Cement restrictors (CRs) are devices that allow occlusion of the femoral canal in order to obtain greater interdigitation of the cement between the bone and a better pressurization, which generates an increase in the survival of cemented stems. The aim of this study was to evaluate the efficacy of the different CRs used and propose a classification of this device.
Materials and methodsAn experimental study was carried out, where 7 CR references of different designs and manufacturers were taken. Later, tests were carried out on 9 chlorinated polyvinyl chloride tubes for each reference, to achieve a total of 63 tests.
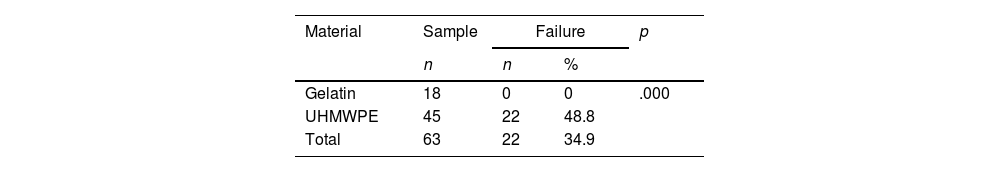
ResultsIn our study, 34.9% of the CRs in ultra high molecular weight polyethylene failed, presenting migration and allowing cement to leak while none of the gelatin RC failed.
ConclusionThe RC with an umbrella design proved to be the less effective, presented a higher incidence of migration and cement leakage, while the gelatin CRs were the best performers. Based on the results of this study, an analysis of the CR design was carried out and a classification was proposed that divides these devices into 2 types.
Los restrictores de cemento (RC) son dispositivos que permiten la oclusión del canal femoral con el fin de obtener una mayor interdigitación del cemento en el hueso y una mejor presurización, lo que genera un incremento en la supervivencia de los vástagos cementados. El objetivo de este estudio fue evaluar la eficacia de los diferentes RC utilizados y proponer una clasificación de este dispositivo.
Materiales y métodosSe realizó un estudio experimental, donde se tomaron 7 referencias de RC de diferentes diseños y fabricantes. Posteriormente se hicieron pruebas en 9 tubos de policloruro de vinilo clorado por cada referencia para conseguir un total de 63 pruebas.
ResultadosEl 34,9% de los RC de nuestro estudio presentaron migración y permitieron la fuga de cemento; todos estos RC eran de polietileno de ultra alto peso molecular (PUAPM), mientras que ninguno de los RC de gelatina falló.
ConclusiónLos RC con diseño en paraguas demostraron ser los menos eficaces, presentando una mayor incidencia de migración y fuga de cemento, mientras que los RC de gelatina fueron los de mejor desempeño. Basado en los resultados de este estudio, se realizó un análisis del diseño de los RC y se propuso una clasificación que divide estos dispositivos en 2 tipos.
