The critical patient affected by SARS-CoV-2 is at risk of malnutrition. The need to avoid volume overload and manoeuvres that delay reaching nutritional requirements such as pronation make the nutritional approach to these patients complex. To ensure adequate treatment, a nutritional support protocol was developed as a clinical practice guideline adapted to the COVID-19 patient.
ObjectiveTo describe and analyse the results of introducing a nutritional support protocol aimed at SARS-CoV-2 patients admitted to the intensive care unit (ICU) of the Consorcio Hospital General Universitario de Valencia (CHGUV) from March to May 2020.
Material and methodsObservational, descriptive, retrospective and longitudinal design to evaluate compliance with a nutritional support protocol.
ResultsThirty-one consecutive patients were included but nutritional follow-up could not be performed in eight. Of the remaining 23 patients, only eight reached 80% of caloric requirements before the tenth day after starting treatment (good compliance group) and 15 after the eleventh day (poor compliance group). In the group with «good compliance» 75% (n=6) were discharged and 25% died (n=2), compared to the group with «bad compliance» where 53% (n=8) were discharged and 47% (n=7) died (Chi square test, p-value=0.019). Those patients who reached 80% of caloric needs during ICU stay had a shorter length of stay compared to those who did not (median days of admission=14, IQR=10-16 and median days of admission=22, IQR=13-39, p-value=0.025).
ConclusionsIntroducing a nutritional protocol during the first weeks of the SARS-CoV-2 pandemic could improve clinical outcomes by promoting healing and reducing associated complications.
El paciente crítico afectado por SARS-CoV-2 tiene riesgo de desnutrición. La necesidad de evitar la sobrecarga de volumen y las maniobras que retrasan el logro de los requerimientos nutricionales como la pronación hacen que el abordaje nutricional de estos sujetos sea complejo. Para asegurar un tratamiento adecuado, se desarrolló un protocolo de soporte nutricional como guía de práctica clínica adaptado al paciente con COVID-19.
ObjetivoDescribir el protocolo de soporte nutricional creado en nuestro centro, el cumplimiento del mismo y analizar los resultados de su aplicación en los pacientes con SARS-CoV-2, ingresados en la unidad de cuidados intensivos (UCI) del Consorcio Hospital General Universitario de Valencia (CHGUV) de marzo a mayo del 2020.
Material y métodosDiseño observacional, descriptivo, retrospectivo y longitudinal para evaluar el cumplimiento de un protocolo de soporte nutricional.
ResultadosSe incluyeron 31 pacientes. No se pudo establecer un seguimiento nutricional en ocho de ellos. De los 23 restantes, ocho alcanzaron 80% de los requerimientos calóricos antes del décimo día tras el inicio del tratamiento (grupo buen cumplimiento) y 15 a partir del undécimo día (grupo mal cumplimiento). El grupo con buen cumplimiento obtuvo 75% (n=6) de curación y 25% de éxitus (n=2), en comparación con el grupo con «mal cumplimiento» donde 53% (n=8) fueron dados de alta a planta vs 47% (n=7) que fallecieron (prueba X2, valor p=0,019). Aquellos pacientes que alcanzaron 80% de las necesidades calóricas en algún momento del ingreso en UCI tuvieron menor duración de la hospitalización frente a los que no lo obtuvieron (mediana de días de ingreso=14, rango intercuartílico [IQR]=10-16 y mediana de días de ingreso=22, IQR=13-39, valor p=0,025).
ConclusionesLa creación de un protocolo nutricional durante las primeras semanas de la pandemia por SARS-CoV-2 podría estar asociada con una mejoría de los resultados clínicos al favorecer la curación y disminuir las complicaciones asociadas.
Critically ill patients requiring long-term admissions, mechanical ventilation, and immobility are at risk of malnutrition and muscle atrophy. In these patients, the energy deficit caused by inadequate nutrient intake prolongs hospital stays, increases care costs, and is associated with a higher risk of morbidity and mortality.1,2
Nutrition therapy plays a key role in the clinical evolution of patients receiving intensive care, and implementing a nutrition protocol has been shown to improve calorie and protein intake, which in turn attenuates the stress response and minimizes the complications associated with nutrition therapy (nutritrauma).3
Given the scant scientific evidence on nutrition therapy in patients with SARS-COV-2, following the outbreak of the pandemic our multidisciplinary critical care nutrition team of anaesthesiologists, endocrinologists, pharmacists and intensive care nurses created a nutrition therapy protocol for SARS-COV2 patients admitted to intensive care.
