Prosthetic joint infection is one of the most serious complications in orthopedics. Prognostic systematic reviews (SRs) detecting and assessing factors related to prosthetic joint infection, allow better prediction of risk and implementation of preventive measures. Although prognostic SRs are increasingly frequent, their methodological field presents some knowledge gaps.
PurposeTo carry out an overview of SR assessing risk factors for prosthetic joint infection, describing and synthesizing their evidence. Secondarily, to assess the risk of bias and methodological quality.
Material and methodsWe conducted a bibliographic search in 4 databases (May 2021) to identify prognostic SR evaluating any risk factor for prosthetic joint infection. We evaluated risk of bias with the ROBIS tool, and methodological quality with a modified AMSTAR-2 tool. We computed the overlap degree study between included SR.
ResultsTwenty-three SRs were included, studying 15 factors for prosthetic joint infection, of which, 13 had significant association. The most frequently studied risk factors were obesity, intra-articular corticosteroids, smoking and uncontrolled diabetes. Overlapping between SR was high for obesity and very high for intra-articular corticoid injection, smoking and uncontrolled diabetes. Risk of bias was considered low in 8 SRs (34.7%). The modified AMSTAR-2 tool showed important methodological gaps.
ConclusionsIdentification of procedural-modifiable factors, such as intra-articular corticoids use, can give patients better results. Overlapping between SR was very high, meaning that some SRs are redundant. The evidence on risk factors for prosthetic joint infection is weak due to high risk of bias and limited methodological quality.
La infección protésica es una de las complicaciones más graves en cirugía ortopédica, por lo que es importante detectar factores relacionados con su aparición. Las revisiones sistemáticas (RS) pronósticas detectan y evalúan factores relacionados con enfermedades, lo que permite una mejor predicción de los riesgos y la implementación de medidas preventivas. Aunque estas RS pronósticas son cada vez más frecuentes, su campo metodológico presenta algunas lagunas de conocimiento.
ObjetivoRealizar una revisión de RS de factores pronósticos para infección protésica y describir la evidencia sintetizada. Secundariamente, evaluar el riesgo de sesgo y la calidad metodológica.
Material y métodosBúsqueda bibliográfica en 4 bases de datos (en mayo de 2021) para identificar RS pronósticas que evaluaran factores pronósticos para infección protésica. Evaluamos el riesgo de sesgo mediante ROBIS y la calidad metodológica con la herramienta modificada AMSTAR-2. Se realizó un estudio de solapamiento entre RS.
ResultadosIncluimos 23 RS que valoraban 15 factores asociados con la infección protésica; de ellos, 13 mostraron asociación significativa. Los más estudiados fueron obesidad, corticoides intraarticulares, tabaquismo y glucemia elevada. El solapamiento entre RS fue elevado para obesidad y muy alto para corticoides intraarticulares, tabaquismo y glucemia elevada. El riesgo de sesgo fue considerado bajo en 8 RS (34,7%), pero la herramienta de evaluación metodológica demostró una baja calidad en general.
ConclusionesLa identificación de factores procedimentales modificables ofrece mejores resultados a los pacientes. Sin embargo, muchas RS son redundantes. La evidencia en factores pronósticos en cirugía ortopédica es débil debido al alto riesgo de sesgo y a la calidad metodológica limitada.
Prosthetic surgery is one of the most commonly performed surgeries in the field of orthopedics. According to data from the Atlas of Variability in Medical Practice group, the standardized rate for total knee prostheses (TKP) in Spain in 2017 was 25 cases per 10,000 inhabitants, with a variability across health regions of 27%, and 10 cases per 10,000 inhabitants for total hip prostheses (THP), varying by 20%.1 Andalusia, Catalonia, the Basque Country, and Valencia are among the autonomous communities with the highest rates. There is a registry of arthroplasties in Catalonia according to which 9246 TKP and 4772 THP were performed in 2019.2
Infection is one of the most serious complications in joint replacement surgery. The incidence of prosthetic infections differs depending on the joint. In primary prosthetic knee arthroplasties, it ranges between 1 and 2% according to the series3–5 and rises up to 10% for revision surgery3,4; the rates are slightly better for hip and shoulder surgeries, and do not exceed 1% in some series.6 The incidence of prosthetic infections also varies according to the type of prosthetic joint. According to data from the Vigilància de les Infeccions Nosocomials als Hospitals de Catalunya (VINCat, abbreviation for the Nosocomial Infection Surveillance Service in Catalan Hospitals) and the programa de Prevenció de les infeccions quirúrgiques a Catalunya (PREVINQCat, abbreviation for the Program for the Prevention of Surgical Infections in Catalonia), infection rates in the autonomous community of Catalonia are 1–2% for knee replacement surgery and 1% for hip arthroplasties.2 These complications involve great economic cost and are associated with a high risk of complications.7 Therefore, detecting prognostic factors related to the appearance of these infections has both clinical and healthcare resource management implications.8
There are prognostic factors that are inherent to the patient, which may or may not be modifiable, such as obesity or smoking. Others have to do with the surgical procedure itself, such as changing moving parts in the prostheses,9,10 whereas others are related to the characteristics of the microorganism responsible for the infection.
