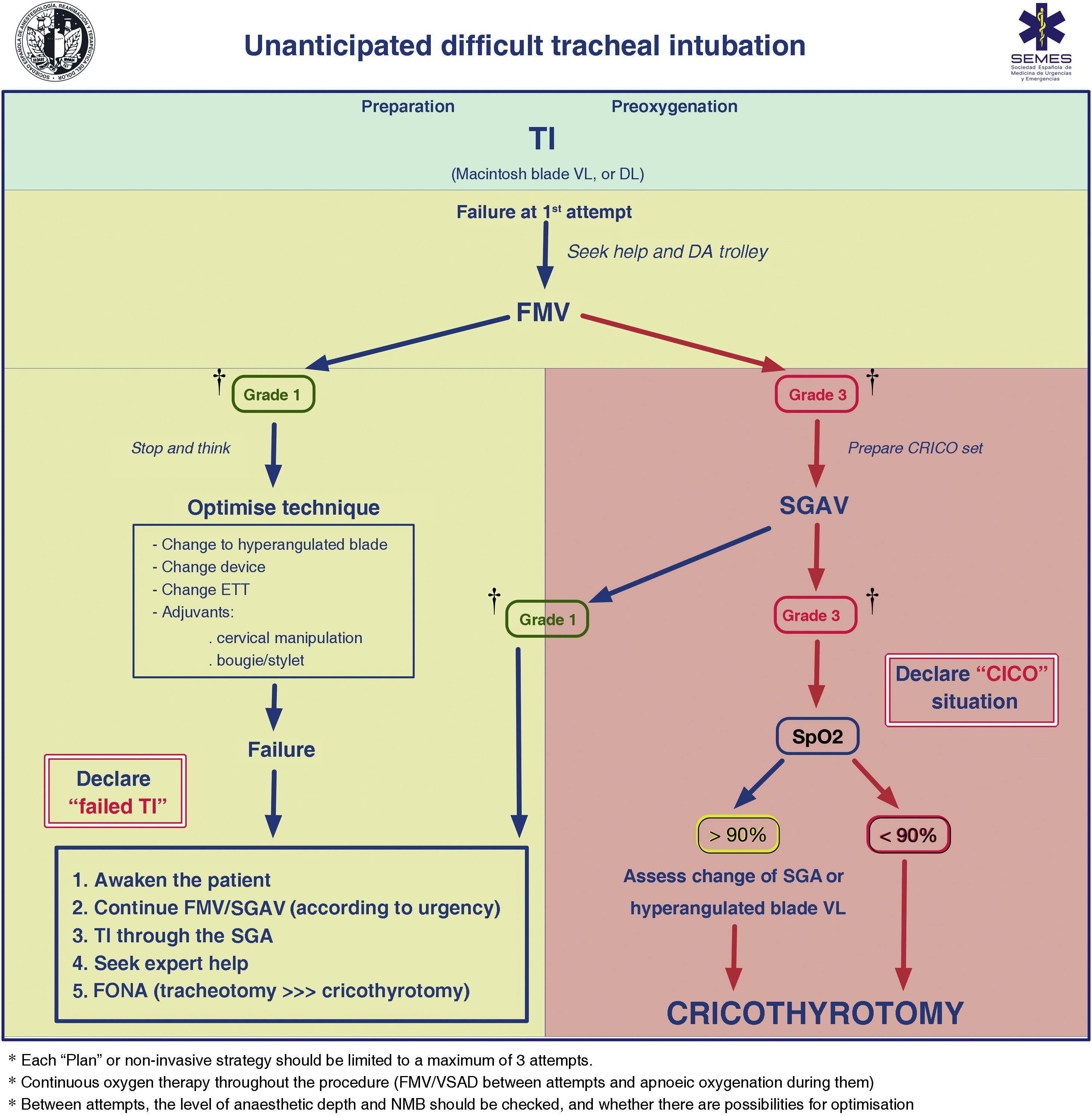
FMV and the depth of anaesthesia and NMB should be verified between attempts. Adequate ventilation between attempts using a face mask or second-generation supraglottic airway device (2GSGA) provides the opportunity to “stop and think”, reformulate the strategy, or bring in new resources while maintaining the initial principles. Fig. 3 shows the unanticipated DA management algorithm.
Unanticipated difficult tracheal intubation algorithm. † Ventilation status on capnography waveform. CICO: Can’t intubate, can’t oxygenate; CRICO: cricothyrotomy; DA: difficult airway; DL: direct laryngoscopy; ETT: endotracheal tube; FMV: face mask ventilation; FONA: front of neck access; SGA: supraglottic airway; SGAV: supraglottic airway ventilation; SpO2: peripheral oxygen saturation; TI: Tracheal intubation; VL: videolaryngoscope.
Failure of non-invasive plans necessitates declaring a CICO situation, ensuring adequate NMB, and immediately performing FONA regardless of SpO2.
Tracheal intubationVideolaryngoscopyFailure of the first TI attempt implies a lower probability of success in subsequent attempts.60,119,128 Multiple attempts can lead to trauma, oesophageal intubation, hypoxaemia, cardiovascular events, a CICO situation, unplanned admission to a critical care unit, or death.129–134 Up to 93% of difficult TIs are unanticipated,121 so the most appropriate first-line technique should be the one that gives the greatest likelihood of achieving success on the first attempt (EO 94.3%).119,122,124–126
Most meta-analyses, despite their heterogeneity,135,136 suggest the superiority of VL over direct laryngoscopy (DL) (Supplementary data 4). Overall, VL compared to DL increases first-attempt success,137–150 improves glottis visualization,140,141,143,145–148,150–158 and reduces the incidence of complications - mainly trauma and oesophageal intubation - 141,142,144–147,149,152,155–157,159–162 by up to 50%.150
The outbreak of the COVID-19 pandemic,135,163–167 cost reduction,18 and the widespread availability of videolaryngoscopes coupled with emerging evidence of their cost-effectiveness168,169 and capacity to improve quality of care,129 teaching, documentation and teamwork (by promoting shared mental models and improving human factors), 122,167,170–173 and mastery of the technique with regular practice129 have overcome resistance to change among clinicians,174,175 and VL is now practically the gold standard for laryngoscopy and DA management.171
Based on all the aforementioned factors, SEDAR SEMES SEORL-CCC recommend the routine use of VL over DL as first-choice TI device (1B). Standard Macintosh blade devices (allowing both direct and indirect laryngoscopy) are appropriate for managing the airway without predictors of difficulty, while hyperangulated blade devices (with or without a guiding channel) are indicated for known or anticipated difficult airway (EO 94.3%).120,135,176,177 Hyperangulated blade devices are first-choice to rescue a failed first attempt.14,178–180 Consequently, it is recommended to have a videolaryngoscope available and the necessary expertise in every location where airway management is performed.
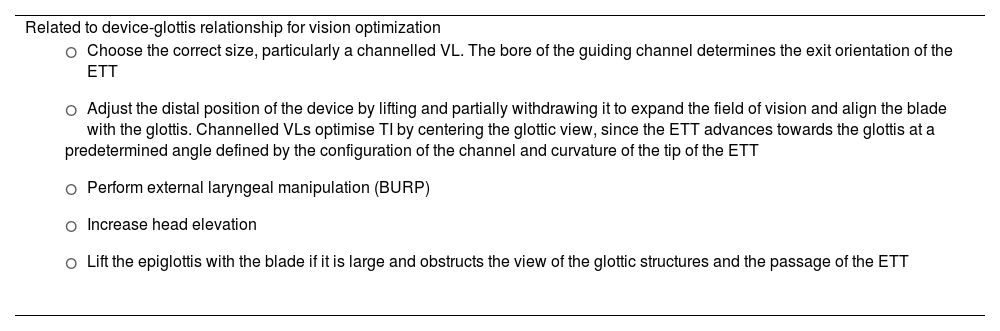
Failure to pass the ETT through the glottis is the most common cause of failed VL-guided TI166,172,173,181,182; however, a skilled operator is highly likely to overcome this difficulty.171 Recommended maneuvers to address this difficulty are listed in Table 3.182–186
Maneuvers to overcome difficulties in videolaryngoscopy-guided tracheal intubation.
| Related to device-glottis relationship for vision optimization |
|
| Related to the use of adjuncts |
|
| Related to the endotracheal tube |
|
BURP: backward, upward, right lateral position; FB: Fibreoptic bronchoscope; TI: tracheal intubation; ETT: endotracheal tube; VL: Videolaryngoscope.
Introducers are associated with a higher first-attempt success rate in elective and emergency TI, particularly in patients with predictors of DA or impaired glottic views.187–190 The availability of a tracheal tube introducer is recommended at all locations where airway management is performed (EO 97.1%)191 and its use should be considered at the first attempt.120,185,188,192 SEDAR SEMES SEORL-CCC recommend using an articulating introducer instead of a conventional stylet for TI in patients with DA (1C), as it improves the rate of first-attempt success and expedites TI, thus reducing instrumentation and the use of alternative adjuncts.60,69,186,193,194
RecommendationThe routine use of VL is recommended over DL as the first-choice TI device.
Strong recommendation; moderate level of evidence (⊕⊕⊕⊝)
A dynamic or articulating introducer (Flex-tip or FB) is recommended instead of a conventional stylet for TI in patients with difficult airway.
Strong recommendation; low level of evidence (⊕⊕⊝⊝)
FOI in unconscious or under GA patients can be highly effective in expert hands195–198; however, it is technically more challenging than in awake patients, it is not fail-safe, leading to episodes of desaturation or complete obstruction of the airway.105 The presence of blood, emesis, or secretions in the airway of patients requiring emergency TI further reduces the likelihood of success.
Manoeuvres such as tongue pulling and jaw thrusting open the pharynx and larynx, respectively, improving vision and the likelihood of success.197,199 Oral cannulas, anterior displacement of the tongue base with laryngoscopy, or placing the patient in the left semilateral position with left head rotation are strategies that facilitate passage of the FB and improve view.200 When ETT encounters resistance, jaw thrust and counterclockwise rotation of the ETT can facilitate passage through the glottis.198
In the NAP4 Audit, TI failed and an emergency FONA was necessary in all patients undergoing FOI after induction of GA as the primary technique or after failure of DL.