The protocol was based on the ESPEN guideline4 on clinical nutrition in the intensive care unit (ICU), and tailored to the needs of patients with respiratory distress and severe respiratory infection. The protocol was developed to help the overworked, stressed doctors and nurses in the CCU, many of whom were brought in to meet the growing demand for additional staff and were therefore not trained in critical care, to comply with nutritional recommendations and ensure that each patient received the protein and calorie intake required at each stage of their disease. Our aim was to standardize nutritional care in order to minimize the incidence of nutritrauma, particularly overfeeding and volume overload in patients with respiratory distress. We placed particular emphasis on the importance of treatment during prone positioning and on strategies to minimize nutrition therapy-related complications.
In this study, we describe the protocol created by our team, determine the level of compliance, and analyse the effect of applying the protocol in COVID-19 patients admitted to the ICU of the Hospital General Universitario de Valencia (CHGUV).
Materials and methodsObservational, descriptive, retrospective, longitudinal study. After the protocol had been distributed to staff members, all adult patients diagnosed with COVID-19 who were admitted to the CHGUV ICU between March and May 2020 were included. The study was presented by the nutrition board of the CHGUV and approved by the Ethics and Research Committee of our hospital (Registration: 61/2020).
The clinical records of each study patient were reviewed retrospectively to determine the effect of applying the following nutrition support protocol.
Brief description of the protocolAdmission staff record the patient’s weight and height and calculate their body mass index (BMI). In the case of patients with a BMI greater than 30kg/m2, the protocol is adjusted for obesity. The energy target (GET) is defined using a simple formula based on weight (20-25kcal/kg/day). Disease stage (early acute stage <70% GET, late acute stage increase intake up to 70% of estimated GET, and post-acute stage >7 days from 80% to 100% of GET) and recommended protein intake (1.3g/kg/day, which may be increased to 2g/kg/day in patients with high demand). Oral intake is prioritised over enteral or parenteral nutrition whenever feasible, provided it meets 70% of requirements. If oral intake is infeasible (risk of vomiting or aspiration, need for orotracheal intubation), enteral nutrition is started through a silicone or PVC nasogastric tube (NT) once the patient is haemodynamically stable. We used a hypercaloric, hyperproteic formula supplemented with enteral modular protein supplements, according to protein requirements. To facilitate compliance with nutrition therapy, we created one nutrition table for supine patients (Supplement 1) and another for patients during pronation cycles (Supplement 2). Nutrition intake was individualised by weight and adapted to each stage of the disease. Each nutrition cycle lasted 24hours, and a dose of supplemental protein was administered between 7 and 10 days of starting the regimen in order to achieve the nutritional target. If nutritional requirements were not met or the patient was not able to follow the protocol, complementary central parenteral nutrition should be started between 4 and 7 days after starting the protocol. Nutritional deficits are supplemented according to the needs of each patient.
Intestinal tolerance was monitored daily (NT output every 12h, presence of abdominal distension or pain, diarrhoea) to detect complications and improve adherence to the therapy.
Study variables and statistical analysisWe collected demographic variables, such as gender, age, date of admission and origin of transfer, and overall clinical variables, such as cardiac, respiratory. Endocrine and metabolic pathologies, SOFA score on admission, and SAPS II score, days on mechanical ventilation, number of consecutive pronations, and days receiving continuous neuromuscular blocking agents. On admission to the ICU, the patient’s approximate weight (kg) and height (cm) were obtained directly from the patient or from a member of their family.
To evaluate the nutrition therapy, protocol compliance was defined as achieving 80% of estimated weight-adjusted calories after the start of enteral nutritional in the ICU. The deadline for protocol compliance was set at 10 days from the start of nutrition therapy, and to determine whether it had been achieved we consulted the patient’s medical charts from the day nutrition therapy started and noted their average daily intake. This variable was then categorized as good compliance (GC), defined as patients who achieved 80% of their caloric intake by day 10, and poor compliance (PC) when this goal was not achieved by day 11.