Interest in studies regarding prognostic factors, including in the field of prosthetic infections, has increased in recent years. Such studies seek to identify prognostic factors with the aim of predicting the course and outcome of patients with a given health status11; that is, they intend to identify factors associated with the occurrence of a condition or event, to understand the course of that condition over time, its determining factors, or the probability of a given outcome. Prognostic information has become increasingly important for evidence-based decisions in health care and is key to personalized, precision, risk-based medicine.12
Studies of prognostic factors feature particularities with regard to their design and the methodological tools to assess the risk of bias compared to studies of interventions or diagnostic testing.11–13 Systematic reviews (SRs) of prognostic factors are a methodological field currently being developed,14–17 although there are still knowledge gaps.18–22 For example, there is no specific tool to evaluate the methodological quality of this type of prognostic revision. There are, however, tools in this sense for reviews of intervention studies, such as the A Measurement Tool to Assess Systematic Reviews (AMSTAR-2).21
The aim of this study was to carry out a review of SRs appraising prognostic factors for the occurrence of prosthetic joint infection, so as to describe and synthesize the evidence. Our secondary objective was to develop a modification of the AMSTAR-2 tool to assess the risk of bias and methodological quality of prognostic SRs.
Material and methodsThis review of reviews was conducted following established methodological proposals and was reported in accordance with the PRISMA 2020 reporting standards.21,23,24 A protocol was previously developed and registered in Open Science Framework (Registration DOI: 10.17605/OSF.IO/HZSQB).
SearchWe performed an electronic search of PubMed, Cochrane Library, Embase, and Epistemonikos to identify SRs published on or before May 5, 2021. We reviewed the bibliography of the SRs included to identify other potentially eligible SRs. There were no language restrictions. The search strategy was designed with the help of an experienced documentalist and was based on the following Boolean operators: “joint arthroplasty,” “joint replacement,” “arthroplasty,” “prosthetic joint infection,” “arthroplasty infection,” “joint infection,” and “systematic review.” The complete electronic search strategy is provided in Anexo 1.12
Inclusion and exclusion criteriaThe inclusion criteria are defined below according to the PICO structure. We included SRs of studies of any design, performed among patients undergoing prosthetic surgery of any joint (population), in which subjects with a prognostic factor (prognostic index factor) were compared with those without the factor under study (comparison) and the incidence of prosthetic infection, be it acute or chronic (outcomes), was observed. We only looked at SRs that conducted their bibliographic search in at least two databases, contained an explicit, detailed method, and that evaluated the quality of the studies included.
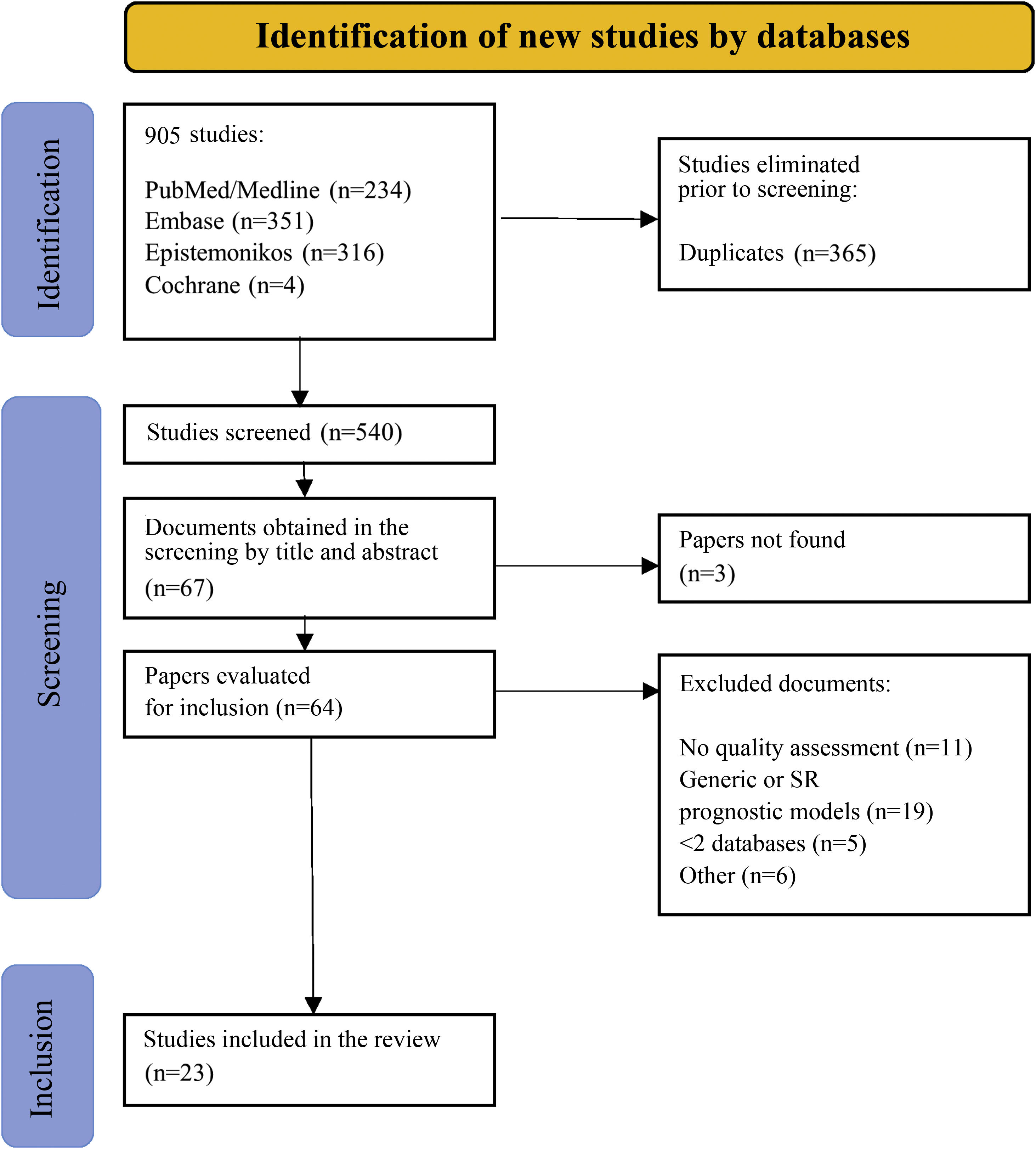