FB, either alone or as part of a multimodal strategy, serves as a highly effective rescue approach following failure of most devices.6 However, its availability, preparation, and execution are more labor-intensive and time-consuming than VL in an emergency,43,47 so its use as a rescue device is less widespread.201
Confirmation of tracheal intubationAfter successful TI, it is crucial to immediately rule out oesophageal intubation - a common complication that can have devastating consequences.167,202–204
None of the available techniques for confirming TI are 100% reliable in all circumstances, so they should be used in combination.205,206
Waveform capnography is the most accurate (sensitivity of 98%–100% and specificity of 100%) and rapid method of confirming TI,4,120,205,207–212 and is therefore the gold standard (1B), Although penetration of waveform capnography as a standard of care remains low,204 it is mandatory to use this technique to confirm ETT placement, and it should be available wherever intubation is performed or may be required.167,203,211,213–216 A flat capnography trace (grade 3 ventilation) indicates failed TI until proven otherwise (EO 80%)211,217 and active steps should be taken to exclude oesophageal intubation.218 The capnography trace remains present, though attenuated (not flat), even during cardiac arrest.211,212,217–219 EtCO2 has a lower predictive value when perfusion is low or absent,212 and can give false positive readings when the tip of the ETT is in the hypopharynx.205 Confirming TI using point-of-care ultrasound is a valid alternative in these cases, because it has a sensitivity of 99% and a specificity of 97%, is not affected by pulmonary blood flow, takes only 13 seconds on average, and even allows real time visualization of insertion of the ETT into the trachea or oesophagus.220,221 The “double trachea sign” allows oesophageal intubation to be detected before starting ventilation.216,222
Colorimetric capnography should only be used when waveform capnography is not available, such as in pre-hospital or emergency settings.205
Clinical examination alone has an unacceptably high false positive rate,223 particularly in emergency situations,224 and confirmation bias can lead to seeing and hearing what one expects,225 although clinical examination combined with capnography can be useful. Ultrasound or FB examination through the ETT are alternative methods of confirming TI. Other methods include the oesophageal detector device, transtracheal illumination, pulse oximetry, and chest x-ray.205
Capnography waveform monitoring during mechanical ventilation is highly recommended in all settings (EO 100%),207,209,211,226–230 as it allows continuous monitoring of the ETT position, confirms airway patency, and provides early diagnosis of accidental extubation or partial displacement of an artificial airway.211,218,227,229,231–233 The NAP4 audit found that absence of monitoring might have contributed to more than 70% of airway-related deaths in the ICU,234 so the widespread adoption of capnography in critical care units and training medical and nursing staff in its use211,217,218 could be the single most impactful change in preventing morbidity and mortality arising from TI or the use of other artificial airways outside the operating room.211,234
RecommendationVentilation with a supraglottic airwaySGAs, in addition to their use as a primary technique in elective surgical procedures or cardiopulmonary resuscitation,235–237 are essential for rescuing a difficult or failed TI, since they enable ventilation and oxygenation, maintain airway patency with a certain degree of protection against aspiration, and act as conduits for FOI.238–243 Insertion and function of an SGA is not usually affected by the anatomical and/or technical factors that prevent effective FMV and TI.239 Therefore, an SGA should be inserted without delay to preserve alveolar oxygenation in the event of difficult or failed TI (EO 85.7%).
2GSGAs possess most of the ideal characteristics: they are easy to insert, provide high oropharyngeal sealing pressures, facilitate gastric decompression, and can be used as a conduit for TI.49,239,244 Thus, they have demonstrated superior performance compared to first-generation devices and are more suitable for advanced uses and as rescue devices.244–246 Therefore, the immediate availability of a 2GSGA and the necessary competence for its use are recommended in all locations where airway management is performed (EO 100%).
The selection of an SGA for rescuing a DA should be made prior to the procedure. Devices with a high first-attempt ventilation success rate that can be used as a conduit for TI are the first choice238,247: i-gel™ (Intersurgical Ltd., Wokingham, United Kingdom), Ambu® AuraGain™ (Ambu A/S, Ballerup, Denmark), LMA® Protector™ (The Laryngeal Mask Company Limited, Mahé, Seychelles) and iLTS-D (VBM Medizintechnik GmbH, Sulz, Germany).
An optimal attempt or a maximum of 3 attempts is recommended before declaring a failed plan, since the success rate significantly decreases with successive attempts,248,249 and these increase trauma and delay the transition between plans. If cricoid pressure has been applied, it must be released during placement of an SGA (EO 80%). Each new attempt must include a change that improves the chances of success. Periprocedural oxygenation methods must continue both between and during attempts.
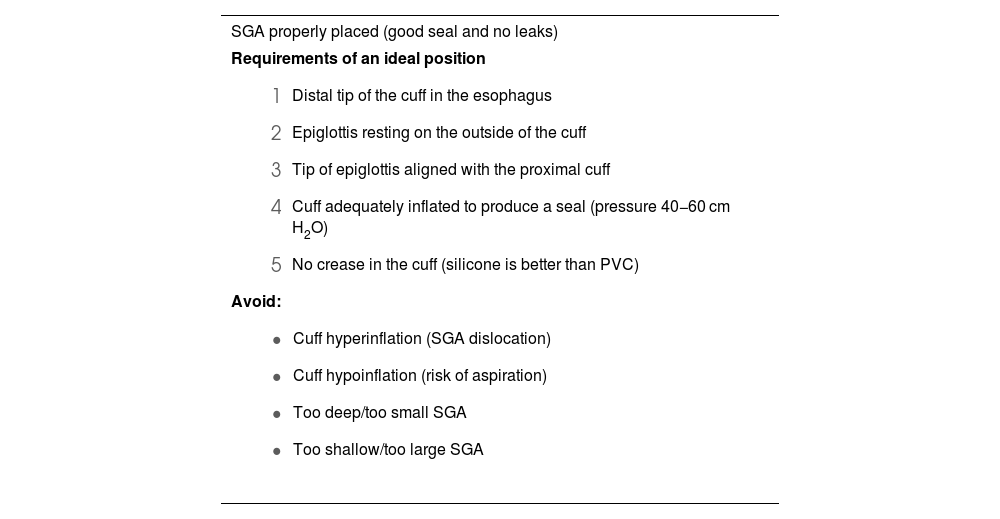
Rapid insertion and correct placement of the SGA is essential for securing the airway.250 Ninety degree rotation, jaw thrust, and the use of DL or VL (first choice) with the “insert-detect-correct as you go” technique facilitate SGA placement, increase the first-attempt success rate while reducing pharyngeal trauma (EO 82.9%),246,250–257 and can prevent malpositioning.257,258 Blind insertion, however, results in malpositioning in 50%–80% of cases,246,256,257 leading to suboptimal airway control, leaks or obstruction, increased risk of further displacement, and increased morbidity.251,255,259 Malpositioned SGAs are 26 times more likely to cause gastric insufflation followed by aspiration.255Table 4 shows the requirements for an ideal SGA position, the causes of malpositioning, and how to correct them.246,255 The recently developed video laryngeal mask (VLM)259 can be placed under direct vision, and malpositioning can be immediately corrected without the need for additional devices,66 although more evidence on their use is required.259–264
Requirements of an ideal position of a SGA, causes of malpositioning and how to correct them.
| SGA properly placed (good seal and no leaks) |
|---|
Requirements of an ideal position
|
| Malpositioned SGA confirmed with video laryngoscopy255 (leaks and airway obstruction) |
|---|
Causes
|
SGA: suplaglottic airway.
Adapted from Van Zundert AA, et al.246
Correct placement of an SGA is confirmed clinically by a normal capnography waveform (grade 1 ventilation) and the maintenance of adequate alveolar oxygenation with peak inspiratory pressures of 20 cmH2O and an oropharyngeal leak pressure >25 cm H2O. Lung auscultation and successful passage of a tube through the gastric canal are complementary signs.259
Achieving effective ventilation and oxygenation provides time to stop and think about the next step, depending on the degree of urgency and type of procedure:
- 1
If the situation is not urgent (e.g., elective surgical procedure), the safest option is to wake the patient and perform the surgery under regional anaesthesia or postpone the intervention in order to perform ATI.
- 2
If the situation is urgent due to: (a) emergency surgery: the team may decide to go ahead with surgery with the 2GSGA, although this is a high-risk option due to the potential compromise of airway patency during surgery; (b) critically ill patient: a definitive airway is likely to be required, so a FONA (tracheostomy) can be performed to prevent potentially fatal hypoxaemia.
- 3
In intermediate cases, FOI through the SGA can be performed if the situation is stable, NMB is adequate, and the operator has the required skills (EO 97.1%, success rates close to 100%238). Blind TI is not recommended due to its low success rate (10%–20%), the need for repeated attempts, and the potential for causing greater trauma and deterioration of oxygenation.265,266 A VLM allows the operator to perform TI without FB guidance,66 so it can shorten intubation time and may be particularly advantageous when FB is not available, such as in the prehospital and emergency setting.267
Grade 2–3 ventilation or ineffective oxygenation after exhausting all attempts requires immediate progression to a new plan.
Face mask ventilationFMV is an essential transition technique during anaesthesia induction and management of the emergent airway, and an indispensable rescue plan when other techniques fail.271
The presence of predictors of difficult FMV, as well as its use during an emergent airway or as a rescue from failed plans, makes it especially advisable to use the optimal technique from the outset (the triple airway manoeuvre of head extension, jaw thrust and mouth opening, placement of a nasopharyngeal or oropharyngeal cannula272 and the two-handed “VE” technique 271,273 performed either by 2 operators or with pressure-controlled ventilation from a ventilator or other device274,275 in a patient with optimal positioning under intense NMB271,276–280) (EO 80%) in order to limit airway obstruction and optimize mask sealing to achieve grade 1 alveolar ventilation without causing gastric insufflation.126,275,281 This speeds up the transition between plans and reduces the maximum ventilation pressure.282 Face mask ventilation with a modified triple airway manoeuvre is recommended over the “CE” technique in the general population (1C).