We also measured other variables, such as the start of enteral nutrition (day), type of initial enteral nutrition, intake of protein supplements, use of prokinetics, and need for complementary parenteral nutrition. Clinical outcomes and protocol-related complications were evaluated with in-ICU death, time to ICU discharge, gastric residual volume >250ml, diarrhoea (defined as 3–5 daily bowel movements or more than 750ml/24h), constipation (7 days without bowel movements), gastrointestinal bleeding, aspiration pneumonia, and peritonitis.
The following laboratory parameters were collected for nutritional follow-up: sodium, phosphorus and magnesium, kidney function, albumin, prealbumin, blood glucose, and lipids. All these parameters were measured at weeks 1, 2 and 3 from admission. Deficits were treated with supplements, and the formula or dose was modified if refeeding syndrome, hypertriglyceridemia, or hyperglycaemia were suspected or the patient was unable to tolerate the regimen and developed diarrhoea or constipation. The patient’s response to treatment was evaluated, and vitamin D levels were determined to assess whether supplementation was required.
Statistical analysis was performed on R.5 The baseline characteristics of the sample were compared overall, and then patients were divided into 2 groups: those that did not achieved 80% of their metabolic requirements within the first 10 days and those that did. For the secondary analysis, we divided patients into a further 2 groups: those that never achieved 80% of their caloric requirements; and those that achieved 80% of their caloric requirements at some point during their stay. Patient characteristics are described as number and percentage, in the case of categorical variables, and as mean and standard deviation (SD) or median and interquartile range (IQR) in the case of continuous variables, depending on normality. Normality was testing with the Shaphiro–Wilk statistical test. Categorical variables were compared using the Chi-square test. Continuous variables were compared using the Student's t test and the Wilcoxon rank test, depending on normality.
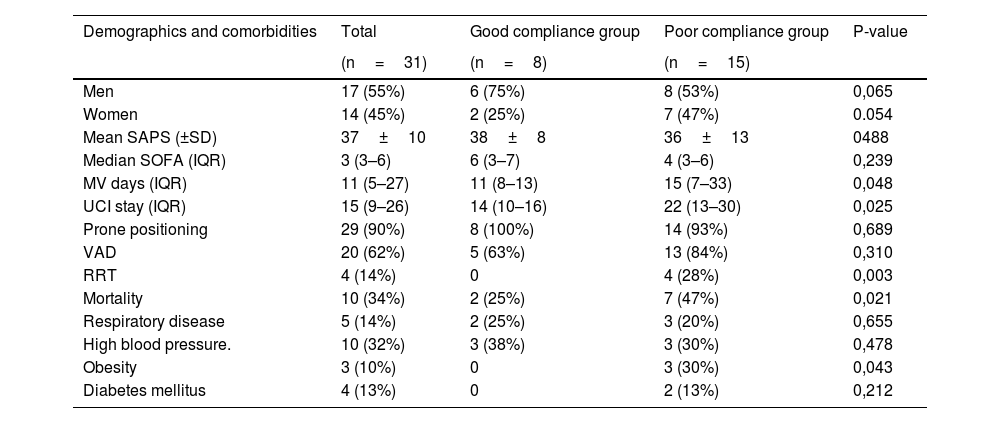
ResultsA total of 31 patients were included; mean age was 63±10 years; 55% (n=17) were men and 45% (n=14) women. The median SOFA score on admission was 3 (3-6) and the SAPS II was 37 (30–45). Nearly all patients (83%) were transferred from the ward, and only 4 patients had a history of lung disease (17%): 3 (13%) with asthma, and 1 (4%) with chronic obstructive pulmonary disease. The most common comorbidity was high blood pressure, found in 10 patients (43%), followed by diabetes mellitus (4 patients [17%]) and obesity (3 patients [13%]). The average weight was 80±13kg. The mean number of prone positioning days was 4±2. The median length of stay in days was 15 (9–26); 68% (n=21) of patients were discharged and 32% (n=10) died in the ICU (Table 1).