We defined prognostic factor as any measure that, among patients with a certain baseline disease, is associated with a particular outcome.11 We extended the definition of prognostic factor to the use of medications prior to surgery because they may influence the preoperative risk of developing an infection following prosthetic implantation. No limitations were established by language or by type of studies included in the SR. Studies of predictive models or prognostic scales and SRs that included more than one factor (general prognostic SRs) were excluded given that the inclusion of all types of prognostic studies would require a bibliography that would be impossible to analyze in a single study. The results were initially imported into Mendeley to eliminate redundancies. Duplicate articles were imported into the Rayyan program (intelligent systematic review) to finalize the selection process.25 Two independent investigators (MSM and AM) reviewed the titles and abstracts and, consequently, the full texts based on the inclusion and exclusion criteria. Discrepancies were resolved by consensus. Fig. 1 illustrates the study selection process.
Two investigators (MSM and JCMP) extracted data by duplicate. Discrepancies were resolved by consensus. We collected the following data: first author, year of publication, search period, number of studies included and their identification, design of the studies included, population characteristics, prognostic factor studied, prosthetic infection outcomes (RR, OR, HR), meta-analysis (MA) and subgroup or sensitivity analyses, heterogeneity index (I2) of the studies included in the MA, reporting guidelines, and funding. We also compiled information to assess risk of bias and methodological quality.
Data extractionTwo independent researchers (MSM and JCMP) assessed the risk of bias in SR using the risk of bias in systematic reviews (ROBIS) tool.22 In a first phase, this scale assesses 4 domains of potential conflict in the SR of prognostic studies: the eligibility criteria of the studies included in the SR; the identification and selection of the studies; data extraction, and synthesis of the results. In a second phase, it assesses the overall risk of bias, taking into account how potential conflicts in the domains have been addressed. Discrepancies were resolved by consensus.
Synthesis of informationWe produced a narrative description of the data extracted, classifying prognostic factors as modifiable or non-modifiable, and individual (patient-dependent) or procedural (clinician-dependent).
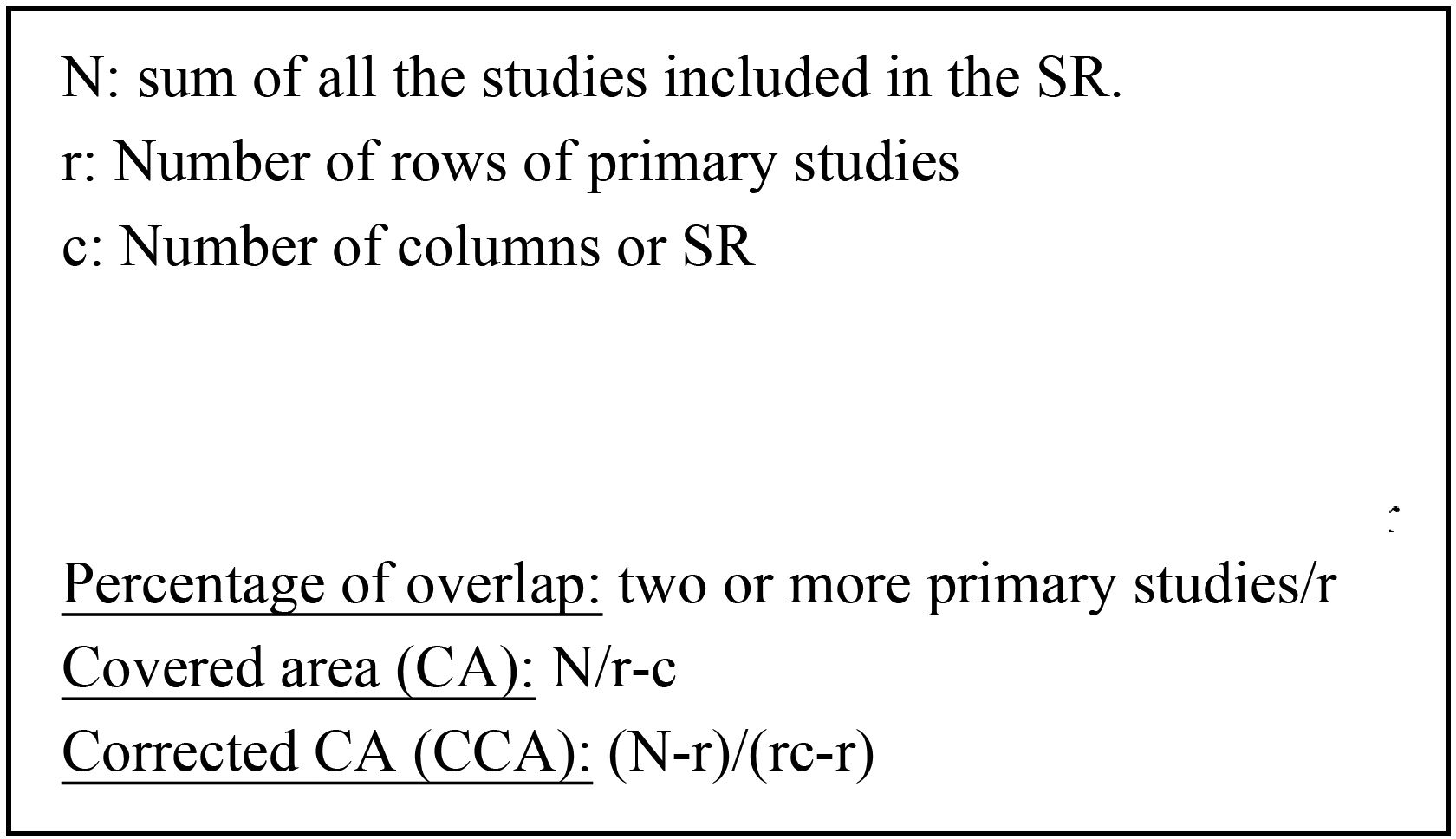
We analyzed the data extracted to construct a map of evidence of overlap, using cross-tabulations of the SRs included and the individual studies to determine the degree of overlap across the SRs included.26 We calculated the percentage of overlap and the corrected covered area (CCA) of the SRs included (Fig. 2), only for those prognostic factors studied in at least two SRs. We defined mild overlap if the CCA ranged between 0 and 5%, moderate between 6 and 10%, high between 11 and 15% and very high when it exceeded 15%.
