Aseptic total knee arthroplasty (TKA) failure has been associated with radiolucent lines. This study aimed to determine the impact of the early appearance of radiolucent lines (linear images of 1, 2, or >2mm at the cement–bone interface) around the TKA on prosthetic survival and functional outcomes in rheumatoid arthritis (RA) patients during a 2–20 years follow-up.
MethodsWe retrospectively analyzed a consecutive series of RA patients treated with TKA between 2000 and 2011. We comparatively analyzed patients with and without radiolucent lines around implants. Clinical outcomes were assessed with the knee society score (KSS) collected before surgery, at years 2, 5, and 10, and at the last postoperative follow-up. The knee society roentgenographic evaluation system was used to analyze the impact of radiolucent lines around the implants at 1, 2, 5, and more than ten years of follow-up. The reoperation and prosthetic survival rates were calculated at the end of the follow-up.
ResultsThe study series included 72 TKAs with a median follow-up of 13.2 years (range: 4.0–21.0), of which 16 (22.2%) had radiolucent lines. We did not observe aseptic failure, and prosthetic survival at the end of the study was 94.4% (n=68). The KSS improved significantly (p<0.001) between preoperative values at 2, 5, and 10 years and the end of follow-up, with no differences between patients with and without radiolucent lines.
ConclusionsOur study demonstrates that the early appearance of radiolucent lines around a TKA in RA patients does not significantly impact prosthetic survival or long-term functional outcomes at 13 years of follow-up.
El fracaso aséptico de la artroplastia total de rodilla (ATR) se ha asociado a las líneas radiolúcidas. Este estudio tiene como objetivo determinar el impacto en la supervivencia protésica y en los resultados funcionales a largo plazo de la aparición temprana de líneas radiolúcidas (imágenes lineales de 1, 2 o >2mm en la interface cemento-hueso) alrededor de la ATR en pacientes con artritis reumatoidea (AR).
MétodosAnalizamos retrospectivamente una serie consecutiva de pacientes con AR tratados con ATR entre los años 2000 y 2011. Se analizaron comparativamente los pacientes con y sin líneas radiolúcidas alrededor de los implantes. Los resultados clínicos se evaluaron con el Knee Society Score (KSS) registrado antes de la cirugía, a los 2, 5 y 10 años, y en el último seguimiento postoperatorio. Se utilizó el Sistema de Evaluación Roentgenográfica de la Sociedad de la Rodilla para analizar el impacto de las líneas radiolúcidas alrededor de los implantes a 1, 2, 5 y más de 10 años de seguimiento. Se calcularon las tasas de reoperación y supervivencia protésica al final del seguimiento.
ResultadosLa serie de estudio incluyó 72 ATR con una mediana de seguimiento de 13,2 años (rango: 4,0-21,0), de las cuales 16 (22,2%) presentaban líneas radiolúcidas. No se observaron fallos asépticos, y la supervivencia protésica al final del estudio fue del 94,4% (n=68). El KSS mejoró significativamente (p<0,001) entre los valores preoperatorios a los 2, 5 y 10 años y el final del seguimiento, sin diferencias entre los pacientes con y sin líneas radiolúcidas.
ConclusionesNuestro estudio demuestra que la aparición precoz de líneas radiolúcidas alrededor de una ATR en pacientes con AR no afecta significativamente a la supervivencia protésica ni a los resultados funcionales a largo plazo a los 13 años de seguimiento.
Despite the improvements in pharmacological therapies, the number of total knee arthroplasties (TKA) for rheumatoid arthritis (RA) has not decreased in the last years,1,2 being the third leading cause of arthroplasty in the US and Europe.3,4
Survival of TKA over ten years has been reported to vary from 81 to 98%, and aseptic loosening represents one of the leading causes of revision.4,5 Aseptic failure of the TKA has been associated with radiolucent lines and its incidence ranges from 29% to 44% in patients with RA in the short and medium term.6–8 Although the progress of demarcations could harm long-term prosthetic survival,9,10 little is known about its real impact on revision rates regarding the onset time after TKA in RA patients. Furthermore, although these demarcations are a common phenomenon around knee arthroplasty,6–8,10 little is known about whether they impact functional outcomes in RA patients.
We hypothesized that the early appearance of radiolucent lines would negatively impact prosthetic survival beyond ten years of follow-up. Therefore, the primary objective of this study was to determine the impact of radiolucent lines around the TKA on prosthetic survival in RA patients during a 2–20-year follow-up and secondarily to see if the appearance of these demarcations affects functional scores.
Materials and methodsWe retrospectively analyzed our department's database, where we systematically recorded all surgeries and follow-up information. The patients’ selection criteria were RA diagnosis and primary TKA.