Baseline characteristics and parameters measured in COVID-19 patients during hospital stay.
| Demographics and comorbidities | Total | Good compliance group | Poor compliance group | P-value |
|---|---|---|---|---|
| (n=31) | (n=8) | (n=15) | ||
| Men | 17 (55%) | 6 (75%) | 8 (53%) | 0,065 |
| Women | 14 (45%) | 2 (25%) | 7 (47%) | 0.054 |
| Mean SAPS (±SD) | 37±10 | 38±8 | 36±13 | 0488 |
| Median SOFA (IQR) | 3 (3–6) | 6 (3–7) | 4 (3–6) | 0,239 |
| MV days (IQR) | 11 (5–27) | 11 (8–13) | 15 (7–33) | 0,048 |
| UCI stay (IQR) | 15 (9–26) | 14 (10–16) | 22 (13–30) | 0,025 |
| Prone positioning | 29 (90%) | 8 (100%) | 14 (93%) | 0,689 |
| VAD | 20 (62%) | 5 (63%) | 13 (84%) | 0,310 |
| RRT | 4 (14%) | 0 | 4 (28%) | 0,003 |
| Mortality | 10 (34%) | 2 (25%) | 7 (47%) | 0,021 |
| Respiratory disease | 5 (14%) | 2 (25%) | 3 (20%) | 0,655 |
| High blood pressure. | 10 (32%) | 3 (38%) | 3 (30%) | 0,478 |
| Obesity | 3 (10%) | 0 | 3 (30%) | 0,043 |
| Diabetes mellitus | 4 (13%) | 0 | 2 (13%) | 0,212 |
The total sample is compared with the two study groups, the good compliance group (defined as achieving nutritional goal by the tenth day of ICU stay) and the poor compliance group (nutritional goal not achieved, or achieved from the eleventh day of ICU stay).
ICU: intensive care unit, IQR: interquartile range; MV: Mechanical ventilation, RRT: renal replacement therapy; SD: standard deviation; VAD: vasoactive drugs.
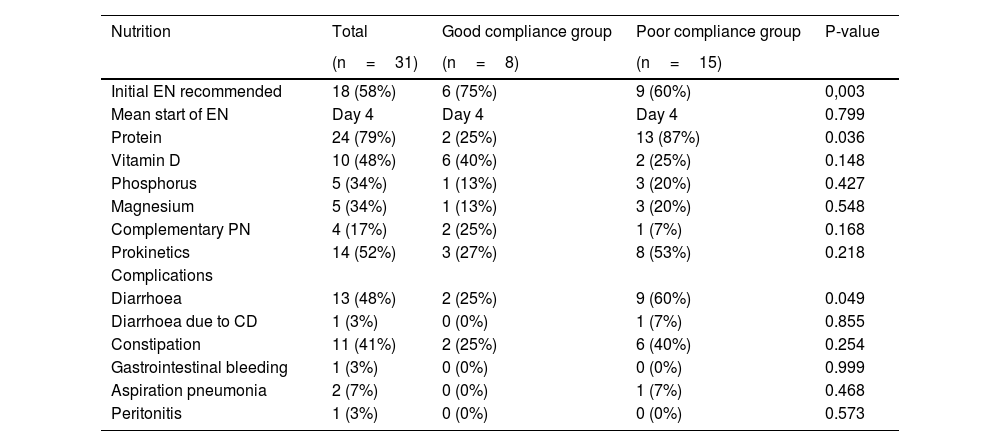
Enteral nutrition was started, on average, on day 4 of admission, and the recommended nutritional regimen was applied in 58% of cases. In total, 79% (n=18) of patients received modular protein supplements, and 17% (n=5) required complementary parenteral nutrition (Table 2).
Characteristics of nutrition therapy and associated complications during ICU stay in COVID-19 patients.
| Nutrition | Total | Good compliance group | Poor compliance group | P-value |
|---|---|---|---|---|
| (n=31) | (n=8) | (n=15) | ||
| Initial EN recommended | 18 (58%) | 6 (75%) | 9 (60%) | 0,003 |
| Mean start of EN | Day 4 | Day 4 | Day 4 | 0.799 |
| Protein | 24 (79%) | 2 (25%) | 13 (87%) | 0.036 |
| Vitamin D | 10 (48%) | 6 (40%) | 2 (25%) | 0.148 |
| Phosphorus | 5 (34%) | 1 (13%) | 3 (20%) | 0.427 |
| Magnesium | 5 (34%) | 1 (13%) | 3 (20%) | 0.548 |
| Complementary PN | 4 (17%) | 2 (25%) | 1 (7%) | 0.168 |
| Prokinetics | 14 (52%) | 3 (27%) | 8 (53%) | 0.218 |
| Complications | ||||
| Diarrhoea | 13 (48%) | 2 (25%) | 9 (60%) | 0.049 |
| Diarrhoea due to CD | 1 (3%) | 0 (0%) | 1 (7%) | 0.855 |
| Constipation | 11 (41%) | 2 (25%) | 6 (40%) | 0.254 |
| Gastrointestinal bleeding | 1 (3%) | 0 (0%) | 0 (0%) | 0.999 |
| Aspiration pneumonia | 2 (7%) | 0 (0%) | 1 (7%) | 0.468 |
| Peritonitis | 1 (3%) | 0 (0%) | 0 (0%) | 0.573 |
CD: clostridium difficile; EN: enteral nutrition; PN: parenteral nutrition.