Successful FMV must be defined as stable or improved SpO2, acceptable tidal volume and airway pressure (4−5 ml/kg and <15–20 cmH2O, respectively273,275,283), and the presence of a plateau phase on capnography.284 It is advisable to use scales to objectively stratify the difficulty of FMV, such as the instrument developed by the Japanese Society of Anaesthesiologists (Fig. 2, Part I). This allows the declaration of a failed FMV attempt before desaturation occurs (late sign).275
FMV is a dynamic procedure that must be continuously monitored until the airway has been secured.285 The declaration of failed FMV necessitates an immediate transition to SGAV (EO 85.7%). Clinical deterioration and worsening oxygenation should prompt the declaration of CICO and an immediate transition to FONA if SGAV has also failed.
A CICO situation should be declared using clear language that is easily understood by all and creates a shared model that will facilitate effective transition to FONA.119,286
RecommendationFront of neck access techniquesFailure of all 3 non-invasive supraglottic plans (primary and rescue) in an apneic patient necessitates the verbalization of CICO and the prompt execution of FONA regardless of the SpO2 value (EO 90.6%), because in these circumstances deterioration is either imminent or already evident. In in-hospital CICO situations, the SEDAR SEMES SEORL-CCC recommend asking for an ENT specialist (or surgeon trained in tracheostomy) as soon as CICO has been declared, although no procedure should be delayed while waiting for their arrival.
Delayed FONA is a leading cause of morbidity and mortality.99,234,247,287,288 Situational awareness and shared decision-making, together with good training in technical skills and human factors management will eliminate the psychological barriers to relinquishing further attempts at non-invasive techniques.99,119,287,289–291 Cognitive aids can help alleviate stress and speed up the transition to FONA292 (Fig. 1, Part I).
Teams should be prepared both psychologically and technically before declaring a CICO situation, and for this purpose the “double setup” may help293: one team performs conventional airway management while a second team stands by ready to perform FONA if needed. Multidisciplinary approach is more likely to secure the airway faster and at the first attempt.
It is recommended to continue providing 100% oxygen via a supraglottic route, ensure intense NMB127,294 and the neck must be extended to expose the larynx.295
There are 3 emergency FONA techniques: percutaneous cricothyrotomy, surgical cricothyrotomy, and surgical tracheotomy.
Cricothyrotomy is the technique of choice in a CICO situation (EO 91.4%) because it is relatively simple, fast, and has a high success rate with few complications.100,296 However, the presence of an experienced ENT specialist or surgeon allows for a tracheostomy to be performed with the same efficacy and safety as a scalpel cricothyrotomy.297,298 Therefore, if an ENT specialist is present, he or she should perform FONA using the technique they consider most appropriate.
Cricothyrotomy can be either surgical or percutaneous. The evidence to support any single FONA technique is week. The few comparison studies published have been performed in simulation or animal models,99 therefore, it is impossible to recommend one technique over the other.99,101,299–302 However, surgical cricothyrotomy is successful in practically 100% of cases.234,287,303
The SEDAR SEMES SEORL-CCC recommend performing surgical cricothyrotomy using the scalpel-bougie-tube technique (EO 91.4%) for the following reasons98–100,234,247,287,291,294,301,304,305: (1) the equipment needed is available in all settings; (2) it can be performed by a single operator; (3) it has a short learning curve; (4) although it is psychologically more challenging,300,306,307 it is a simple, rapid technique; (5) it permits a large bore ETT or cuffed cannula to be inserted into the trachea to definitively secure the airway, protect against aspiration, and achieve effective gas exchange with conventional PPV that can be confirmed with capnography; (6) it is both safe and effective. The equipment includes a scalpel with a number 10, 20 or 21 blade, an ETT or cannula with a bore of up to 6 mm, and a bougie.
Other cricothyrotomy techniques are also valid, provided they are performed by an experienced operator using the correct equipment. Percutaneous cricothyrotomy to re-establish oxygenation and adequate ventilation involves inserting either a ≥4 mm tube with a 15 mm connector, or a wide-bore cannula techniques.247,291,294,301,305,308 The SEDAR SEMES SEORL-CCC leave it to the operator to decide whether to use percutaneous cricothyrotomy as their first-choice or rescue technique,309 and therefore recommend acquiring skills in more than one technique.300,302,310 The operator’s experience, the availability of the material, and the characteristics of the patient will determine the technique used.99,101,294,297,309,311 The percutaneous technique, being more familiar and less intimidating, could be initiated earlier.300
The success of the technique relies on identification of the cricothyroid membrane (CTM). Identification by palpation is prone to error. Ultrasound is recommended over palpation to identify the CTM (1C), so the SEDAR SEMES SEORL-CCC recommend acquiring the necessary skills. The laryngeal handshake technique (by palpation), though slightly more time-consuming than conventional techniques, is associated with a higher success rate.312,313 When the anatomy of the neck makes it difficult to identify the CTM by palpation, the operator should perform a vertical cutaneous incision measuring more than 4 cm in the midline of the neck in a distal-proximal direction above the sternal notch to identify the relevant anatomy.98,247,314 Ultrasound, though superior to palpation in identifying the CTM,315–319 can be time consuming, so in a CICO situation it should only be used if it is immediately available and the operator has the required training.
After securing the airway with FONA, adequate ventilation and alveolar oxygenation should be confirmed by capnography waveform, clinical evaluation, pulse oximetry and arterial blood gases when indicated.294 The examination can be complemented by a fibrobronchoscopic and radiological study. Emergency cricothyrotomy should be converted to an ETT or tracheostomy in a timely fashion, as there is insufficient evidence to support its long term use (EO 85.7%).296,320 The cricothyrotomy tube should be replaced with a tracheostomy within 72 h to avoid subglottic stenosis.301,321
Failure of a cricothyrotomy to secure the airway makes it advisable to perform a tracheostomy by an experienced operator (EO 94.3%).100
According to the NAP4 audit, CICO situations account for 39% of critical events and 25% of all anaesthesia-related deaths.287 FONA is the last resort when non-invasive plans have failed, so it is vitally important.322 Each institution should protocolise the technique used, and a commercial or pre-assembled cricothyrotomy kit should be available wherever airway management is performed.99,247,311,323 The kit should be placed in an easily accessible transparent bag, ideally in the DA trolley, so that the team can become familiar with its contents and mentally rehearse the procedure. All clinicians involved in airway management must acquire and maintain the skills needed to perform a surgical or percutaneous cricothyrotomy using the Seldinger technique (EO 100%).99,101,119,247,291,298,301 The performance of FONA should be feasible at any location where airway management is conducted (EO 100%).324
RecommendationThe prehospital settingPrehospital airway management is essential for patient survival, and is a major challenge for Emergency Medical Services (EMS).325,326 Different factors and limitations come together in each scenario to create a high level of uncertainty that transforms any ordinary airway into a DA,327 and it is not always possible for patient and operator to be positioned as described in intubation guidelines (patient trapped, confined, crushed, buried, or inaccessible). Survival depends on effective coordination of the entire emergency care chain. Table 5 shows the distinguishing characteristics of prehospital airway management.328,329
Characteristics of pre-hospital airway management.
| Guidelines Procedures Safety |
|
| Setting | Particularly complex setting (contextual difficult airway)
|
| Patient and pathology |
|
| Operator |
|
| Equipment |
|
| Airway management |
|
FRC: Functional residual capacity; V˙/Q˙ mismatch: ventilation/perfusion mismatch.
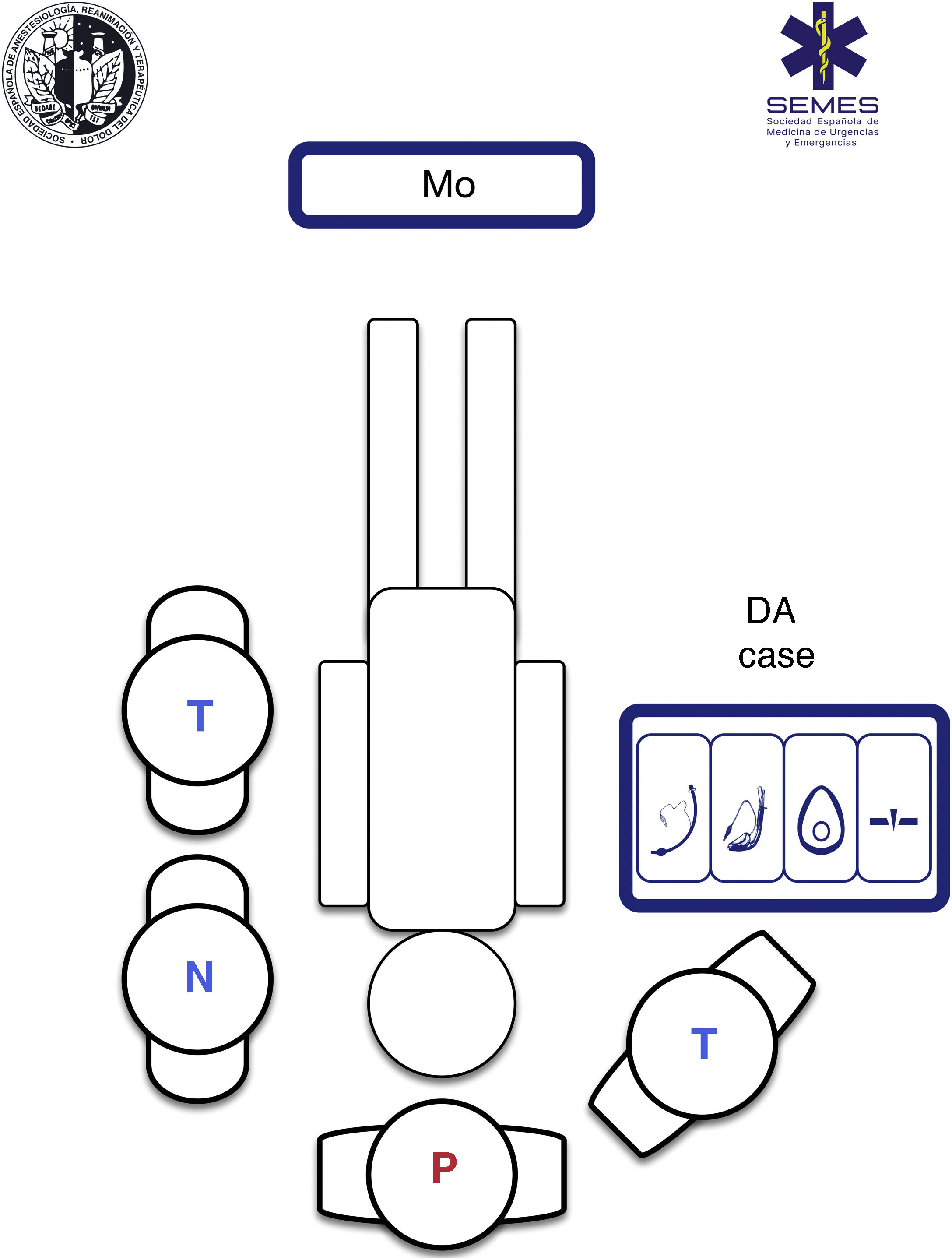
Using a standardised RSI protocol will optimize outcomes, reduce the cognitive burden in high-stress situations, and improve technical and non-technical performance. Therefore, it is advisable to standardize the contents and layout of the airway management bag or “kit dump”, and have a checklist on hand.330Fig. 4 shows our suggested contents and ergonomic layout of the airway management bag suggested by SEDAR SEMES SEORL-CCC for the prehospital setting.