After the approval of the institutional ethics committee (Protocol Number 1409), information from our database was crossed with medical and radiographic records at our hospital to obtain accurate data to form the final cohort. The study cut-off date was December 2020.
We included RA patients consecutively treated with TKA from January 2000 to December 2011. History of previous surgeries on the operated knee and those who did not meet the minimum follow-up of 24 months were excluded. Also, due to their different biomechanical and radiological behavior, arthroplasties with a prosthesis with a higher degree of a constraint than a posterior stabilized (PS) one was used were excluded.
In all cases, RA was diagnosed or confirmed by our institution's Rheumatology Department, according to the American College of Rheumatology (score≥6 assessing joint involvement, serology, acute phase reactants, and duration of symptoms).11
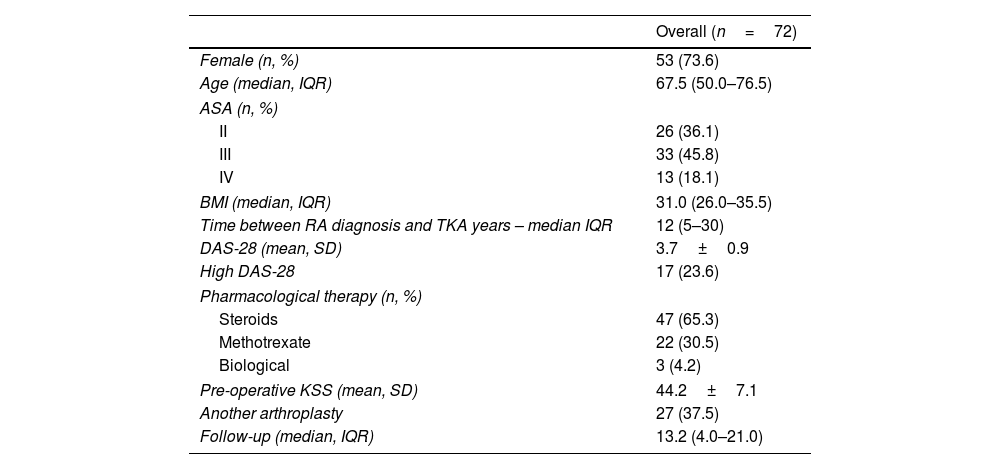
Of 81 TKA identified, nine were excluded (2 for having CCK prosthesis, 1 for having a history of surgery in the operated knee, 3 for not having the radiographic studies to analyze, and 2 for not complying with the minimum follow-up). The study series included 72 TKAs performed on 58 patients, 53 (94.8%) female. The median patient age at surgery was 57.5 (IQR 50–66.5) years, with a mean DAS-28 of 3.7±0.9. Regarding the pharmacological treatment of RA, 65.3% had received corticosteroids, 30.5% methotrexate, and 4.2% biological therapy. The overall time between RA diagnosis and arthroplasty was 12 (range 5–30) years. According to the medical records, 45 (62.5%) PFC Sigma, Johnson & Johnson, Depuy (War, Ind, USA), and 27 (37.5%) Scorpio Stryker (Ma, NJ, USA) were used. All prostheses were posterior stabilized. The summary description of the series is detailed in Table 1.
Description of overall patients and comparative analysis between cohorts.
| Overall (n=72) | |
|---|---|
| Female (n, %) | 53 (73.6) |
| Age (median, IQR) | 67.5 (50.0–76.5) |
| ASA (n, %) | |
| II | 26 (36.1) |
| III | 33 (45.8) |
| IV | 13 (18.1) |
| BMI (median, IQR) | 31.0 (26.0–35.5) |
| Time between RA diagnosis and TKA years – median IQR | 12 (5–30) |
| DAS-28 (mean, SD) | 3.7±0.9 |
| High DAS-28 | 17 (23.6) |
| Pharmacological therapy (n, %) | |
| Steroids | 47 (65.3) |
| Methotrexate | 22 (30.5) |
| Biological | 3 (4.2) |
| Pre-operative KSS (mean, SD) | 44.2±7.1 |
| Another arthroplasty | 27 (37.5) |
| Follow-up (median, IQR) | 13.2 (4.0–21.0) |
ASA: American Society of Anesthesiologists; BMI: body-mass index; DAS: disease activity score; IQR: interquartile range; SD: standard deviation; KSS: knee society score.
The study population was divided into two cohorts for analysis, those with RLL and those without RLL, to assess the impact of the RLL on functional outcomes and prosthesis survival.