Sir John Chanley introduced the technique of femoral cementing in total hip replacement, which has evolved over the past 55 years.1 The quality of cementing has been described as a predictor of femoral stem survival along with other determinants such as pulsatile lavage, cement type, bone quality, retrograde cementing technique, cement pressurisation and cement restrictor (CR) placement, and positioning technique.2–4 CRs are devices that allow occlusion of the femoral canal to prevent cement migration, increase intramedullary pressure, promote bone–cement interface, aid femoral stem orientation and decrease the likelihood of medical complications such as pulmonary thromboembolism, hyperpressure syndrome in the medullary canal, and cardiovascular events.5 Cement pressurisation in the femoral canal enhances interdigitation in spongy bone, and is directly related to tensile and shear strength at the cement-bone interface.2
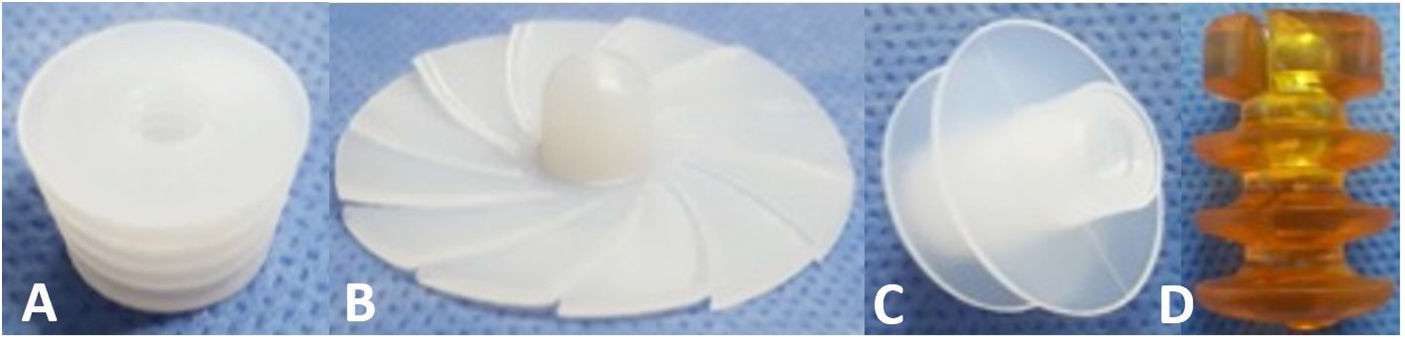
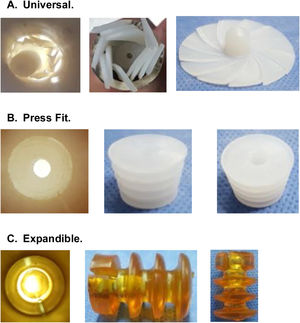
CRs can be made of bone, ultra-high molecular weight polyethylene (UHMWPE) or gelatin, among other materials, and can be classified by design, of which there are 3 types2,6,7 (Fig. 1):
- 1.
Universal: CRs that are available in a single size and adapt to the different internal diameters that the femoral canal may present.
- 2.
Press-fit: CRs that are available in different sizes and are impacted to the required depth within the femoral canal.
- 3.
Expandable: CRs that are available in different sizes that expand within the femoral canal until supported with the endosteal surface.
Radiological images have been found in surgical practice and in postoperative controls with cemented stems, that show evidence of cement leakage and/or migration of the CR, and these factors may impact the survival of the femoral stem.2 The aim of this study was to analyse the performance of different CR materials and designs, considering the variables of cement migration and leakage, and to propose a classification for this device.
Materials and methodsVariablesA non-clinical experimental study was performed analysing CR migration, defined as the presence of this device more than 10cm from the proximal end of the tube. The second variable was cement leakage, defined as a radio-opaque image distal to the CR, in any length of the tube.3
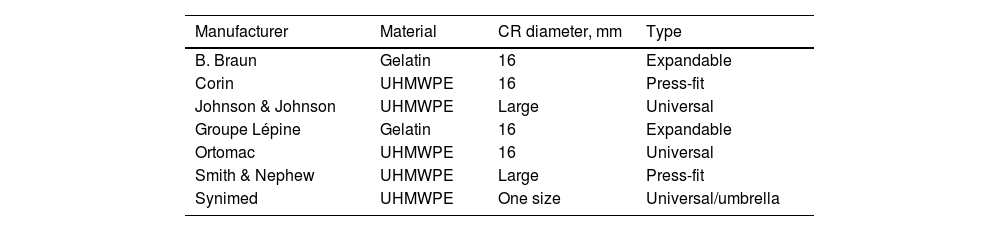
ProcedureSeven types of CR of different brands with a 16mm diameter were used (Table 1). For each CR reference, 9 samples were taken and placed in 20cm long PAVCO chlorinated polyvinyl chloride (PVC) tubes that withstand elevated temperatures and have an internal diameter of 16mm; the RC was placed 10cm away from the proximal edge.