Methodological qualityThe AMSTAR-2 tool is used to assess the methodological quality of SRs of randomized and non-randomized studies of health interventions. This tool consists of 16 assessment domains, seven of which are critical and nine of which are non-critical.
The critical domains are as follows: 2, protocol registration prior to commencing the SR; 4, adequacy of the literature search; 7, justification for exclusion of primary studies; 9, risk of bias of primary studies; 11, appropriateness of meta-analysis methods; 13, consideration of the risk of bias when interpreting the results, and 15, assessment of the presence and likely impact of publication bias.
As for the non-critical domains, we find the following: 1, the question includes the PICO components; 3, the reasons for the study design selected for inclusion in the SR are explained; 5, screening in duplicate; 6, data extraction in duplicate; 8, explanation of the included studies in adequate detail; 10, report of the funding of each primary study; 12, assessment of the risk of bias on the results of the meta-analysis; 14, satisfactory explanation of the possible heterogeneity between primary studies, and 16, report of conflicts of interest when conducting the SR. This tool is not intended to generate an overall score.21
We have no similar tool for SRs that only include prognostic studies. Consequently, we modified the AMSTAR-2 tool to explore the methodological quality of the various prognostic SRs included. Our modified version consists of 16 items with changes in two items, a critical and a non-critical one. As modifications, we added the time and types of primary studies included to item 1 in the question related to inclusion criteria (PICOT-S), whereas we introduced the assessment of bias using a tool for prognostic studies (Quality In Prognosis Studies, QUIPS27 or the Newcastle-Ottawa Scale, NOS,28 as appropriate) to item 9.
ResultsSearchWe identified 905 registries published up and until 5 May 2020 in the main search. After eliminating the duplicates, we examined 540 SRs, according to title and abstract. Of the 67 remaining studies, three were excluded because the full text could not be found, even after being in direct contact with the authors. We reviewed the full text of the remaining 64 SRs; we removed 11 because the methodological quality of the primary studies had not been evalutated, 19 because they were predictive models or general prognostic SRs, 5 because they only searched a single database, and 6 for other reasons put forth in Anexo 2. Upon completion of the bibliographic review of the SRs included, we did not identify other studies that met our eligibility criteria. In the end, we included 23 SRs (Fig. 1).29–51
Characteristics of the systematic reviewsThe 23 SRs included in this review were published betweehn 2011 and 2021; the number of primary studies included in each ST varied from 5 to 29, and the number of participants ranged between 2909 and 8,363,266. For the most part, the type of primary study included was retrospective observational, of both cohorts, as well as case–control. Six SRs included prospective cohorts together with retrospective studies and only three SRs included randomized clinical trials. Table 1 displays the general characteristics of the 23 SRs included.