Surgical techniqueThe same surgical team performed all surgeries under hypotensive spinal anesthesia in a laminar-flow operating room. We use a midline, longitudinal skin incision with a medial parapatellar approach and capsule-synovectomy. Soft tissue balancing was based on preoperative deformity. In all cases, we replaced the patella with an all-polyethylene kneecap. All prosthetic components were cemented in a single-stage approach (femoral component followed by tibial component, including the tibial keel) using antibiotic cement (gentamicin 0.5g per cement dose). Routinely administered antibiotic prophylaxis (cephazolin during 24h) and antithrombotic drugs (low-molecular-weight heparin for three weeks). Postoperative clinical exams were scheduled at 3 and 6 weeks, six months, and yearly after that.
Data collectionWe recorded demographic data (gender and age), American Society of Anesthesiologists (ASA), body mass index (BMI), the time between RA diagnosis and TKA, pharmacological treatment (corticosteroids, methotrexate, biologics), RA activity level at the time of TKA (assessed with the disease activity score-28 – DAS-28),12 and prosthesis used.
Clinical analysisThe knee society score (KSS)13 was used to assess objective clinical outcomes preoperatively, at 2, 5, and 10 years, and the last patient's follow-up visit.
Radiographic analysisThe femorotibial angle was digitally measured before surgery, and during the immediate postoperative period, an anteroposterior (AP) radiographic view was assessed with Synapse software (Fujifilm Corporation, USA).