Cement restrictors used in the study.
| Manufacturer | Material | CR diameter, mm | Type |
|---|---|---|---|
| B. Braun | Gelatin | 16 | Expandable |
| Corin | UHMWPE | 16 | Press-fit |
| Johnson & Johnson | UHMWPE | Large | Universal |
| Groupe Lépine | Gelatin | 16 | Expandable |
| Ortomac | UHMWPE | 16 | Universal |
| Smith & Nephew | UHMWPE | Large | Press-fit |
| Synimed | UHMWPE | One size | Universal/umbrella |
Cementation followed this process, for which we used low viscosity Fix 3™ bone cement from Groupe Lépine. The syringe was filled with this component, and we cemented retrograde to the proximal edge, with a setting time of 2min, at an ambient temperature of 21.9°C and humidity of 46.3% in all cases, simulating an operating theatre (Figs. 2 and 3). The average time between preparing the cement and the cementing procedure was 4.3min (SD .78min).
Finally, the PVC pipes were x-rayed with Shimadzu's MobileArt eco equipment, and from the images obtained, a radiologist performed measurements to establish the results in terms of migration and leakage variables. The radiologist did not know the type of CR.
Statistical analysisQualitative variables are presented as absolute frequencies and percentages. Quantitative variables are described as medians and interquartile ranges assuming that the distribution is non-normal according to the Shapiro–Wilk test (p=.000). To estimate differences in efficacy between CR types by material (gelatin and UHMWPE), Fisher's exact test was used, and Pearson's χ2 statistical test was used for design and manufacturer analysis. A p-value <.05 was interpreted as significant, with 2-tailed hypothesis testing. The analysis was performed in IBM SPSS 21.
ResultsTwenty-two (34.9%) of the 63 CRs in our study failed, and of these, only 3 CRs had migrated; no restrictor showed leakage in isolation.
The analysis of materials found that of the 63 CRs, 45 (71.4%) were UHMWPE and 18 (28.6%) were gelatin. Failure was found in 22 (48.8%) of the 45 UHMWPE CRs, whereas none of the 18 gelatin CRs showed failure (Tables 2 and 3).
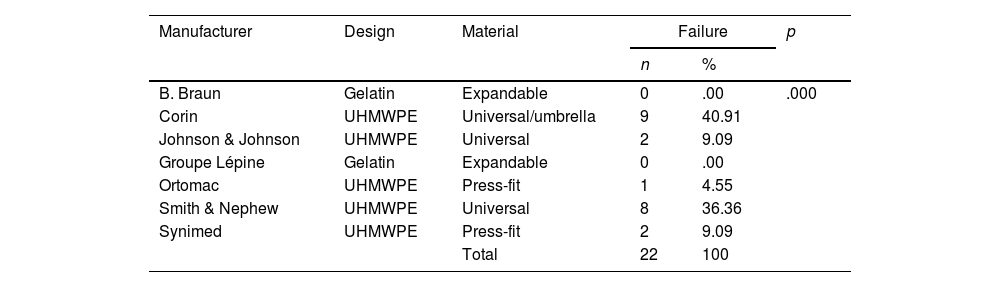
Analysis of cement restrictors according to the manufacturer.