General characteristics of the systematic reviews included.
| Author | Year of publication | Search period | Number of studies | Number of patients | Population characteristics | Diagnosis of infection (number and percentage with prosthetic infection) | Risk factor (number and percentage with RFs) | Report | Funding | ROBIS |
|---|---|---|---|---|---|---|---|---|---|---|
| Haverkamp et al.41 | 2011 | 1970–2010 | 15 | 10,441 patients | Primary THP with and without obesity | Septic loosening (3816; 36.5%) | Obesity (2007; 19.2%) | PRISMA | No | |
| Charalambous et al.45 | 2014 | Up to February 2014 | 8 | 2909 patients | Primary THP and TKP | Acute infection (does not separate superficial and deep) (7500; 71.8%) | Intra-articular corticosteroids (953; 32.8%) | PRISMA | No | |
| Xing et al.38 | 2014 | Up to August 2014 | 8 | 2909 patients | Primary THP and TKP | Separates superficial and deep infection (NA) | Intra-articular corticoids (953; 32.8%) | NA | No | |
| Teng et al.40 | 2015 | Up to August 2014 | 6 (4 deep infection) | 8181 patients | Primary THP | Separates superficial and deep infection (NA) | Smoking (2353; 28.8%) | MOOSE | Yes, grant | |
| Zhou et al.49 | 2015 | To May 2014 | 11 (10 quantitative synthesis) | 5433 patients (33 polymorphisms) | Prosthetic replacement in Caucasian population | Separates superficial and deep infection (2771; 33.9%) | Genetic (NA) | STROBE and STREGA | Yes, grant and foundations | |
| Ma et al.42 | 2015 | Until May 2014 | 15 | 868,017 patients | Primary THP | NA (1736; 31.9% infections) | Obesity (NA) | NA | No | |
| Meng et al.29 | 2016 | Until November 2015 | 11 | 78,308 patients | Primary THP and TKP | NA (897; 0.10%) | Intra-articular corticosteroids (26,021; 33.2%) | MOOSE | Not specified | |
| Lee et al.43 | 2016 | 1 January 1987–30 June 2015 | 13 (7 quantitative synthesis) | 203,256 (75,756 in quantitative synthesis) | Primary PTR with RA vs. primary osteoarthritis | NA (NA) | Rheumatoid arthritis (7810; 10.2%) | Cochrane Review Method | No | |
| Yang et al.33 | 2017 | 1950 up to October 2017 | 6 | 26,901 patients | Primary THP and TKP | CDC criteria (963; 1.2%) | HbA1c and perioperative blood glucose elevation (continuous variables) | PRISMA | Not specified | |
| Shohat et al.46 | 2018 | Up to 1 June 2017 | 17 (10 in the MA) | 56,060 patients in the MA | Primary and revision THP and TKP | NA | Glycaemia (dichotomous variable) (13,876; 24.7%) | MOOSE and PRISMA | Not specified | |
| Bedard et al.31 | 2018 | Up to January 2018 | 14 (13 on prosthetic infection) | 227,289 surgeries | Primary THP and TKP | Prosthetic infection (532; 2.0%) | Smoking (49,538 active and ex-smokers; 21.8%) | NA | Not specified | |
| Xu et al.50 | 2018 | January 1990–December 2017 | 16 | 108,323 patients | Primary THP and TKP | Separates superficial and deep infection or surgical wound problems (827; 1.5%) | Simultaneous bilateral prosthesis (36,765; 33.9%) | Cochrane Book | No | |
| Kunutsor et al.37 | 2019 | Up to 24 April 2019 | 22 | 2,269,428 surgeries | THP | Separates superficial and deep infection (NA) | Cement fixation (1,308,868; 57.7%) | PRISMA and MOOSE | Yes, grants and foundations | |
| Sousa et al.35 | 2019 | Up to 22 April 2018 | 10 | 28,588 surgeries | THP and TKP | Separates superficial and deep infection (1430; 1.3%) | Asymptomatic bacteriuria (1997; 7.0%) | PRISMA | Not specified | |
| Tsantes et al.34 | 2019 | Up to 31 December 2018 | 8 (6 prosthetic infection) | 257,258 patients | THP and primary and revision TKP | Deep infection or revision due to infection (11,463; 0.5%) | Malnutrition (NA) | Cochrane Book | No | |
| Onggo et al.47 | 2019 | Up to 5 May 2019 | 67 (25 deep infection) | 2,190,824 patients | Primary THP | NA (239; 0.8%) | Obesity (581,012; 26.5%) | PRISMA | No | |
| Bojan et al.30 | 2020 | Up to July 2019 | 10 (7 deep infection) | 20,640 patients | Primary THP | Revision; CDC; MSIS | Tobacco (5328; 25.8%) | MOOSE | Yes, grant | |
| Wang et al.32 | 2020 | Up to 11 June 2019 | 5 | 21,819 surgeries | Primary THP and TKP, and hemiarthroplasties | Separates superficial from deep infection | ITU (1144; 5.2%) | PRISMA | No | |
| Liu et al.44 | 2020 | Up to October 2019 | 9 (7 deep infection) | 6,783,990 patients | TKP | Prosthetic infection (456; 0.2%) | Post-traumatic osteoarthritis (74,896; 1.11%) | PRISMA | No | |
| Xu et al.36 | 2020 | Up to September 2019 | 29 | 3,204,887 patients | THP, TKP, THR, PTH and reverse humeral prosthesis | Separates superficial from deep infection (206,586; 9.4%) | BMI (NA) | PRISMA | Yes, foundations | |
| Cheng et al.48 | 2021 | Up to May 2020 | 15 (10 infections, 8 deep infection) | 8,918,380 patients | Primary THP and TKP | Separates superficial and deep infection (NA) | VHC (67,110; 0.7%) | PRISMA | Yes, grant | |
| Feeley et al.51 | 2021 | Up to January 2021 | 29 | 117,358 patients (17,643 THP; 96,595 TKP; 27 spinal material; 3083 prosthetic revisions) | Primary THP and TKP, prosthetic revisions | NA | Perioperative intravenous corticosteroids (8091; 7.0%) | (AMSTAR) | Not specified | |
| Chen et al.39 | 2021 | January 1990–31 January 2020 | 10 (3 prosthetic infection) | NA | Primary THP and TKP | (237; 1.1%) | Preoperative opioids (NA) | PRISMA and MOOSE | Yes, grant |
BMI: body mass index; CDC: control disease center; HbA1c: glycosylated hemoglobin; HCV: hepatitis C virus; MOOSE: meta-analysis of observational studies in epidemiology; MSIS: musculoskeletal infection society; NA: not available; PRISMA: preferred reporting items for systematic reviews and meta-analyses; publ.: publication; SR: systemic review; STREGA: strengthening in the reporting of genetic association studies; STROBE: strengthening in the reporting of observational studies in epidemiology; TAP: total ankle prosthesis; THP: total hip prosthesis; THR: total humeral replacement; TKP: total knee prosthesis; UTI: urinary tract infection.