Airway management bagAll the airway material is stowed in the bag using a standardised, modular design so that it is always visible and accessible in less than a minute. The material needed for each of the four treatment plans is stowed in a separate compartment separated by Velcro® or zippers, and marked with easily recognizable pictograms.331 Each compartment must contain at least 1 airway device for each plan (see Fig. 4).
For field use, bags, cases, or backpacks must be easy to carry (reasonable size and weight), made of officially approved flexible, water- and corrosion-proof material of an easily identifiable colour that is clearly labelled and can be easily washed and sterilize. They must have both handles and straps to enable them to be carried on the back to keep the hands free for care procedures. They should ideally be checked daily, and also at each change of shift, following a checklist.
Ergonomics of positions during airway managementPre-hospital care of critically ill patients relies on teamwork, usually made up of a physician, a nursing component, and one or more technicians. Therefore, there is a single operator.
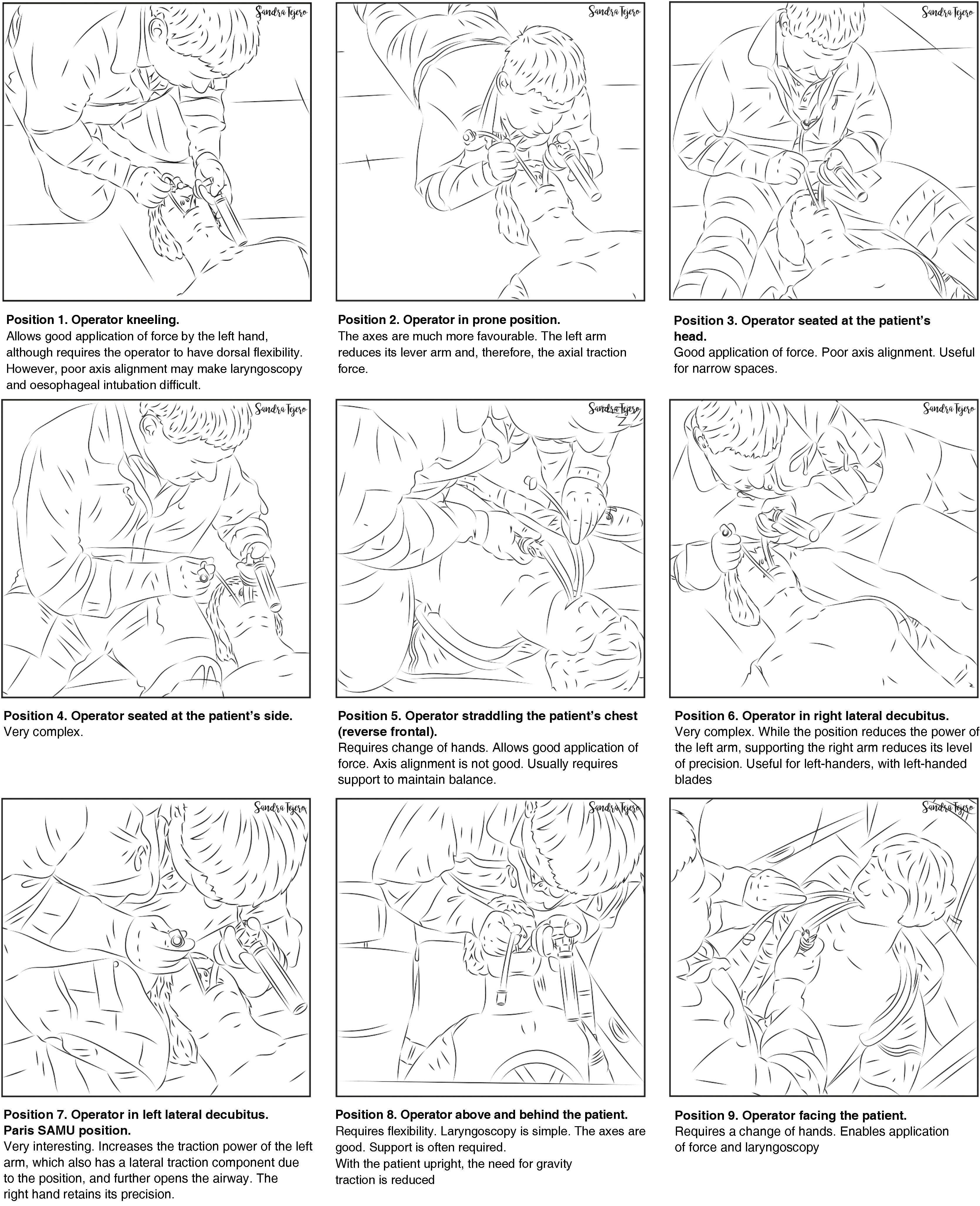
The airway approach will vary greatly, depending on the many different scenarios, risks, patients, positions, and obstacles encountered. Each intervention is unique and unrepeatable. The emergency team must access and control the scene, identify the most seriously ill patient, determine their priorities, and establish more than one approach plan. The patient will usually be lying on the ground allowing for a safety roll, although this is not always possible. Tracheal intubation on the ground is a predictor of DA.332 Non-standard positions create varying degrees of airway access difficulties, so it is important to bear in mind the anatomical changes and the pathophysiology associated with each position (Fig. 5). However, in an ideal scenario, the SEDAR SEMES SEORL-CCC suggest arranging team members as shown in Fig. 4. The physician is situated at the head of the patient and controls ventilation and airway. A technician is situated on his or her right to assist with the technique (Sellick, BURP, jaw thrust, handing over material, etc.). The nursing member and the second technician are positioned at the patient's left shoulder/arm, while the airway management bag is placed on the patient's right, and the monitor is placed at the patient’s feet where it can be clearly seen by all team members. If the patient is in cardiac arrest, the monitor should be placed by the patient's left shoulder to facilitate manoeuvres.
Pre-procedure assessment and planningA DA encountered in the pre-hospital setting is, by definition, unanticipated. However, it is still essential to evaluate the airway in order to anticipate possible difficulties and plan the treatment.331 Predictive tests can be challenging to use, and often there is only time for a “one second look”. Although this quick evaluation can be useful, it should be combined with other tests as far as possible.333
The following independent predictors of DA in the pre-hospital setting have been described in the literature: (1) airway obstruction, patient lying on the ground, and thyromental distance of less than 3 fingers,332 (2) cramped conditions, short neck, obesity, facial and neck injuries, mouth opening less than 3 cm, and ankylosing spondylitis,334 (3) Glasgow scale >3, limited neck movement, trismus/jaw clenched, inability to palpate landmarks on the neck, presence of blood or vomit in the airway.335
Teams dealing with an unanticipated DA should also have experience in ATI, although the indications are more limited in emergency TI.336,337
Periprocedure oxygenationApnoeic oxygenation significantly reduces hypoxaemia during emergency TI,338,339 so this, together with preoxygenation, is essential in prehospital airway management.340 Patients should be oxygenated using a standard nasal cannula at 15 l/min before induction and until the ETT is in place, except in the case of epistaxis, severe head trauma with possible skull base fracture, or complex facial fractures, because it could worsen intubating conditions and potentially cause pneumocephalus.331
Any factor that may affect airway management must be optimized before attempting TI to make sure that the first attempt will be the best.