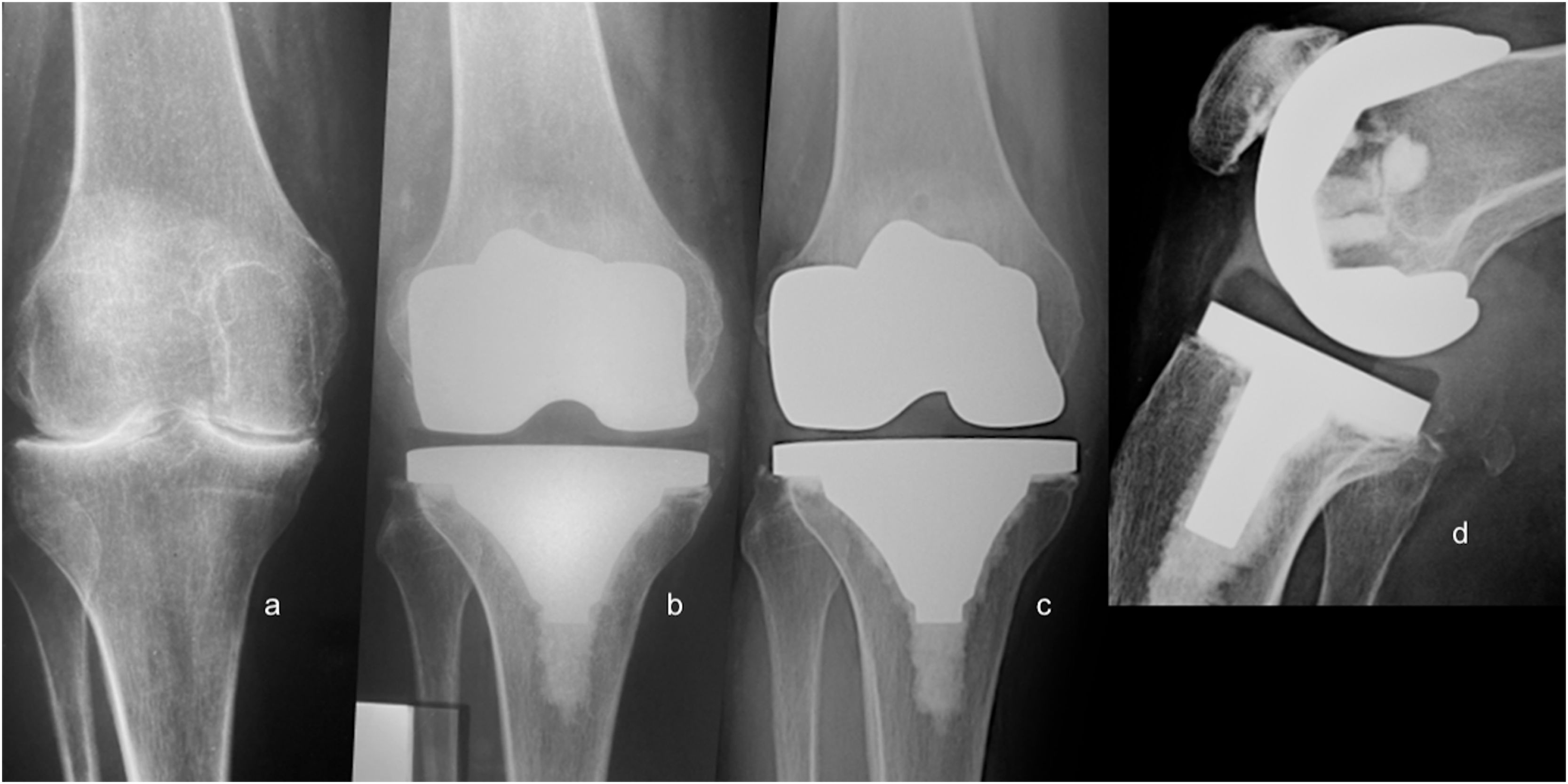
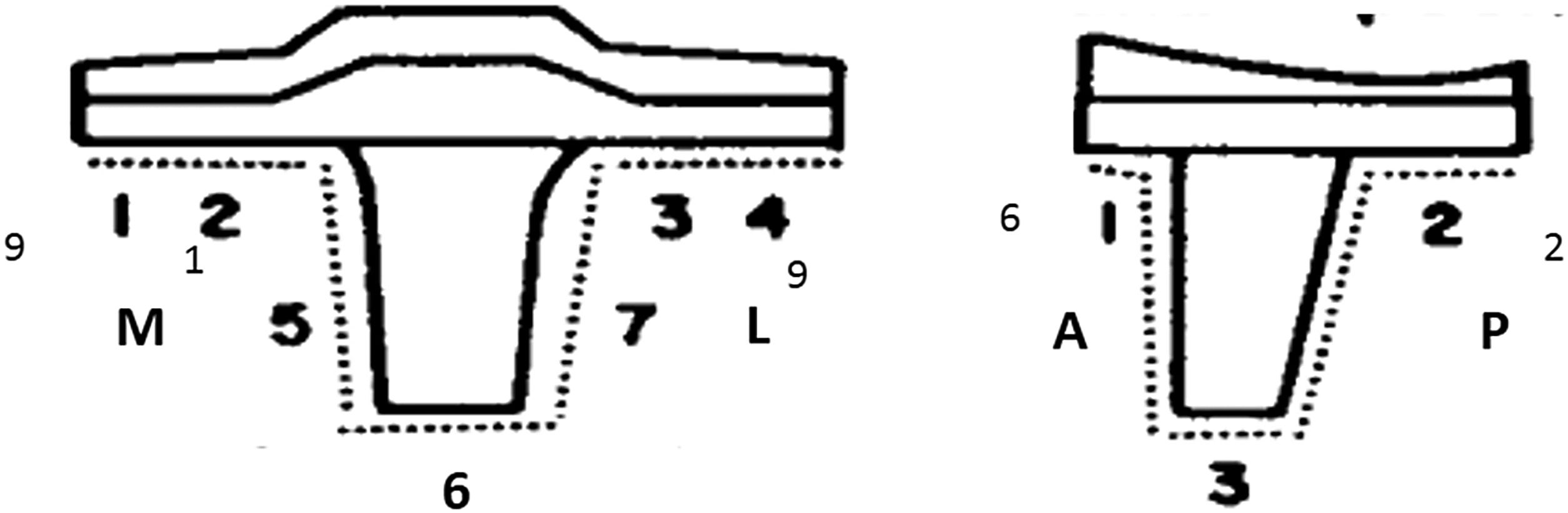
Radiolucent lines in the bone cement interface (linear images 1, 2mm, or >2mm thick), osteolysis (nonlinear areas of bone destruction thicker than 2mm) around the prosthesis, and migration of the implant (>2mm) were assessed by analyzing the postoperative AP and lateral views performed at 1, 2, 5, 10 years and the end of follow-up.14 The affected zone, time of appearance, and progression over time were recorded. The knee society roentgenographic evaluation system was used to describe demarcation lines around the tibial and femoral components and determine mechanical loosening.15,16 Two independent observers and differences performed a radiographic assessment were settled by consensus.
We also recorded the date, type, and number of postoperative complications that required reoperations and prosthetic survival until the end of follow-up. Prosthesis survival was measured, considering revision for any reason as the endpoint.
Statistical analysisQuantitative variables were expressed as mean, standard deviation, median, interquartile range (IQR), and categorical variables as frequencies or percentages. A “t” test and a Chi-square test were used to compare quantitative variables and proportions among groups. A probability of <0.05 was considered statistically significant. Statistical analysis was conducted using SPSS software version 23.0 (IBM, Chicago, IL, USA).