In the SRs included, the quality and ris of bias of the primary studies were assessed using the following tools: the Cochrane Collaborative Back Review Group (CCBG) tool,52 the Newcastle-Ottawa Scale (NOS) in non-randomized studies in zx MA,22 the Methodological Index for Non-Randomized Studies (MINORS)53 and the Downs and Black Scale.54 None of the SRs used a specific scale for prognostic studies.27
Upon examination of the risk of bias, only 8 of the 23 SRs (34.7%) included exhibited a low risk of bias.31,34,36,37,40,42,44,46 Of the other 15, one failed to explain the details of the final bias evaluation transparently.32 The remaining 14 reviews were at high risk of bias because at least one domain revealed serious unresolved conflicts in the overall bias assessment phase.29,30,33,35,38,39,41,43,45,47–51 Ten of these 14 SRs displayed conflicts in at least 2 domains. Overall, there were 5 out of 23 SRs that presented conflicts in domain 2 of study selection and identification, 8 in domain 3 of data extraction, and 9 in domain 4 of synthesis. None of the SRs exhibited conflicts in domain 1, as all SRs clearly and properly stated the eligibility criteria of the primary studies included. Table 2 illustrates the risk of bias assessment for domains 1 through 4, with a final overall risk assessment phase.
ROBIS risk of bias rating.
| Author | Inclusion criteria | Study identification and selection | Data extraction | Synthesis | Overall rating |
|---|---|---|---|---|---|
| Haverkamp et al.41 | No conflict | Conflicts | No conflict | Conflict | |
| Charalambous et al.45 | No conflict | No conflict | Conflicts | No conflict | |
| Xing et al.38 | No conflict | No conflict | No conflict | Conflicts | |
| Teng et al.40 | No conflict | No conflict | No conflict | No conflict | |
| Zhou et al.49 | No conflict | No conflict | Conflicts | Conflicts | |
| Ma et al.42 | No conflict | No conflict | No conflict | No conflict | |
| Meng et al.29 | No conflict | Conflict | Not clear | No conflict | |
| Lee et al.43 | No conflict | No conflict | No conflict | Conflicts | |
| Yang et al.33 | No conflict | No conflict | Conflicts | Conflicts | |
| Shohat et al.46 | No conflict | No conflict | No conflict | No conflict | |
| Bedard et al.31 | No conflict | No conflict | Conflicts | No conflict | |
| Xu et al.50 | No conflict | Not clear | Conflicts | No conflict | |
| Kunutsor et al.37 | No conflict | No conflict | No conflict | No conflict | |
| Sousa et al.35 | No conflict | No conflict | Conflicts | Conflict | |
| Tsantes et al.34 | No conflict | Conflicts | No conflict | No conflict | |
| Onggo et al.47 | No conflict | Conflicts | No conflict | Conflicts | |
| Bojan et al.30 | No conflict | Conflicts | Conflicts | No conflict | |
| Wang et al.32 | No conflict | No conflict | Unclear | No conflict | |
| Liu et al.44 | No conflict | No conflict | No conflict | No conflict | |
| Xu et al.36 | No conflict | Unclear | No conflict | No conflict | |
| Cheng et al.48 | No conflict | Unclear | Conflicts | No conflict | |
| Feeley et al.51 | Unclear | Conflicts | No conflict | Conflicts | |
| Chen et al.39 | No conflict | No conflict | Conflicts | Conflicts | |
| Low risk of bias | |||||
| High risk of bias | |||||
| Unclear risk of bias |
We included a total of 15 different prognostic factors. Table 3 summarizes the odds ratio (OR) or relative risk (RR) found for each factor for prosthetic infection. The most frequent prognostic factors were obesity in four SRs,36,41,42,47 intra-articular corticosteroids in three,29,38,45 smoking in another three,30,31,40 and lack of diabetes control in two SRs.33,46 The remaining factors were assessed in only one SR each: the presence of certain genetic polymorphisms,49 rheumatoid arthritis,43 concurrent surgery,50 prosthetic fixation,37 asymptomatic bacteriuria,35 urinary tract infection (UTI),32 malnutrition,34 post-traumatic osteoarthritis,44 hepatitis C virus (HCV),48 intravenous corticosteroid administration,51 and opioid intake.39 In the study by Zhou et al.,49 33 different polymorphisms were studied for a possible association of each polymorphism with prosthetic infection. Table 4 illustrates the prognostic factors studied in each SR.