Rapid sequence inductionRSI is the most widely used induction technique in the pre-hospital setting. However, alternative techniques should also be available.95 It is advisable to follow a standardized checklist-based RSI protocol that includes drugs and dose calculation, and all the material required.341
Cardiopulmonary resuscitationAny abnormal situation increases the delay in achieving ventilation and alveolar oxygenation, interrupts chest compressions, and delays the return of spontaneous circulation (ROSC).342
According to current evidence, there are no significant differences between non-invasive airway management plans.343–345 Outcomes are determined by TI success rates. Therefore, if the desired level of TI efficacy is not achieved, preference should be given to establishing ventilation and alveolar oxygenation over a specific treatment plan, and airway management should not interfere with resuscitation interventions, such as chest compressions, defibrillation, and treatment of the reversible causes of the cardiac arrest.328
Severe traumaSevere traumatic brain injury carries a high risk of airway obstruction, pulmonary aspiration, hypoxia, brain injury, and death.346 Pre-hospital TI is beneficial when performed by experienced practitioners following standardized protocols.346,347 Pre-hospital TI together with air transport could reduce overall mortality by 47%.326
Various factors can complicate airway management,348 including: (1) possibility of an unstable cervical spine injury requiring bimanual alignment; (2) contamination or flooding of the airway by tissue, vomit, secretions, blood, etc. This requires aggressive management with manual removal of fragments and aspiration of secretions using the SALAD (suction assisted laryngoscopy and airway decontamination) technique349; (3) uncooperative or agitated patient; (4) facial bone fractures; (5) Direct Airway Trauma: Both penetrating and blunt trauma (burnt airway and/or inhalation syndrome). The presence of penetrating cervical trauma is the most common indication for ATI, while the highest incidence of FONA is reported in patients with maxillofacial trauma.348,350
In these conditions, RSI and VL-guided TI with a hyperangulated blade and preconfigured stylet are recommended348,351 The procedure can be affected by sunlight reflecting off the screen or contamination of the airway by blood or vomit.352 If a VL is not available, an SGA or DL with reduced traction can be used.329 The use of DL may increase the risk of cervical spine injury.348,350
Patient trapped, buried, crushed, or inaccessibleThis represents the paradigm of contextual difficult airway. To deal with this setting, teams need to acquire additional skills in order to take part in rescue support manoeuvres (8 modalities) and work in hostile environments.353
Cuff pressure monitoringA large percentage of laryngotracheal morbidity is related to improper establishment of cuff pressure.354 Underinflation can cause hypoventilation or increase the risk of aspiration, while excessive pressure, even for short periods, can cause hoarseness, sore throat, impaired ciliary motility, and injuries such as mucosal inflammation and ischaemia, laryngeal oedema, ulcers, stenosis, tracheoesophageal fistula, tracheomalacia, tracheal rupture, vocal cord paralysis, or nerve injury.241,355–357 The incidence of these complications has declined since the introduction of low-pressure, high-volume cuffs358; however, these devices still cause preventable injuries.94,359
Intermittent monitoring with manometry of cuff pressure is desirable after establishment and periodically during maintenance360–364 (not applicable in crisis situations). The use of continuous monitoring to constantly maintain cuff pressure within a desired range in critically ill patients could reduce the risk of ventilator-associated pneumonia.365–367 A manometer should be used for continuous monitoring of cuff pressure (1C).
Cuff pressure must be established with the minimum pressure needed to guarantee a safe, effective seal. Cuff pressure should remain between 20−30 cm H2O (ideally lower than 25 cm H2O) in ETTs and tracheostomy and cricothyrotomy cannulas, and <60 cm H2O in the case of SGAs (EO 94.3%)241,354,358,368–371 from insertion to extraction. If the seal is inadequate even at these pressures, it may be necessary to reposition or re-size the device.372
Nitrous oxide spreads into the cuff,359 so pressure should be re-measured after 20 min, at which time the pressure will have stabilized, and again if nitrous oxide concentration increases.373
RecommendationExtubationTracheal extubation is a high-risk procedure that has a major physiological impact.374 It may trigger a haemodynamic stress response, coughing, laryngospasm or agitation which may in turn increase intracranial or intraocular pressure.375–378 Laryngotracheal protective reflexes remain impaired for several hours after extubation, facilitating aspiration. Extubation failure occurs when a patient is unable to maintain adequate oxygenation and ventilation, clear secretions, or maintain airway patency, and can have catastrophic consequences, particularly in patients with a DA.376 Airway obstruction is the main cause, and is usually associated with post-obstructive pulmonary oedema and severe hypoxia.378 Failed extubation, generally defined as the need for reintubation within 24−72 hours, occurs in approximately 0.1%–0.45% of patients undergoing general anaesthesia.379 Prevalence increases 10-fold in patients with sleep apnoea-hypopnea syndrome (SAHS) or in those undergoing airway surgery, and another 10-fold in patients treated in the ICU.380 Failed extubation can potentially lead to severe adverse outcomes.381–383 One third of all cases of anaesthesia-related deaths or permanent brain damage are due to failed extubation.288,384 Poor anticipation and planning for “at risk” extubation are core issues.288,384 Extubation is an elective procedure that must be prepared, executed a stepwise strategy, and meticulously followed up.375,376,378,385Fig. 6 shows a cognitive aid for planning extubation based on risk assessment.
The main objective in tracheal extubation is to preserve oxygenation and avoid reintubation. Reintubation should always be considered potentially difficult due to the additional complexity involved (EO 97.1%, distorted anatomy with restricted access, secretions, blood or oedema, time constraints, and highly stressful setting).375,376,385 Anatomical, physiological and contextual risk stratification376,383,386,387 determines the likelihood of successful extubation on the basis of tolerance to extubation and the feasibility of reintubation, and allows to establish an individualized strategy and optimise the patient’s status in anticipation of airway-related and physiological factors, such as hypoxaemia, hypercapnia, residual NMB, hypothermia, or airway oedema.355,383,387 The leak test,388 preferably quantitative (leak volume <110 ml or <12%–24% of delivered tidal volume determines diminished airway patency and risk for postextubation stridor due to laryngeal oedema387,389), ultrasound evaluation,390,391 and laryngeal visualization with VL or FB can be used to evaluate airway patency and identify periglottic oedema or bleeding before extubation, thereby facilitating decision making (EO 97.1%),355 although none of these are specific predictors of successful extubation.392
Once the decision has been made to proceed with extubation, the first step is to review the original TI and re-evaluate the airway and overall risk factors.383 Careful advance preparation, notifying the team of potential problems and early warning signs, and establishing appropriate rescue plans for oxygenation and reintubation if the primary strategy fails will help ensure safe, successful extubation.378,383 Important considerations before extubation include378: (1) preoxygenate until extubation; (2) aspirate visible airway secretions or blood, ideally using a laryngoscope to avoid soft tissue trauma; (3) place a bite bloc386; (4) properly positioning the patient; (5) reverse NMB. Neuromuscular monitoring in combination with reversal agents to achieve a train-of-four ratio of ≥0.90 is essential to avoid residual NMB393–396; (6) minimise head and neck movements and reduce noxious stimuli to avoid laryngospasm; (7) emergence to an awake state.377,378,385–387 Deep extubation is inappropriate in patients with DA or risk of aspiration377,386; (8) apply positive pressure, deflate the cuff, and remove the ETT; (9) administer 100% oxygen, confirm airway patency and adequate spontaneous breathing; (10) maintain continuous oxygen therapy until full recovery, along with surveillance, monitoring, and qualified assistance to handle potential emergent tracheal reintubation.385 DA equipment must be prepared and immediately accessible.
Prophylactic administration of corticosteroids before elective extubation significantly reduces the incidence of airway-related post-extubation adverse events and reintubation, so patients at high risk of obstruction could benefit from this strategy.397–399 In patients with an absent or reduced cuff leak, administration of a corticosteroid at least 4 h before extubation is advised.376,387,389 Prophylactic administration of corticosteroids is recommended before extubation in patients at high risk of airway obstruction (1B).
Various advanced techniques have been developed for high-risk extubation, but these should only be performed by clinicians experienced in each technique.378 Administration of pharmacological adjuvants,400–405 such as infusion of remifentanil,406 or performing the Bailey manoeuvre, which involves the placement of a SGA over the ETT followed by the removal of the ETT,407 may be considered when smooth emergence and attenuation of undesirable cardiovascular or respiratory responses are required.408–410 The use of VLM for TI could play a significant role in making this manoeuvre safer and simpler, as it allow for the easy withdrawal of the ETT through its ventilation channel under direct vision and reinsert it immediately if required.66 Both techniques require a deep level of anesthesia for their execution, so they may be inappropriate in patients at risk of aspiration and in whom reintubation may be difficult.1,378
Awake extubation and the use of advanced techniques is the most suitable approach in patients with a difficult airway (EO 94.3%), as the maintain airway patency and muscle tone, protective reflexes, and spontaneous breathing.410 Airway exchange catheters are usually used to rescue extubation in patients with anticipated or known DA.1 These devices can be left in place after extubation if reintubation is likely,376 in which case an ETT can be railroaded over the catheter under direct vision.185 They can also be used to deliver low-flow oxygen or jet ventilation in the event of life-threatening hypoxaemia, although this should be avoided as far as possible, since even low flows have been known to cause barotrauma.411 Reintubation with an airway exchange catheter is successful in 92% of cases, with a first attempt success rate of 87% vs 14%. They are associated with a low incidence of oesophageal intubation and desaturation, bradycardia, and hypotension.412 The main risks of the technique are airway stimulation, injury due to subcarinal insertion, and accidental dislodgement of the catheter. In adults, they should never be inserted deeper than 25 cm from the lips.378 Staged extubation sets, consisting of a guide wire and reintubation catheter,413,414 are associated with an overall success rate of 93%415 and appear to improve tolerance at the expense of increased risk of accidental dislodgement.416 If reintubation over an intubation catheter is necessary, reducing the gap between the ETT and the catheter, administering adequate NMB, and using a VL to visualise the advance of the ETT facilitate the procedure by preventing arytenoid and epiglottic impingement.383,417 Other recommendations to facilitate FOI are applicable to exchange catheter-guided TI. If re-intubation fails, the operator must follow the unanticipated DA algorithm.