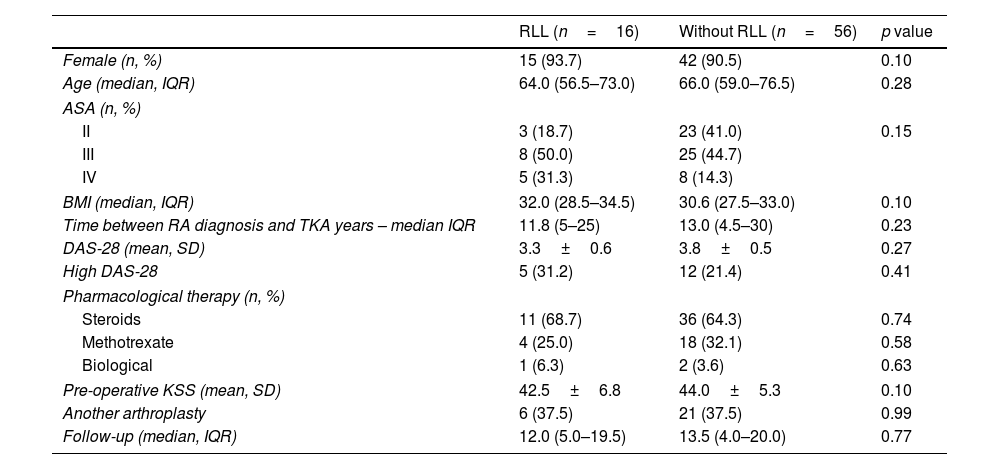
ResultsOf the 72 TKAs analyzed, we observed radiolucent lines around 16 (22.2%) components. The comparative analysis of both groups is shown in Table 2.
Comparative analysis between cohorts.
| RLL (n=16) | Without RLL (n=56) | p value | |
|---|---|---|---|
| Female (n, %) | 15 (93.7) | 42 (90.5) | 0.10 |
| Age (median, IQR) | 64.0 (56.5–73.0) | 66.0 (59.0–76.5) | 0.28 |
| ASA (n, %) | |||
| II | 3 (18.7) | 23 (41.0) | 0.15 |
| III | 8 (50.0) | 25 (44.7) | |
| IV | 5 (31.3) | 8 (14.3) | |
| BMI (median, IQR) | 32.0 (28.5–34.5) | 30.6 (27.5–33.0) | 0.10 |
| Time between RA diagnosis and TKA years – median IQR | 11.8 (5–25) | 13.0 (4.5–30) | 0.23 |
| DAS-28 (mean, SD) | 3.3±0.6 | 3.8±0.5 | 0.27 |
| High DAS-28 | 5 (31.2) | 12 (21.4) | 0.41 |
| Pharmacological therapy (n, %) | |||
| Steroids | 11 (68.7) | 36 (64.3) | 0.74 |
| Methotrexate | 4 (25.0) | 18 (32.1) | 0.58 |
| Biological | 1 (6.3) | 2 (3.6) | 0.63 |
| Pre-operative KSS (mean, SD) | 42.5±6.8 | 44.0±5.3 | 0.10 |
| Another arthroplasty | 6 (37.5) | 21 (37.5) | 0.99 |
| Follow-up (median, IQR) | 12.0 (5.0–19.5) | 13.5 (4.0–20.0) | 0.77 |