Review of odds ratios and relative risk for different risk/prognostic factors.
| Factor | Author | OR/RR | 95% CI | Heterogeneity (I2) | ROBIS |
|---|---|---|---|---|---|
| Obesity | Haverkamp et al.41 | OR 0.3 | 95% CI 0.19–0.49 | 0% | |
| Ma et al.42 | BMI>30kg/cm2 vs. BMI<30kg/cm2 (14 studies): prospective RR 2.26; retrospective RR 2.31BMI>40kg/cm2 vs. BMI<30kg/cm2 (4 studies): prospective RR 9.86; retrospective RR 7.32Overweight vs. normoweight (4 studies): prospective RR 1.34; retrospective RR 1.23Overweight vs. normoweight (6 studies): prospective RR 1.52; retrospective RR 1.46 | 95% CI 1.60–3.295% CI 1.41–3.895% CI 2.76–35.1895% CI 2.09–25.6795% CI 1.09–1.6495% CI 0.39–3.9195% CI 1.21–1.9095% CI 0.54–3.92 | BMI>30 vs. <30kg/cm2: 43.6%Otros studies: 0% | ||
| Onggo et al.47 | Deep infection obese vs. not obese (25 studies):OR 2.71Prospective OR 4.34Retrospective OR 2.40Case–control OR 4.89Deep infection morbid obesity vs. not obese (13 studies):OR 3.69Prospective OR 4.64Retrospective OR 3.66Case–control OR 5.07 | 95% CI 2.08–3.5395% CI 2.26–8.3395% CI 1.78–3.2495% CI 1.85–12.9295% CI 3.16–4.3095% CI 1.26–17.0495% CI 3.14–4.2895% CI 1.13–22.82 | 43.6%0% | ||
| Xu et al.36 | Obese vs. not obese (20 studies): OR 1.51Morbid obesity vs. no morbid obesity (14 studies): OR 3.27BMI>35kg/cm2 vs. <35 (5 studies): OR 1.64BMI>50kg/cm2 vs. <50 (2 studies): OR 1.68 | 95% CI 1.30–1.7495% CI 2.46–4.3495% CI 1.39–1.9495% CI 1.25–2.24 | 78.6%69%13.2%0% | ||
| Intra-articular corticosteroids | Charalambous et al.45 | Deep infection: RR 1.87Deep infection in THP: RR 1.59 | 95% CI 0.80–4.3595% CI 0.66–3.83 | 0% | |
| Xing et al.38 | Deep infection: OR 2.13 | 95% CI 1.02–4.45 | NA | ||
| Meng et al.29 | RR 1.43 | 95% CI 1.08–1.9 | 53.5% | ||
| Teng et al.40 | Deep infection: RR 3.71 | 95% CI 1.86–11.84 | 0% | ||
| Tobacco | Bedard et al.31 | Smokers vs. non-smokers (13 studies): OR 2.02Active smokers vs. non-smokers: OR 2.16Former smokers vs. non-smokers: OR 1.52Active smokers vs. non-smokers: OR 1.52 | 95% CI 1.47–2.7795% CI 1.57–2.9795% CI 1.16–1.9995% CI 1.07–2.14 | NA | |
| Bojan et al.30 | Overall infection in smokers vs. non-smokers (10 studies): OR 1.54Deep infection: (7 studies): OR 1.81 | 95% CI 1.25–1.9195% CI 1.39–2.36 | 39%6.5% | ||
| Yang et al.33 | Perioperative glucose (6 studies): DM 2.365Perioperative HbA1c (4 studies): DM 3.266 | 95% CI 1.802–2.92995% CI 2.858–3.674 | 0%52.6% | ||
| Poorly controlled diabetes | Shohat et al.46 | HbA1C and deep infection (10 studies): OR 1.49HbA1C 7% subgroup analyses (6 studies): OR 0.87 | 95% CI 0.94–2.3795% CI 0.57–1.32 | 81.3%54.2% | |
| Zhou et al.49 | Consult primary study | ||||
| Lee et al.43 | Deep infection: OR 2.04Septic review: OR 1.89 | 95% CI 1.37–3.0595% CI 1.34–2.66 | 22–44% | ||
| Genetics | Xu et al.50 | Simultaneous vs. separate: OR 0.57Comparable demographic groups: OR 0.55 | 95% CI 0.49–0.6695% CI 0.21–1.40 | 0% | |
| Rheumatoid arthritis | Kunutsor et al.37 | All cemented vs. not cemented: RR 1.10Subgroup analysis, 6-month follow-up: RR 0.75Cement without antibiotic vs. not cemented: RR 1.50Cement with antibiotic vs. not cemented: RR 1.07Cement without antibiotics vs. with antibiotic: RR 1.52 | 95% CI 1.04–1.1795% CI 0.63–0.8995% CI 1.27–1.7795% CI 0.97–1.1895% CI 1.36–1.70 | 39–53% | |
| Simultaneous bilateral prosthesis | Sousa et al.35 | Association of asymptomatic bacteriuria and prosthetic infection: OR 3.64Association of infection between treated and not treated with antibiotics: OR 0.98 | 95% CI 1.40–9.4295% CI 0.39–2.44 | 0–73% | |
| Fixation | Tsantes et al.34 | Prosthetic infection: OR 3.62TKP (2 studies): OR 2.55PTH (1 study): OR 3.10 | 95% CI 2.33–5.64 | 75% | |
| Asymptomatic bacteriuria | Wang et al.32 | RR 3.17 | 95% CI 2.19–4.59 | 0% | |
| Malnutrition | Liu et al.44 | OR 1.98 | 95% CI 1.52–2.57 | 58% | |
| UTI | Cheng et al.48 | Prosthetic infection: OR 2.72PTR (4 studies) OR 2.06PTC (5 studies) OR 1.71 | 95% CI 1.50–4.9395% CI 1.90–2.2495% CI 1.50–1.94 | 0–98% | |
| Post-traumatic arthrosis | Feeley et al.51 | Infection with single low dose (7 studies): OR 0.00Infection with single high dose (6 studies): OR 0.00Infection with multiple low dose (13 studies): OR 0.00PTR infection any dose (14 studies): OR 0.01PTC infection any dose (7 studies): OR 0.01 | 95% CI 0–095% CI 0–095% CI 0.02–0.0295% CI 0.04–0.0895% CI 0.03–0.02 | 0% | |
| HCV | Chen et al.39 | Deep infection (3 studies): OR 1.36 | 95% CI 1.08–1.71 | 78% | |
BMI: body mass index; CI: confidence interval; DM: difference of means; NA: not available; OR: odds ratio; RR: relative risk; SR: systematic review; THP: total hip prosthesis; THR: total humeral replacement; TKP: total knee prosthesis; HCV: hepatitis C virus; UTI: urinary tract infection.