Waveform capnography should be available in recovery units and should be used in high-risk patients (EO 97.1%). After extubation, oxygen therapy with nasal prongs and face mask with capnography waveform monitoring will promptly detect respiratory depression, hypoventilation, hypercapnia or post-extubation airway obstruction.379,387
Strategies to prevent extubation failure include head tilt and administration of supplemental oxygen. A good oxygenation strategy with NIV or HFNO can avoid reintubation in at-risk patients.374,418–421
RecommendationDocumentationThe airway management process must be detailed in a report that can orient future intubations. In addition to the informed consent and the results of the preintubation pre-procedure airway evaluation, the report should detail the techniques used for periprocedure oxygenation, topicalization, awake sedation, induction, the devices, adjuncts and ETT used, the approach (right nasal, left nasal or oral), number of attempts, the extubation strategy, and any difficulties or complications encountered. Supplementary data 5 shows an airway management report template.
A history of failure in previous procedures is the most accurate predictor of failure in subsequent treatments (EO 97.1%).422 Reporting difficulty is one of the most important ways of preventing future complications. The report facilitates decision-making and forms the basis for a structured, targeted approach that ensures an efficient transition and reduces airway instrumentalization by steering away from plans that have been unsuccessful in the past.422,423 Using a variety of resources to report a DA will increase the likelihood of this critical information fulfilling its purpose.423,424 For example11,294,424: (1) a detailed description of the successful technique used together with a visual record with images and/or video of the anatomy and the technique can be added to the patient’s clinical history425; (2) the patient and their next of kin or caregiver can be notified verbally in person; (3) the patient can be given a written report. Supplementary data 6 shows our suggested DA notification template; (4) the patient can be issued with a medical alert bracelet, necklace or ID card with a QR code for access to clinical information. Supplementary data 7 shows our suggested DA alert wearables; (5) a DA alert can be programmed to pop up whenever the patient's electronic record is accessed; and (6) the patient can be registered in national or international DA databases.426 The registration form should be standardised, and should include mandatory fields describing the characteristics of the airway, the nature of the difficulty, the techniques that failed and succeeded, and a free text field for additional information.294,424 Using structured difficult airway notes in electronic records has been shown to reduce the number of adverse events.427,428
Organisational aspects of airway managementThe implementation of airway strategies requires not only individual commitment, but also a proactive attitude on the ministerial, institutional and departmental levels.429,430 The action plan to improve the safety, cost-effectiveness and quality of airway management includes: (1) standardising the techniques and equipment used across all locations wherever airway management is performed, effective adherence to guidelines, standardising the DA trolley,431 eliminating barriers and promoting facilitators to encourage compliance with guidelines432–434; (2) coordinating all departments involved in airway management (anaesthesia, intensive care, emergency, otolaryngology, and prehospital care); (3) acquiring and ensuring accessibility of the right equipment; (4) providing staff with training and opportunities for continuous professional development; (5) performing periodic audits and introducing a human error prevention program: a) analysing airway-related incidents, b) identifying and addressing departmental and institutional factors that may have contributed to the event in order to learn from mistake and make improvements, c) reviewing cases and discuss alternative plans; (6) implementing a system for reporting and notifying airway-related incidents435; (7) introducing a system of coding, registering, identifying and warning of DAs; (8) drafting organizational guidelines on the use of resources and continuous improvement.436,437
Each institution should appoint an “Airway Lead” (EO 100%)11,429,430,438 - a clinician with experience in the field who liaises with hospital management to ensure local policies and organisation are implemented. The airway lead’s primary objective is to provide each member of staff with the tools they need.429,439 In order to improve safety, some hospitals have formed multidisciplinary specialist teams with clearly defined roles.440–442 The creation of a national network of airway leads and airway teams would further improve airway management and patient safety.438,439
Decisions to acquire airway devices must be supported by a formal, evidence-based evaluation.24,443,444
Education and trainingAirway management is an essential skill in anaesthesiology, critical care, and prehospital and emergency medicine.445,446 Poor training is a common causal or contributing factor in complications234,384 and undermines the operator’s confidence in performing essential techniques, such as FOI and cricothyrotomy.447–449 Optimizing teaching and training are key to improving safety.322
There is scant evidence on airway management curricula, and training is not standardized,324,450 so strategies used in other specialist fields have been adapted to airway management training. Good training should include both cognitive skills based on theoretical principles, and technical and non-technical skills.322,451 Skills must be acquired gradually through a process of theoretical, simulation-based, and clinical training with problem solving until the learning curve has been completed. Instructors must give evaluation and feedback in each phase of learning (EO 100%).437,452,453 Competency-based training must be individualized.454 Training should follow a mastery learning approach that consists of achieving predefined standard learning objectives involving mastery of a particular skill before moving on to a higher level of difficulty and stress.455 Mastery learning is a student-centred, evidence-based method456 that could give better results.457,458
Education and training must be structured in such a way as to address all aspects of airway management,37,322,445,455 with emphasis on core, versatile skills, and skills to be used in critical emergency situations.445 Standardization of a training program is highly recommended.322 Simulation-based training is important for acquiring non-technical skills, such as teamwork, using guidelines and cognitive aids in clinical practice, and practising procedures and resolving life-threatening issues that would rarely be encountered in real-world practice.437,448,455,459–464 Guidelines and algorithms are tools for learning skills and strategies, and can be used as a reference during debriefing,322,465,466 while cognitive aids are useful when practising procedures in simulation-based training.127,322 Structured debriefing improves clinical knowledge and the acquisition and implementation of skills in clinical practice.455,467
More senior operators may be at risk of providing lower quality care,446,468 and therefore require a continuing education and training program, preferably annual, with refresher courses to acquire the new skills needed to use the latest devices or new techniques and to maintain their existing skills322,445,455,469,470 (EO 97.1%).471 Both hospital management and individual clinicians must take a proactive approach to training. It is highly recommended that each service appoint a local airway leader to organise top quality interprofessional, multidisciplinary training programs with defined learning goals, evaluations, and supervision, and to ensure that guidelines and cognitive aids are disseminated and followed.322,324,439,445,448,472 Training must be diversified to provide the operator with several alternatives.43Supplementary data 8 shows the skills that we believe should be included in all airway management training programs, as well as the teaching methods needed to achieve these objectives.
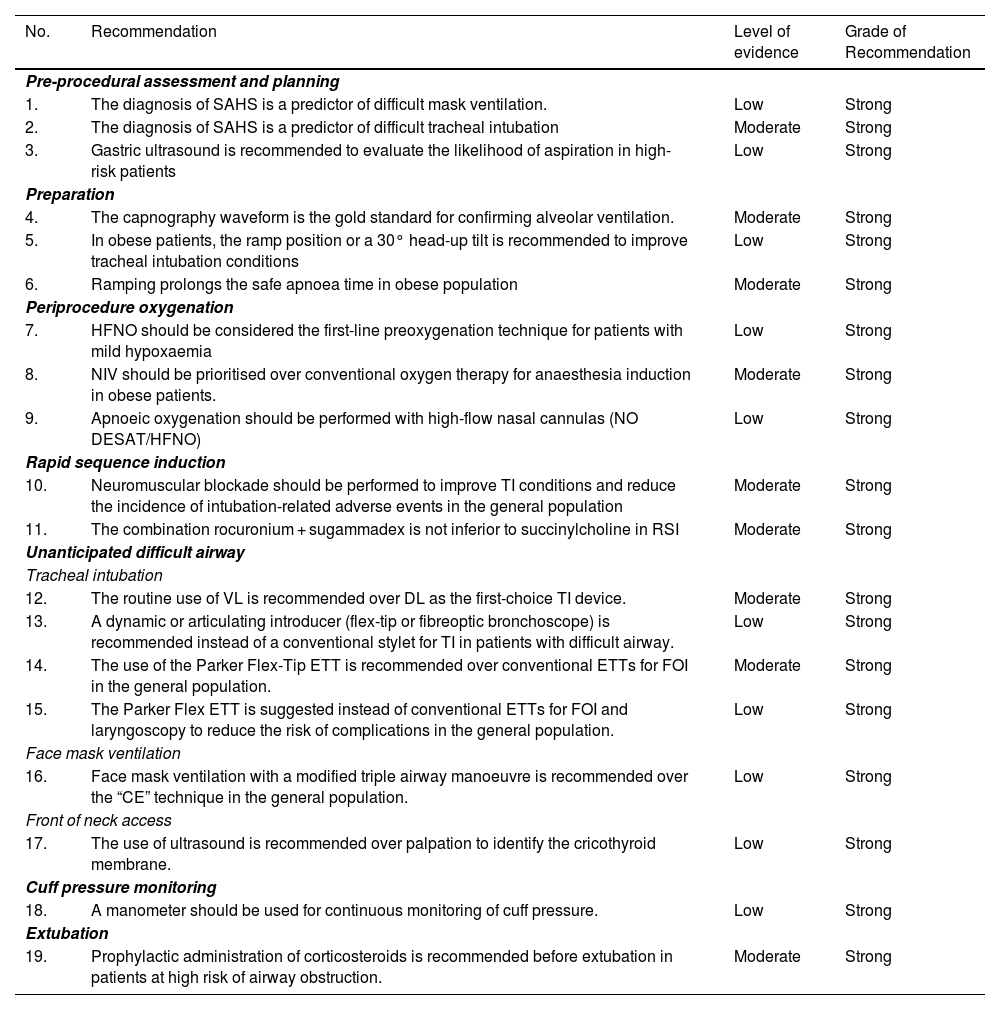
Summary of recommendations derived from the systematic literature searchSearch strategies and GRADE tables are shown in supplementary data.