| Manufacturer | Design | Material | Failure | p | |
|---|---|---|---|---|---|
| n | % | ||||
| B. Braun | Gelatin | Expandable | 0 | .00 | .000 |
| Corin | UHMWPE | Universal/umbrella | 9 | 40.91 | |
| Johnson & Johnson | UHMWPE | Universal | 2 | 9.09 | |
| Groupe Lépine | Gelatin | Expandable | 0 | .00 | |
| Ortomac | UHMWPE | Press-fit | 1 | 4.55 | |
| Smith & Nephew | UHMWPE | Universal | 8 | 36.36 | |
| Synimed | UHMWPE | Press-fit | 2 | 9.09 | |
| Total | 22 | 100 | |||
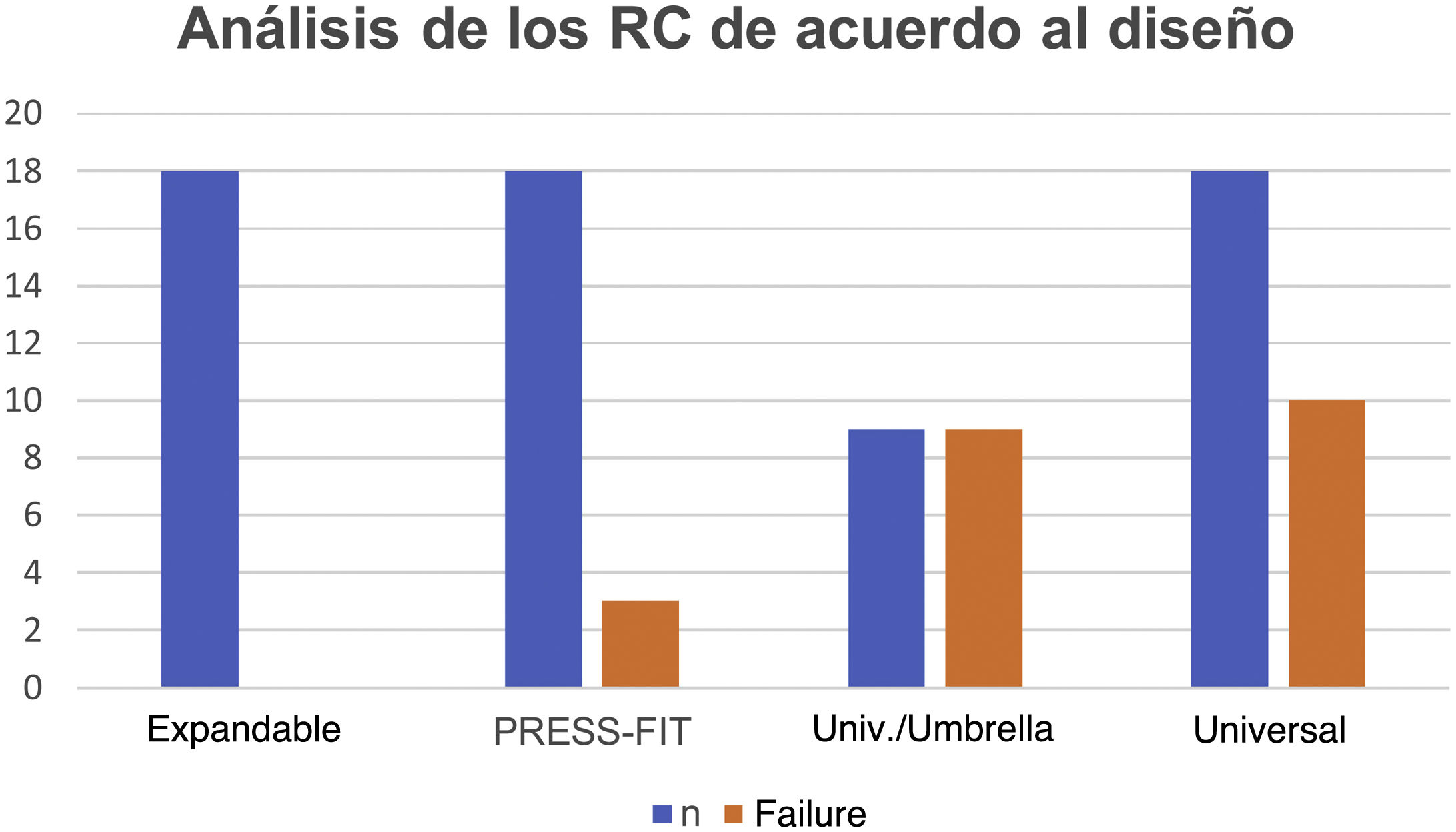
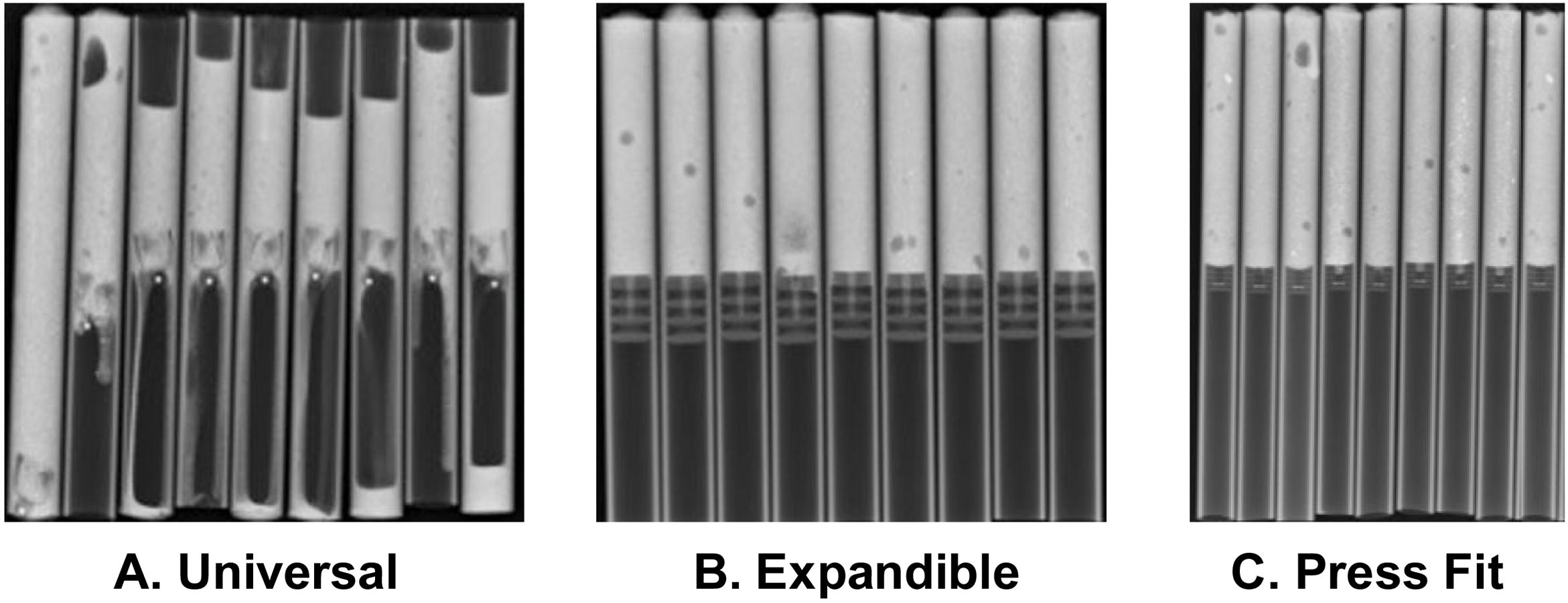
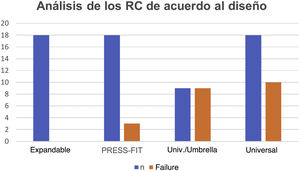
In the analysis according to design, we found the CRs were distributed as follows: 18 (28.5%) were expandable, 18 (28.5%) press-fit, and 27 (42.8%) universal. We found failure in 19/27 (70.3%) of the universal and 3/18 (16.6%) of the press-fit CRs; none of the expandable CRs failed. Of the 27 universal CRs, 9 were umbrella-shaped, of which 100% failed, while only 10 failed (55.5%) of the remaining 18 universal CRs (Figs. 4 and 5).
Fig. 5 gives an example of the 3 groups of CRs analysed in our study. Of the CRs that showed migration and leakage, the median displacement was 10.15mm (interquartile range 8-10.43); however, 3 cases showed higher migration (100, 102 and 279mm); these 3 CRs were UHMWPE and universal/umbrella in design.