RLL: radiolucent lines; ASA: American Society of Anesthesiologists; BMI: body-mass index; DAS: disease activity score; IQR: interquartile range; SD: standard deviation; KSS: knee society score.
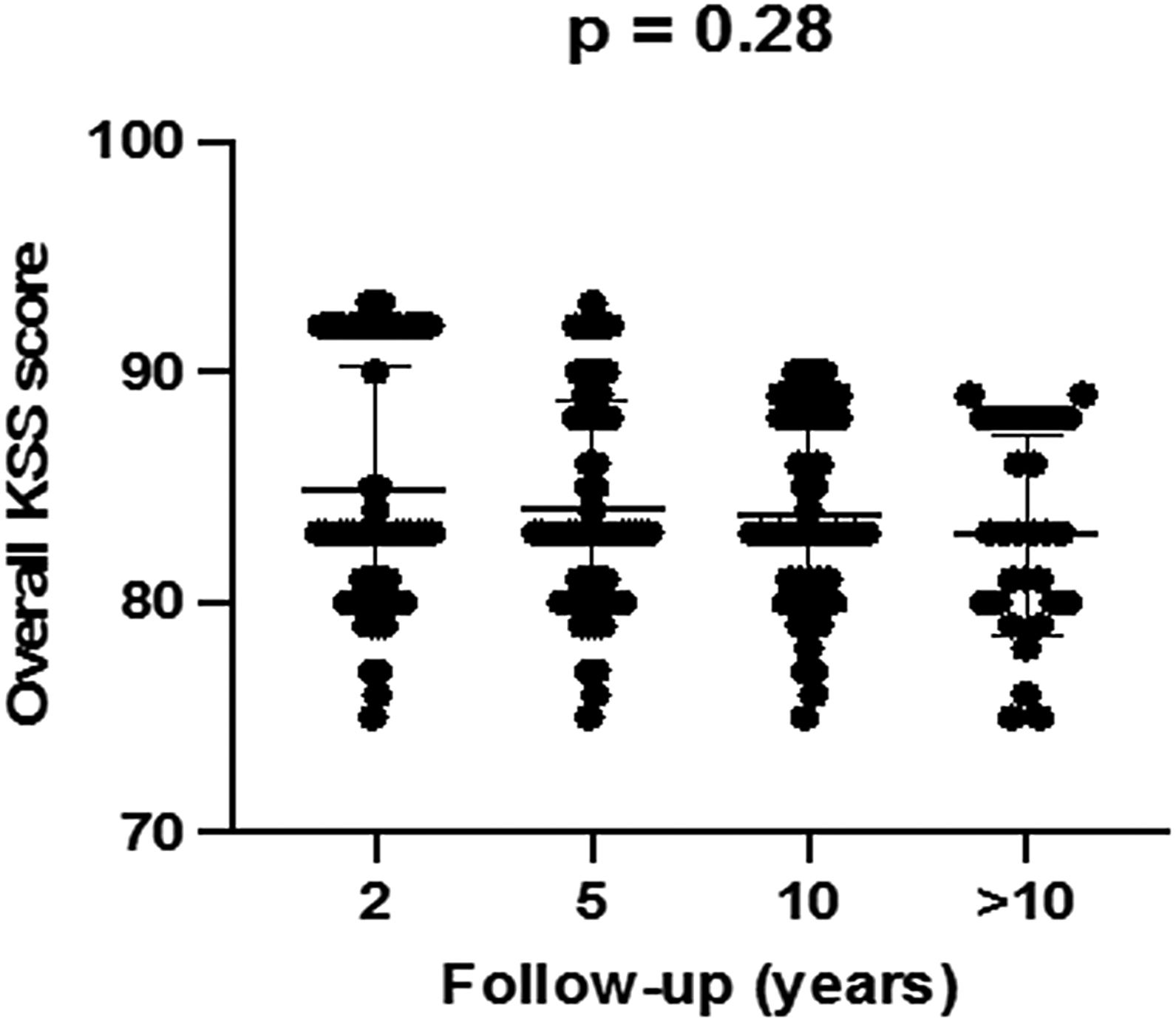
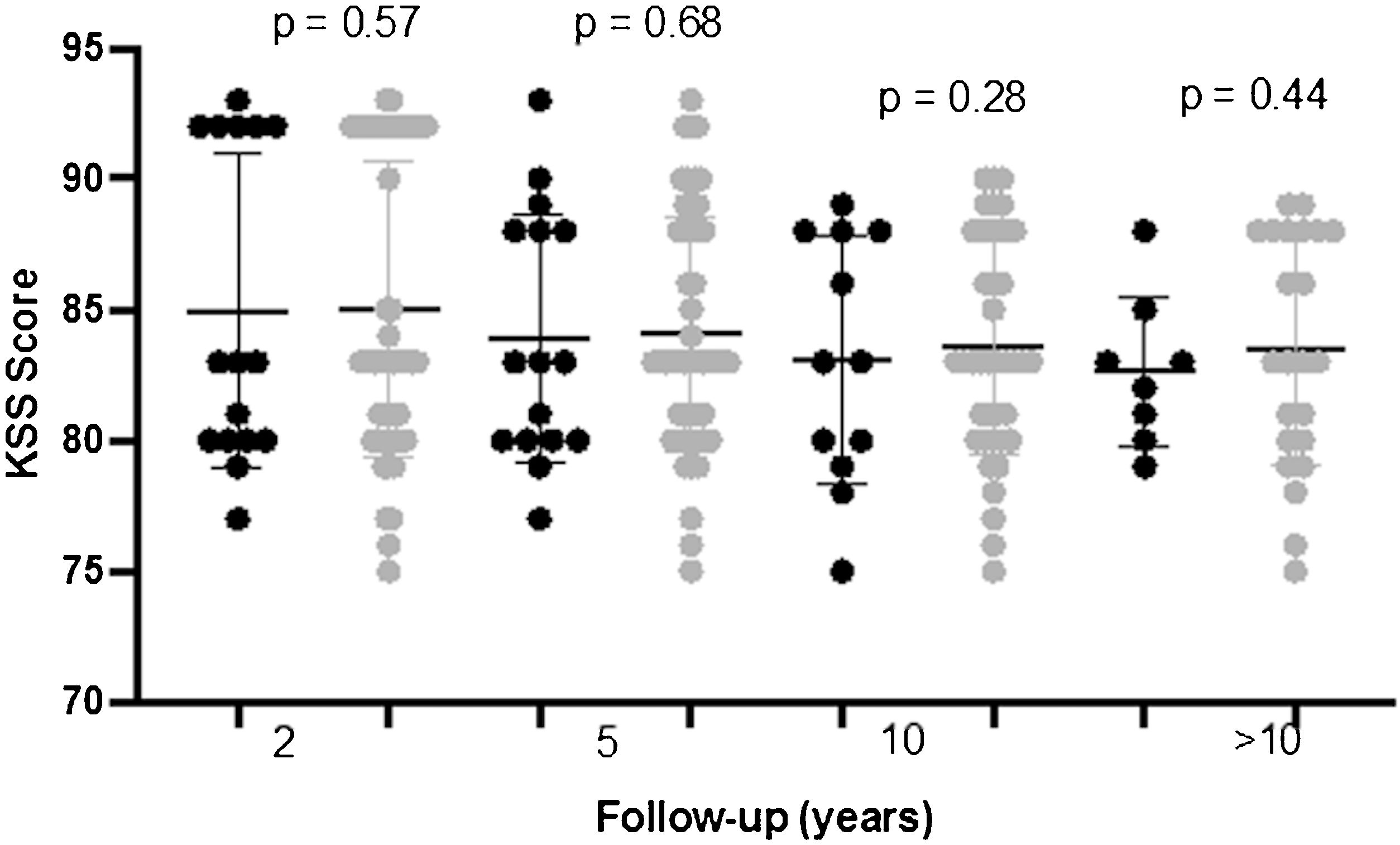
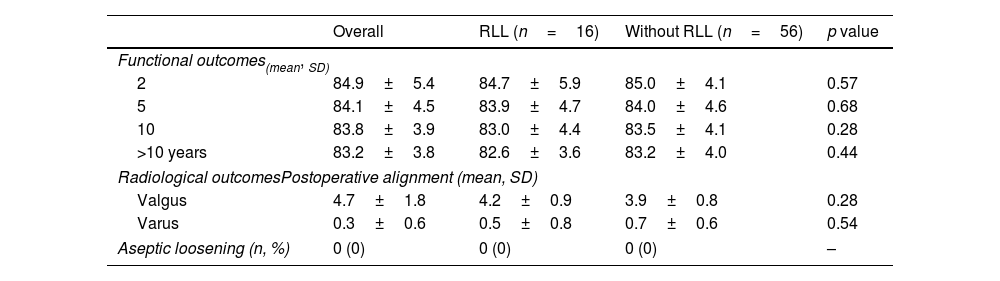
KSS values improved significantly between baseline and the first two postoperative years. These improvements were sustained over time with no significant differences at 2-, 5-, 10- and more than 10-year postoperative scores (p=0.28), and no significant differences were observed between patients with and without RLL (Figs. 1 and 2).
Radiological outcomesThe radiological assessment showed preoperative valgus malalignment in 91.66% (n=66) and varus in 8.4% (n=6) cases. The postoperative alignment was valgus in 60 (83.33%) cases and varus in 12 (16.67%) (Table 3).
Clinical and radiological assessment.
| Overall | RLL (n=16) | Without RLL (n=56) | p value | |
|---|---|---|---|---|
| Functional outcomes(mean, SD) | ||||
| 2 | 84.9±5.4 | 84.7±5.9 | 85.0±4.1 | 0.57 |
| 5 | 84.1±4.5 | 83.9±4.7 | 84.0±4.6 | 0.68 |
| 10 | 83.8±3.9 | 83.0±4.4 | 83.5±4.1 | 0.28 |
| >10 years | 83.2±3.8 | 82.6±3.6 | 83.2±4.0 | 0.44 |
| Radiological outcomesPostoperative alignment (mean, SD) | ||||
| Valgus | 4.7±1.8 | 4.2±0.9 | 3.9±0.8 | 0.28 |
| Varus | 0.3±0.6 | 0.5±0.8 | 0.7±0.6 | 0.54 |
| Aseptic loosening (n, %) | 0 (0) | 0 (0) | 0 (0) | – |
RLL: radiolucent lines; SD: standard deviation.
We found 27 radiolucent lines around 16 (22.22%) prosthetic components in 16 patients. All appeared during the first two postoperative years around of tibial component (Fig. 3). Ten patients presented one demarcated zone, 3 two zones, 2 three zones, and 1 four zones. All of them were <2mm and did not progress during follow-up. The frequency of RLL distribution is detailed in Fig. 4. No femoral component showed demarcation. No patients showed signs of osteolysis, migration of prosthetic components, or more than four areas with RLL.
The reoperation rate was 6.94% (n=5). All reoperations were performed within two years of the procedure (median 12 months; range 5–20). Four were due to prosthetic joint infections (PJI) and were successfully treated with a 2-stage revision. The remaining patient suffered an extensor apparatus rupture, as he fell from his height seven months after TKA and was successfully treated with a direct repair procedure. No mechanical failures were registered until the end of the study. Prosthetic survival at the end of the study was 94.4% (n=68).