| No. | Recommendation | Level of evidence | Grade of Recommendation |
|---|---|---|---|
| Pre-procedural assessment and planning | |||
| 1. | The diagnosis of SAHS is a predictor of difficult mask ventilation. | Low | Strong |
| 2. | The diagnosis of SAHS is a predictor of difficult tracheal intubation | Moderate | Strong |
| 3. | Gastric ultrasound is recommended to evaluate the likelihood of aspiration in high-risk patients | Low | Strong |
| Preparation | |||
| 4. | The capnography waveform is the gold standard for confirming alveolar ventilation. | Moderate | Strong |
| 5. | In obese patients, the ramp position or a 30° head-up tilt is recommended to improve tracheal intubation conditions | Low | Strong |
| 6. | Ramping prolongs the safe apnoea time in obese population | Moderate | Strong |
| Periprocedure oxygenation | |||
| 7. | HFNO should be considered the first-line preoxygenation technique for patients with mild hypoxaemia | Low | Strong |
| 8. | NIV should be prioritised over conventional oxygen therapy for anaesthesia induction in obese patients. | Moderate | Strong |
| 9. | Apnoeic oxygenation should be performed with high-flow nasal cannulas (NO DESAT/HFNO) | Low | Strong |
| Rapid sequence induction | |||
| 10. | Neuromuscular blockade should be performed to improve TI conditions and reduce the incidence of intubation-related adverse events in the general population | Moderate | Strong |
| 11. | The combination rocuronium + sugammadex is not inferior to succinylcholine in RSI | Moderate | Strong |
| Unanticipated difficult airway | |||
| Tracheal intubation | |||
| 12. | The routine use of VL is recommended over DL as the first-choice TI device. | Moderate | Strong |
| 13. | A dynamic or articulating introducer (flex-tip or fibreoptic bronchoscope) is recommended instead of a conventional stylet for TI in patients with difficult airway. | Low | Strong |
| 14. | The use of the Parker Flex-Tip ETT is recommended over conventional ETTs for FOI in the general population. | Moderate | Strong |
| 15. | The Parker Flex ETT is suggested instead of conventional ETTs for FOI and laryngoscopy to reduce the risk of complications in the general population. | Low | Strong |
| Face mask ventilation | |||
| 16. | Face mask ventilation with a modified triple airway manoeuvre is recommended over the “CE” technique in the general population. | Low | Strong |
| Front of neck access | |||
| 17. | The use of ultrasound is recommended over palpation to identify the cricothyroid membrane. | Low | Strong |
| Cuff pressure monitoring | |||
| 18. | A manometer should be used for continuous monitoring of cuff pressure. | Low | Strong |
| Extubation | |||
| 19. | Prophylactic administration of corticosteroids is recommended before extubation in patients at high risk of airway obstruction. | Moderate | Strong |
AW: airway; DL: direct laryngoscopy; ETT: endotracheal tube; FOB: fiberoptic bronchoscopy; HFNO: high flow nasal oxygen therapy; NIV: non-invasive ventilation; NOT DESETT: Nasal oxygen therapy during efforts to secure an ETT; RSI: Rapid sequence induction; SAHS: Sleep apnoea-hypopnoea syndrome; TI: tracheal intubation; VL: videolaryngoscopy.
Expert statement derived from the results of the Delphi questionnaire| No. | Statement | % in favour [for; neutral; against] |
|---|---|---|
| Human factors | ||
| 1. | No more than 3 attempts should be made in each non-invasive airway management plan | 88.6 [31; 2; 2] |
| 2. | The first attempt must be made under optimal conditions | 100 [35; 0; 0] |
| 3. | The most appropriate first-line technique should be the one that gives the greatest likelihood of achieving success on the first attempt. | 94.3 [33; 1; 1] |
| 4. | Clinicians should use visual cognitive aids to deal with emergencies | 97.1 [34; 1; 0] |
| 5. | A standardized difficult airway trolley should be placed near any room where airway management is performed | 100 [35; 0; 0] |
| 6. | Checklists should be used to reduce human error, speed up tasks, and promote an airway management safety culture | 100 [35; 0; 0] |
| 7. | Systems that promote ergonomics and communication models should be implemented | 91.4 [32; 3; 0] |
| Pre-procedural assessment and planning | ||
| 8. | A pre-procedure evaluation should be performed in all patients who require airway management | 100 [35; 0; 0] |
| 9. | Pre-procedural airway assessment should be multifactorial, structured, and aimed at detecting anatomical, physiological, and contextual difficult airway | 97.1 [34; 1; 0] |
| 10. | The airway assessment can begin by detecting predictors of difficulty or failure for the primary plan and subsequently for the three alternative plans | 97.1 [34; 1; 0] |
| 11. | Multivariate models could have greater predictive capacity | 97.1 [34; 1; 0] |
| 12. | Decision-making must be individualized to the patient, operator, context, and time of day | 97.1 [34; 1; 0] |
| 13. | Intake of food and liquids should be restricted according to preoperative fasting guidelines | 97.1 [34; 1; 0] |
| 14. | A full stomach is an indication for TI to protect the airway | 88.6 [31; 2; 2] |
| Preparation | ||
| 15. | Waveform capnography must be available at all locations where airway management is performed to confirm successful ventilation with any of the 4 plans used | 97.1 [34; 1; 0] |
| Difficult airway management: basic options | ||
| 16. | A pre-intubation risk-benefit analysis must be performed to determine the suitability of the airway management technique | 97.1 [34; 1; 0] |
| 17. | Awake airway management is recommended when TI is likely to be highly difficult or impossible, or when there are composite predictors of difficulty, physiological alterations, and negative contextual issues | 82.9 [29; 5; 1] |
| 18. | General anaesthesia with preserved spontaneous breathing should be induced whenever awake tracheal intubation is advisable but general anaesthesia is unavoidable due to lack of cooperation or the urgency of the situation, and when there are no predictors of physiological or context-related difficulties or obstructive airway pathology | 91.4 [32; 2; 1] |
| 19. | In patients with predictors of physiological or context- difficulties, postponement can be considered if it outweighs the risk of proceeding with airway management, or alternative anaesthesia strategies can be considered | 85.7 [30; 5; 0] |
| Known or anticipated difficult airway | ||
| 20. | Awake tracheal intubation is the technique of choice in patients with known or anticipated difficult airway | 85.7 [30; 4; 1] |
| 21. | High-flow nasal oxygen therapy is recommended over conventional low-flow cannulas | 91.4 [32; 3; 0] |
| 22. | NIV through an endoscopy face mask could be useful when intubating critically ill patients with hypoxaemia | 82.9 [29; 6; 0] |
| 23. | Premedication with an antisialogogue, preferably glycopyrrolate, is recommended to optimize the local anaesthetic effectiveness and improve the field of vision. | 80 [28; 5; 2] |
| 24. | Sedation is an optional complement to adequate topical anaesthesia in awake tracheal intubation | 88.6 [31; 2; 2] |
| 25. | The goals of conscious sedation in awake tracheal intubation are effective amnesia, patient satisfaction, and analgesia to reduce coughing, gagging, and haemodynamic reflexes while preserving airway patency, spontaneous breathing, and protective laryngeal reflexes. | 94.3 [33; 2; 0] |
| 26. | If the first-line technique (FB or VL) fails, the alternative technique should be used. | 80 [28; 6; 1] |
| 27. | A third attempt may benefit from a multimodal approach (VL + FB) | 100 [35; 0; 0] |
| 28. | The combination of intubating SGA and FOI can be a useful rescue technique to maintain oxygenation and airway patency and be used as a conduit to facilitate TI | 100 [35; 0; 0] |
| 29. | A smaller calibre ETT than usual is recommended when performing FB and VL. | 85.7 [30; 4; 1] |
| 30. | There should be less difference between the external diameter of the FB and the internal diameter of the ETT in order to facilitate FOI. | 85.7 [30; 3; 2] |
| 31. | Standard PVC ETTs are not recommended for FOI as they are more likely to impinge on the glottic structures. | 71.9 [23; 4; 5] |
| 32. | After visual confirmation of tracheal intubation, general anaesthesia should be induced after inflating the cuff and confirming intubation with capnography. | 94.3 [33; 2; 0] |
| 33. | Fallback techniques and approaches should be planned in advance and implemented without delay after failure of the primary technique. | 100 [35; 0; 0] |
| 34. | A FONA plan must be prepared before attempting awake tracheal intubation if it is likely to fail and result in complete airway obstruction. | 88.6 [31; 4; 0] |
| 35. | Awake tracheostomy under local anaesthesia should be performed in patients with pre-existing critical airway compromise. | 82.9 [29; 6; 0] |
| 36. | Awake cricothyrotomy would be the most appropriate technique in the event of emergency critical airway compromise. | 91.4 [32; 3; 0] |
| 37. | Awake ECMO under local anaesthesia may be the safest option when all 4 conventional plans are likely to be impossible, to fail, or to be ineffective and the patient is at risk of complete airway obstruction. | 90.6 [29; 1; 2] |
| Unanticipated difficult airway | ||
| Periprocedure oxygenation | ||
| 38. | HFNO should be considered the first-line preoxygenation technique for patients with mild hypoxaemia (PaO2/FiO2 > 200 mmHg), while NIV is the technique of choice in those with severe hypoxaemia (PaO2/FiO2 ≤ 200 mmHg). | 87.5 [28; 3; 1] |
| 39. | Preoxygenation with NIV + HFNO and apnoeic oxygenation with HFNO should be a priority in critically ill patients undergoing TI. | 85.7 [30; 4; 1] |
| Rapid sequence induction | ||
| 40. | RSI is the recommended technique when there is a considerable risk of aspiration in an airway without predictors of difficulty | 97.1 [34; 1; 0] |
| 41. | RSI, with or without the Sellick manoeuvre, should be performed in in all emergency TIs | 84.4 [27; 1; 4] |
| 42. | An RSI checklist should be used to improve patient safety | 97.1 [34; 1; 0] |
| 43. | Patients with high risk of aspiration should be premedicated with a nonparticulate antacid immediately before induction or an H2 receptor antagonist or a proton pump inhibitor 40−60 min before induction to increase pH and reduce the volume of gastric contents | 82.9 [29; 5; 1] |