Nutritional follow-up was impossible in 8 (25%) patients — 3 received exclusively oral nutrition from Day 1 with no nutritional support, and 5 were unable to receive nutritional therapy due to the severity of their illness. Of the 23 remaining patients, 8 (34%) were included in the GC group, in other words, they achieved 80% of their caloric requirements by day 10; however, 10 (44%) patients did not achieve this goal until day 11, and 5 (21%) did not achieve it at any time during their ICU stay. Therefore, 15 (66%) patients were included in the PC group (see Flowchart).
Groups were evenly matched in terms of age, weight prognostic scales, days on mechanical ventilation, number of pronations, duration of neuromuscular relaxation, and mortality, both globally and by sex (p>0.05).
The percentage of discharges was significantly higher and the number of in-ICU deaths significantly lower in the GC group. Specifically, 75% (n=6) of patients in this group were discharged and 25% died in the ICU (n=2), compared with 53% (n=8) discharged and 47% (n=7) in-ICU deaths in the PC group (Chi square test, p-value 0.019). Length of ICU stay did not differ between groups. However, in patients that achieved their caloric requirements at some point during their stay, even 11 or more days after the start of nutrition therapy (18 patients), the length of ICU stay was significantly shorter compared with those that did not reach this goal at any time during their ICU stay (5 patients), (median 14 days, IQR 10–16 vs median 22 days, IQR 13–39, Kruskal Wallis test p-value 0.025).
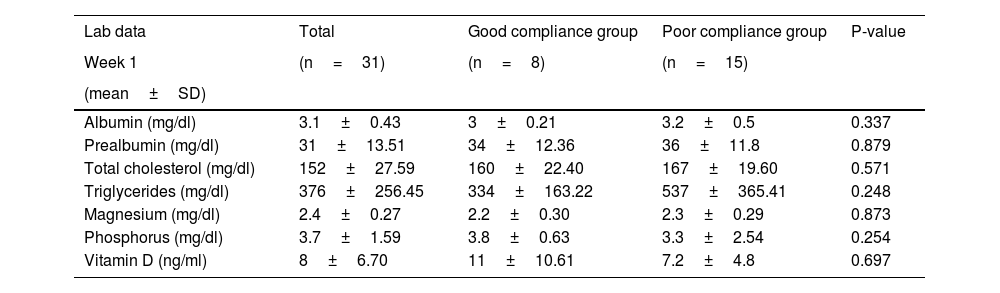
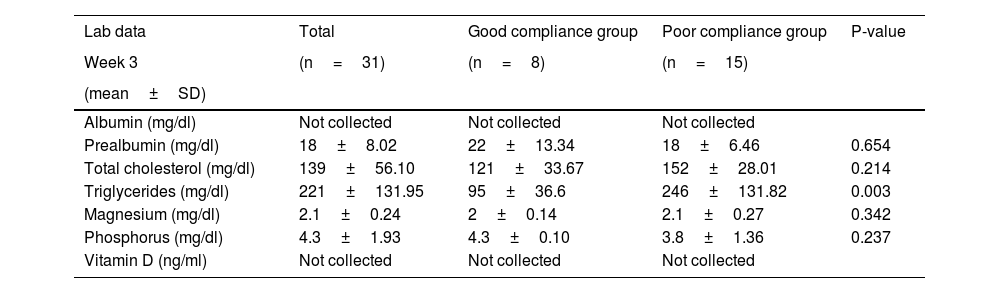
Mean prealbumin on admission was 31±13.51mg/dl, and remained at normal values during the first 3 weeks, albeit with a tendency to progressive decline that was greater in the PC vs the GC group. Mean triglycerides on admission was 376±257mg/dl, and was significantly higher in the PC (initial levels 537±365mg/dl) vs the GC (initial levels of 334±163mg/dL) group. These levels were normalised in the GC group by the third week, but were persistently above normal in the PC group. Although vitamin D is not requested on admission in all patients, when these tests were performed both group showed severe deficiency (Table 3). Tables 3 and 4 show weekly determinations of ions and other parameters required to assess nutrition.