DiscussionThe most relevant finding of our study was that the early appearance (in the first two postoperative years) of radiolucent lines around the TKA implants did not impact long-term prosthetic survival in RA patients.
Radiolucent lines on follow-up radiographs are a common finding in TKA analysis, and their appearance has been associated with mechanical loosening and prosthetic failure in non-RA patients.6,10,17 Most previous studies describing these radiolucent lines do so at the end of follow-up.18,19 Although using cementless prostheses and diagnoses other than RA, a few have evaluated the evolution of these lines at more a than ten years of follow-up.6–9 To our knowledge, this study is the first to evaluate this topic in RA patients. In the present study, 22.22% of the prosthetics components presented these RLL during the first two postoperative years. After a median follow-up of 13.5 years, we did not observe progression or impact on prosthetic survival. These RLL were located around the tibial component, which has been described as the most frequent location even in non-RA patients.6,10,20,21 Another interesting point of our analysis is that, although the inflammatory component of RA can be aggressive (25% of the patients analyzed had high levels of disease activity at the time of TKA), the RLL showed no progression between two and more than ten years of follow-up. It is in contrast to that reported by Bohler et al.22 who observed that patients with higher disease activity levels at the time of surgery had a higher risk of aseptic failure in the future.
The absence of revisions for mechanical loosening in our series contrasts with those studied by Lee et al.5 who reported a revision rate of 12.87% at 15 years of follow-up. However, our findings are consistent with Yamanaka et al.’s,8 who found no revisions due to mechanical loosening at 12 years of follow-up. The absence of revisions due to mechanical loosening in our patients could be explained by the involvement of other joints (37.5% of the patients underwent another arthroplasty), generating a lower demand for the implant.2 It also could be explained because our study population was relatively young (median age 67.5 years) and mostly females (94.8%), which agrees with that reported by Rand et al.,23 who highlighted these variables as protective of long-term revision. Finally, another possible explanation could be related to the cemented fixation used in the series, which, even in this particular group of patients, is shown to be an alternative that allows efficient long-term fixation. Furthermore, prosthesis survival in our series was consistent with the ones reported by Kristensen et al.20 (81% after ten years), Rodriguez et al.24 (91% after 15 years), and Lee et al.5 (98.7% after ten years).
Another relevant finding of our study was that the improvements in functional outcomes achieved two years after TKA in RA patients did not deteriorate over time or with the appearance of RLL. We observed no significant changes when we analyzed functional outcomes at 5, 10, or even above ten years of follow-up. In addition, we also observed no significant differences in the short or long term when comparing these scores with those of patients without RLL. Because we found no studies correlating functional outcomes with the occurrence of RLL in RA patients, we can only compare this with TKA in non-RA patients. Our results were comparable to those reported by Ng et al.25 who reported the apparition of radiolucent lines in 48% of their series and found that it did not affect early functional outcomes. In contrast, they were opposite to those reported recently by Sadoghi et al.,26 who reported a direct relationship between RLL and pain. Their series reported that 28 patients reported postoperative pain, of which 27 had RLL.
Few studies have focused on characterizing functional outcomes’ evolution in RA patients.27 While different series have reported improvements in functional scores and quality of life in RA patients after TKA, most only describe outcomes at the end of follow-up.5,7,8,20 Thus, this analysis fills a gap in the available literature on TKA in RA patients.
The limitations of this study lie in its retrospective design and the small number of patients. It may have affected the statistical significance of the analysis (beta-type error). Another limitation may be represented by the low number of patients treated with biological agents, which is very frequent nowadays. However, we understand this limitation as logical due to the postoperative follow-up that the series presented. Another limitation was the absence of similar studies, which would allow us to compare our findings accurately. Its strength is that it is the first to compare RA patients with and without RLL around a knee prosthesis regarding their functional impact and prosthetic survival at 13 years of follow-up.
ConclusionsThe results of our study suggest that the early appearance of radiolucent lines around a TKA in RA patients does not necessarily impact long-term prosthetic survival and functional outcomes.
Level of evidenceLevel of evidence iv.
Authors’ contributionsAll authors contributed to the study's conception and design. Material preparation, data collection, and analysis were performed by German Garabano, Joaquín Rodriguez, and Leonel Perez Alamino. The first draft of the manuscript was written by German Garabano and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Ethics approvalAll procedures performed in this study, which involved the use of data from human participants, were in accordance with the ethical standards of the 1964 Helsinki Declaration and its later amendments. The Ethics committee of the British Hospital of Buenos Aires approved this study (N° 1409).
Informed consentInformed consent was obtained from all individual participants included in the study.
Availability of data and materialAll data generated and analyzed during this study are included in this published article and are available from the corresponding author upon reasonable request.
FundingThis research has not received specific support from public sector agencies, the commercial sector, or non-profit organizations.
Conflict of interestNone.
None.