| 44. | Use of a nasogastric tube should be individualized | 88.6 [31; 4; 0] |
| 45. | High-efficiency, large-calibre, multi-hole suction instruments must be readily available to treat possible regurgitation | 100 [35; 0; 0] |
| 46. | A 20–30° head-up position is recommended to prevent passive regurgitation. Should this occur, the patient should be placed in the Trendelenburg position with the head turned to one side to aspirate fluid from the pharynx and trachea before starting PPV | 94.3 [33; 2; 0] |
| 47. | The choice, dose, and rate of administration of hypnotic agent must be individualized | 91.4 [32; 3; 0] |
| 48. | In agitated, non-cooperative patients, delayed sequence induction can be considered to achieve adequate preoxygenation. | 71.9 [23; 3; 6] |
| 49. | The routine use of cricoid pressure cannot be recommended | 81.3 [26; 2; 4] |
| 50. | A “modified RSI” can be used in patients at high risk of hypoxia who are not candidates for awake tracheal intubation | 85.7 [30; 5; 0] |
| Tracheal intubation | ||
| 51. | Videolaryngoscopes with standard Macintosh blade (which allow for both direct and indirect laryngoscopy) are appropriate for tracheal intubation in patients without predictors of difficulty, while those with hyperangulated blade (with or without a guiding channel) are indicated in patients with known or anticipated difficult airway. | 94.3 [33; 1; 1] |
| 52. | The availability of a tracheal tube introducer is recommended at all locations where airway management is performed. | 97.1 [34; 1; 0] |
| 53. | A flat capnography trace (grade 3 ventilation) indicates failed TI until proven otherwise. | 80 [28; 6; 1] |
| 54. | Capnography waveform monitoring during maintenance of mechanical ventilation is highly recommended in all settings | 100 [35; 0; 0] |
| Ventilation with a supraglottic airway | ||
| 55. | An SGA should be inserted without delay to preserve alveolar oxygenation in the event of difficult or failed TI | 85.7 [30; 3; 2] |
| 56. | Immediate availability of a 2GSGA is recommended, along with the necessary proficiency for its use in all locations where airway management is conducted. | 100 [35; 0; 0] |
| 57. | If cricoid pressure has been applied, it must be released during placement of an SGA | 80 [28; 5; 2] |
| 58. | A 90-degree rotation, jaw thrust, and the use of DL or VL (of choice) with the “insert-detect-correct-as-you-go” technique enhance the efficacy and safety of SGAs by facilitating insertion, increasing the success rate on the first attempt, and reducing pharyngeal trauma. | 82.9 [29; 4; 2] |
| 59. | FOI can be performed through the SGA if the situation is stable, NMB is adequate, and if the operator is skilled in the technique | 97.1 [34; 1; 0] |
| Mask ventilation | ||
| 60. | The optimal mask ventilation technique should be used as first-line the triple airway manoeuvre of head extension, jaw thrust and mouth opening, placement of a nasopharyngeal or oropharyngeal cannula and the two-handed “VE” technique in a patient with optimal positioning under intense NMB. | 80 [28; 3; 4] |
| 61. | The declaration of failed mask ventilation necessitates an immediate transition to SGAV. | 85.7 [30; 2; 3] |
| Front of neck access | ||
| 62. | The failure of the 3 non-invasive plans (primary and rescue), regardless of the SpO2 value, requires the verbalization of CICO and subsequent execution of FONA. | 90.6 [29; 0; 3] |
| 63. | Cricothyrotomy is the technique of choice in a CICO situation | 91.4 [32; 2; 1] |
| 64. | The scalpel-introducer-tube cricothyrotomy technique is recommended | 91.4 [32; 2; 1] |
| 65. | The performance of FONA should be feasible at any location where airway management is conducted. | 100 [35; 0; 0] |
| 66. | Emergency cricothyrotomy should be converted to ETT or tracheostomy, as there is insufficient evidence to support its use as long-term treatment | 85.7 [30; 3; 2] |
| 67. | Failure of a cricothyrotomy to secure the airway makes it advisable to perform a tracheostomy by an experienced operator. | 94.3 [33; 1; 1] |
| 68. | All clinicians involved in airway management must acquire and maintain the skills needed to perform a surgical or percutaneous cricothyrotomy using the Seldinger technique. | 100 [35; 0; 0] |
| Cuff pressure monitoring | ||
| 69. | Cuff pressure should be established with the minimum pressure needed to guarantee a safe, effective seal. Cuff pressure should remain between 20−30 cm H2O in ETTs and tracheostomy and cricothyrotomy cannulas, and <60 cm H2O for SGAs | 94.3 [33; 1; 1] |
| Extubation | ||
| 70. | Reintubation should always be considered potentially difficult due to the addition complexity involved | 97.1 [34; 1; 0] |
| 71. | The cuff leak test, preferably using a quantitative evaluation technique, ultrasound evaluation and laryngeal visualization with VL or fibreoptic bronchoscope can facilitate decision-making. | 97.1 [34; 1; 0] |
| 72. | Awake extubation and the use of advanced techniques is the most suitable approach in patients with difficult airway | 94.3 [33; 2; 0] |
| 73. | Waveform capnography should be available in recovery units and should be used in high-risk patients | 97.1 [34; 1; 0] |
| Documentation | ||
| 74. | A history of failure in previous procedures is the most accurate predictor of failure in subsequent treatments. | 97.1 [34; 1; 0] |
| Organisational aspects of airway management | ||
| 75. | Each institution should appoint an “Airway Lead” | 100 [35; 0; 0] |
| Teaching and training | ||
| 76. | Skill acquisition should be gradual, involving a cognitive phase, simulation, and clinical training with problem-solving until the learning curve is completed, with evaluation and feedback from the instructor at each stage. | 100 [35; 0; 0] |
| 77. | Continuing education and regular training are required for the development of new skills or techniques and the maintenance of competencies, preferably on an annual basis. | 97.1 [34; 1; 0] |
- •
Manuel Á. Gómez-Ríos: drafting of the manuscript, preparation of all cognitive aids and graphic material, tables and annexes, literature review, critical reading, levels of evidence, final revision of the document.
- •
José Alfonso Sastre: Draft of RSI sections, SGA, and checklist, risk factor tables, information document models, literature review, critical reading, levels of evidence, final revision of the document.
- •
Xavier Onrubia-Fuertes: FONA contribution, unanticipated difficult tracheal intubation, literature review, final revision of the document.
- •
Teresa López: Draft of SGA and ECMO sections, literature review, critical reading, levels of evidence, final revision of the document.
- •
Alfredo Abad-Gurumeta: literature review, critical reading, levels of evidence, final revision of the document.
- •
Rubén Casans-Francés: literature review, critical reading, final revision of the document.
- •
David Gómez-Ríos: literature review, critical reading, final revision of the document.
- •
José Carlos Garzón: literature review, critical reading, final revision of the document.
- •
Vicente Martínez-Pons: Algorithm for unanticipated difficult tracheal intubation, literature review, final revision of the document.
- •
Marta Casalderrey-Rivas: literature review, critical reading, final revision of the document.
- •
Miguel Ángel Fernández-Vaquero: Algorithm for unanticipated difficult tracheal intubation, literature review, and critical reading aimed at airway predictors and assessment, final revision of the document.
- •
Eugenio Martínez-Hurtado: Algorithm for unanticipated difficult tracheal intubation, final revision of the document.
- •
Ricardo Martín-Larrauri: Algorithm for unanticipated difficult tracheal intubation, final revision of the document.
- •
Laura Reviriego-Agudo: Algorithm for unanticipated difficult tracheal intubation, literature review, final revision of the document.
- •
Uxía Gutierrez-Couto: literature search strategies.
- •
Javier García-Fernández: critical reading, final revision of the document.
- •
Alfredo Serrano Moraza: prehospital setting section, literature review, critical reading, final revision of the document.
- •
Luis Jesús Rodríguez Martín: prehospital setting section, final revision of the document.
- •
Carmen Camacho Leis: prehospital setting, final revision of the document.
- •
Salvador Espinosa Ramírez: prehospital setting, final revision of the document.
- •
José Manuel Fandiño Orgeira: critical reading, final revision of the document.
- •
Manuel José Vázquez Lima: critical reading, final revision of the document.
- •
Miguel Mayo-Yáñez: FONA contribution, final revision of the document.
- •
Pablo Parente-Arias: FONA contribution, final revision of the document.
- •
Jon Alexander Sistiaga-Suárez: critical reading, final revision of the document.
- •
Manuel Bernal-Sprekelsen: critical reading, final revision of the document.
- •
Pedro Charco-Mora: Coordination, Algorithm for unanticipated difficult tracheal intubation, draft ergonomic options, draft teaching and training, literature review, critical reading, final revision of the document.
MAGR received lecture honoraria from Medtronic.
XOF received honoraria for lecture and practical workshop on neuromuscular block from Merck Sharp & Dohme.
RCF received honoraria for lectures from Fresenius Kabi.
AAG received honoraria for lectures from Merck Sharp &Dohme and 3 M Edwards.
Delphi expert panelManuel Á. Gómez-Ríos, José Alfonso Sastre, Xavier Onrubia-Fuertes, Teresa López, Alfredo Abad-Gurumeta, José Carlos Garzón, Vicente Martínez-Pons, Marta Casalderrey-Rivas, Miguel Ángel Fernández-Vaquero, Eugenio Martínez-Hurtado, Ricardo Martín-Larrauri, Laura Reviriego-Agudo, Javier García-Fernández, Pedro Charco-Mora, Raquel García Álvarez, Alfredo Panadero Sánchez, Alejandra Prieto Gundín, María Luisa Santos Marqués, David Domínguez García, Irma, María Barrio, Uxío García-Aldao, Aixa Espinosa, Carmen M. Holgado Pascual, Jesús Carazo Cordobés, Cristobal Añez Simón, Natividad Quesada Gimeno, Marina Gómez-Morán Quintana, Silvia Bermejo, Pilar Cabrerizo Torrente, Francisca Llobell, Roque J. Company Teuler, Teresa del Castillo Fdez de Betoño, Carlos González Perrino, and Paola Hurtado.
External reviewersJaideep Pandit, Luis Gaitini, Tomasz Gaszyński, and Pavel Michalek.
ContributorsA list of contributors can be found in the supplementary data.
We would like to thank Sandra Tejero Muñoz for illustrating Fig. 5 of this consensus document.