Laboratory parameters in critically ill COVID-19 patients during the first week of ICU stay.
| Lab data | Total | Good compliance group | Poor compliance group | P-value |
|---|---|---|---|---|
| Week 1 | (n=31) | (n=8) | (n=15) | |
| (mean±SD) | ||||
| Albumin (mg/dl) | 3.1±0.43 | 3±0.21 | 3.2±0.5 | 0.337 |
| Prealbumin (mg/dl) | 31±13.51 | 34±12.36 | 36±11.8 | 0.879 |
| Total cholesterol (mg/dl) | 152±27.59 | 160±22.40 | 167±19.60 | 0.571 |
| Triglycerides (mg/dl) | 376±256.45 | 334±163.22 | 537±365.41 | 0.248 |
| Magnesium (mg/dl) | 2.4±0.27 | 2.2±0.30 | 2.3±0.29 | 0.873 |
| Phosphorus (mg/dl) | 3.7±1.59 | 3.8±0.63 | 3.3±2.54 | 0.254 |
| Vitamin D (ng/ml) | 8±6.70 | 11±10.61 | 7.2±4.8 | 0.697 |
SD: standard deviation.
Laboratory parameters in critically ill COVID-19 patients during the third week of ICU stay.
| Lab data | Total | Good compliance group | Poor compliance group | P-value |
|---|---|---|---|---|
| Week 3 | (n=31) | (n=8) | (n=15) | |
| (mean±SD) | ||||
| Albumin (mg/dl) | Not collected | Not collected | Not collected | |
| Prealbumin (mg/dl) | 18±8.02 | 22±13.34 | 18±6.46 | 0.654 |
| Total cholesterol (mg/dl) | 139±56.10 | 121±33.67 | 152±28.01 | 0.214 |
| Triglycerides (mg/dl) | 221±131.95 | 95±36.6 | 246±131.82 | 0.003 |
| Magnesium (mg/dl) | 2.1±0.24 | 2±0.14 | 2.1±0.27 | 0.342 |
| Phosphorus (mg/dl) | 4.3±1.93 | 4.3±0.10 | 3.8±1.36 | 0.237 |
| Vitamin D (ng/ml) | Not collected | Not collected | Not collected |
SD: standard deviation.
The most common complication was diarrhoea (46% of patients), predominantly in the PC group (60% vs 25% in the GC group). There was only 1 case of clostridium difficile-induce diarrhoea. The second most common complication was constipation (41% of patients), predominantly in the PC group. Lastly, gastric residuals >250ml with or without associated symptoms were recorded in 20% of the PC group and in none of the patients in the GC group; 53% of patients in the PC group required prokinetics during their stay vs 27% in the GC group; only 1 patient developed aspiration pneumonia (Table 2).
DiscussionAccording to the ESPEN guidelines,4 all critically ill patients staying for more than 48h in the ICU should be considered at risk for malnutrition. The risk is even higher in critically ill SARS-CoV2 patients due to anorexia, COVID-19-related intestinal symptoms,6,7 and the severe inflammation that characterises this disease.8 Patients with respiratory distress also require frequent ventilation manoeuvres in the prone position to improve oxygenation, and intake must be reduced to avoid volume overload. This is why nutrition therapy is such an important factor in the favourable evolution of these patients. Our objective was to develop a protocol to help clinical staff implement and follow-up nutrition therapy and adapt it to each stage of the disease and each patient’s specific requirements and complications.
A review of the results obtained after implementing our protocol in the ICU during the months of April and May has allowed us to draw a number of conclusions.
Compliance with the protocol was low. Instead of starting nutrition therapy early, as recommended in clinical guidelines, it was not started until the fourth day in most patients, and only 8 patients achieved their caloric goals within 10 days of starting the therapy. Fifteen patients did not achieve their nutritional goal within the deadline, even though their severity scores, number of pronations, and clinical characteristics were similar.