DiscussionAn adequate cement mantle around the femoral component maintains load distribution across the interface between the stem and the cement and between the cement and the femoral cortices. To achieve this quality of cementation it is essential to have a CR that does not allow leakage or migration of more than 3cm, above this distance cementation defects can be seen in Gruen zones 3, 4 and 5.8 Some CRs allow cement leakage or migrate distally, affecting the quality of cementing and thus the survival of the femoral stem.3,7
Wembridge and Hamer9 conducted a prospective randomised clinical trial in which they evaluated 2 CRs: a UHMWPE CR and a gelatin CR. They concluded that the UHMWPE CR showed less migration compared to the gelatin CR; however, they mention that they have reservations about using the UHMWPE CR due to the potential risks of osteolysis and aseptic loosening. In this study the gelatin CR was not shown to be suitable for good femoral cement pressurisation, unlike the results obtained in our study, where none of the gelatin CRs showed migration.
Schauss et al.10 conducted a prospective randomised clinical trial comparing UHMWPE and gelatin CRs. They found greater migration in patients using the biodegradable gelatin Biostop G (DePuy) CR compared to the non-degradable UHMWPE Allopro CR (Sulzer Medica) with a statistically significant difference (p=.031). Although, when assessing the quality of cementation with the Barrack classification, it did not differ significantly between the 2 groups, it is worth noting that in the conclusion of the study they mention that the insufficient intramedullary fixation of biodegradable CRs is probably due to the elastic properties of the material, which may lead to imprecision in the choice of CR size. They add that the result may be related to imprecision during the surgical procedure and not to the design or material of the CR.
The studies by Wembridge and Hamer9 and Schauss et al.11 differ from our results, where the 2 types of degradable gelatin CRs used performed best, as they did not migrate or leak. Consistent with this, Downing and Broodryk12 published their clinical experience with the gelatin Biostop G CR and show that it is an effective device that prevents migration and leakage once the correct sizing and insertion technique has been learned. Similarly, Prudhon et al.13 conducted a retrospective review study involving 100 cases of the use of an Air Plug™ gelatin CR and found 100% survival and no adverse events reported, which supports the use of this type of gelatin CR.
Heisel et al.,14 in a non-clinical experimental study, proposed that flexible gelatin CRs (Biostop G, IMSET, Plugin Tech) achieved sufficient occlusion and stability in the canal even at slightly higher insertion pressures and forces. However, the stiffer polyethylene CRs (BUCK, Universal Cement Restrictor) showed reduced stability and poor sealing ability, and they state that the latter devices cannot be recommended for use with modern cementation techniques. The findings of this study are consistent with those reported in our study, where we found optimal results with gelatin CRs.
Faraj and Rajasekar8 conducted a randomised double-blind clinical trial comparing a bone CR and a UHMWPE universal umbrella-shaped CR, and reported that 69.4% of the universal umbrella-shaped CRs migrated; 100% of the universal umbrella-shaped CRs also failed in our study.
The results obtained allowed us to analyse the design of the distinct types of CR, to find different materials and to determine the anatomy of the CR, such as the centre or core and the fins, which have variations in diameter, thickness, and the distance between them.
In view of the above, we propose the following classification:
- I.
CR where the core is more than half the diameter of the overall diameter of the restrictor.
- II.
CR where the core is less than or equal to half the overall diameter of the restrictor.
Having evaluated the design of the CRs and analysed the results found in our study, we conclude that the CRs with thicker cores, i.e., type I CRs, better met the objectives required for proper cementing.
This study has some limitations due to its non-clinical experimental design and because the cementation tests were not performed on trabeculated material or bone. However, the use of PVC tubing made it possible to guarantee the same diameter in all samples and thus ensure better fixation for the different CRs analysed. The studies cited in this section were conducted with different types of stems and in our study, we worked with CRs without placing this device.
To conclude, it is evident that gelatin CRs show better results because they have a thicker centre or core and smaller and thicker but malleable fins with shorter intercalation, which allows better occlusion and coaptation of the canal, reducing migration and leakage; this requires an appropriate surgical technique for sizing and insertion. However, the universal umbrella-shaped CRs showed the highest percentage of migration and leakage of all the designs evaluated in this study. Prospective clinical and radiological studies are required of the different CR models used.
Level of evidenceLevel of evidence i.
Conflict of interestsThe authors have no conflict of interests to declare.