There could be several reasons for this — one being the severe inflammation associated with SARS-COV2 infection.9 The persistently high triglyceride levels in the patients that failed to achieve their nutritional requirements by the tenth day could be attributed to the cytokine storm described in this disease.10 Further studies are needed to evaluate the correlation between the degree of inflammation and nutrition therapy.
We found a higher rate of recovery in the group that met their nutritional requirements according to the protocol, but no significant reduction in the length of ICU stay. However, ICU stay was shorter in patients who at some point achieved the caloric requirements, albeit after the deadline (18), compared with patients that never achieved this goal (5). These is consistent with several studies that suggest that implementing evidence-based protocols to improve nutrition reduces length of ICU stay, days on mechanical ventilation, and mortality11,12; however, further studies are needed to evaluate the correlation between nutrition therapy and ICU stay.13,14
Lab tests showed that prealbumin on admission was within normal limits, indicating that the patients were not malnourished. In the GC group, prealbumin levels remained similar to baseline. We also observed a vitamin D deficiency at admission, a finding reported by other authors, who also observed low levels of vitamin D and vitamin D binding protein and believe that this can be explained in part by high protein consumption and its associated deficit.15 Scant evidence is available on intestinal dysfunction in critically ill patients, due to the difficulty involved in objectively assessing this condition. Intestinal dysfunction occurs as a response to critical illness, and is frequently associated with difficulty in achieving nutritional goals due to frequent treatment interruptions. Diarrhoea, constipation, and high gastric residual volume are signs of poor tolerance,16 and diarrhoea has been described in between 2% and 50% of patients with SARS-COV2.17
In our study, a lower incidence of diarrhoea and constipation and less need for prokinetics in the GC group suggests better tolerance of the nutrition therapy. To minimise diarrhoea, a common complication of SARS-COV2, low-fibre nutrition was the first choice when starting nutrition therapy, but this may have increased the incidence of constipation in our patients. Few patients presented high gastric residual volume, showing that intolerance to enteral nutrition may be an adaptive response at the start of treatment. Clinical guidelines advise against measuring this parameter in the absence of symptoms, as it bears little relation to expected complications.18 Other authors have shown that enteral nutrition therapy is safe during repeated prone positioning and does not increase the risk of complications.19,20
Several studies have shown the benefit of creating nutrition therapy protocols for critically ill patients.21–23 These protocols should also be followed during prone positioning in order to minimise potential complications.19,20,24 The latest clinical guidelines on nutrition therapy in the context ofSARS-COV-2 infection25,26 recommend adapting these protocols in patients with respiratory distress, given the numerous therapeutic strategies used (high-flow oxygen, non-invasive positive pressure ventilation, awake prone positioning, or intubation with the need for prone positioning) and the high prevalence of malnutrition associated with prolonged and often complicated ICU stays.27 Some authors recommend considering parenteral nutrition as a complement to oral or enteral nutritional when patients on high flow or non-invasive positive pressure ventilation fail to achieve their nutritional goals.28
Starting nutrition can facilitate early switch to enteral nutrition, thus preserving the intestinal microbiome and reducing endothelial dysfunction. It also reduces the risk of initial overfeeding, thus inhibiting autophagy,29 helps achieve caloric and protein goals during the first week of admission, minimises malnutrition and muscle weakness,30 minimises unnecessary frequent interruptions, and reduces the risk of intestinal complications, such as diarrhoea, intestinal bleeding and nutritrauma.3
This study has several limitations. It is a retrospective study in a small sample, and some of the variables, such as weight or height, were not measured accurately due to communication difficulties with the patient or incorrect information from the family. The protocol was implemented during unprecedented times. In the first weeks of the SARS-COV2 pandemic, the shortage of protective equipment, the need to staff the ICU with personnel who had little experience in intensive care medicine, the lack of specific nutritional guidelines for SARS-COV2 patients, and the stressful working conditions took a toll on nutritional care quality in our unit.
In conclusion, the creation of a nutrition therapy protocol during the first weeks of the SARS-COV2 pandemic could be associated with better clinical outcomes, as we believe it promoted recovery and reduced the risk of treatment-related complications. Further studies are needed to validate the impact of these protocols on clinical outcomes and compare them with other strategies. However, the poor compliance observed in our study shows that barriers to full implementation of nutrition therapy persist. Identifying these barriers will improve adherence to the protocol.