Overlap across systematic reviews and risk/prognostic factors.
| Systematic review | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| [41] | [45] | [38] | [40] | [41] | [42] | [29] | [43] | [33] | [46] | [31] | [50] | [37] | [35] | [34] | [47] | [30] | [32] | [44] | [36] | [48] | [51] | [39] | |
| Risk/prognostic factor | |||||||||||||||||||||||
| Obesity/BMI | |||||||||||||||||||||||
| Intra-articular corticosteroids | |||||||||||||||||||||||
| Tobacco | |||||||||||||||||||||||
| Genetics | |||||||||||||||||||||||
| Rheumatoid arthritis | |||||||||||||||||||||||
| Glycaemia/Hb1A | |||||||||||||||||||||||
| Simultaneous surgery | |||||||||||||||||||||||
| Fixation | |||||||||||||||||||||||
| Asymptomatic bacteriuria | |||||||||||||||||||||||
| Malnutrition | |||||||||||||||||||||||
| UTI | |||||||||||||||||||||||
| Post-traumatic osteoarthritis | |||||||||||||||||||||||
| HCV | |||||||||||||||||||||||
| Perioperative intraveous corticosteroids | |||||||||||||||||||||||
| Opioates | |||||||||||||||||||||||
BMI: body mass index; HCV: hepatitis C virus; UTI: urinary tract infection.
Degree of overlap in obesity: [% overlap: 16/60=26.6%; AC: 84/60×4=0.35; ACC: (84−60)/(60×4−60)=0.133=13.3%].
Degree of overlap in intra-articular corticosteroids: [% overlap: 8/12=66.6%; AC: 27/12×3=0.75; ACC: (27−12)/(12×3−12)=0.625=62.5%].
Degree of overlap in tobacco: [% overlap: 4/18=22.2%; AC: 25/18×3=0.462; ACC: (25−18)/(18×3−18)=0.194=19.4%].
Degree of overlap in poorly controlled glycaemias: [% overlap: 4/19=21.05%; AC: 23/19×2=0.605; ACC: (23−19)/(19×2−19)=0.2105=21%].
As for the degree of overlap across reviews, the CCA for obesity, intra-articular corticosteroids, smoking, and uncontrolled diabetes were 13.3%, 62.5%, 19.4%, and 21%, respectively (Table 4; see Anexos 3, 4, 5, and 6, respectively), all with a high or very high degree of overlap. In the reviews evaluating intra-articular corticosteroid use, the overall CCA was 62.5%, although two of the three reviews displayed a total overlap (100% CCA).
We classified the 15 prognostic factors into modifiable or non-modifiable and individual or procedural. Modifiable factors were obesity, smoking, asymptomatic bacteriuria, UTI, malnutrition, intra-articular and intravenous corticosteroid injection, opioid intake, fixation type, and concurrent surgery. Non-modifiable factors were post-traumatic osteoarthritis and genetics or certain polymorphisms. There is another group of non-modifiable but controllable factors, such as uncontrolled diabetes, rheumatoid arthritis, or HCV. Factors characterized as individual included obesity, smoking, uncontrolled diabetes, asymptomatic bacteriuria, UTI, malnutrition, post-traumatic osteoarthritis, genetics, rheumatoid arthritis, or HCV. Procedural factors were intra-articular and intravenous steroid injection, opioid intake, fixation type, and concurrent surgery.
The evidence and results of association between each prognostic factor and prosthetic infection are summarized narratively throughout the SR:
- -
Obesity: The four SRs that evaluated obesity demonstrated an increased risk of prosthetic infection associated with obesity. Ma et al.42 found that this association persisted when categorized by body mass index (BMI) and study design (prospective/retrospective), and when comparing overweight and normal weight patients.
- -
Intra-articular use of corticosteroids: the three SRs reported slightly discrepant results, albeit all of them pointing toward a factor-related increased risk of prosthetic infection, with statistical significance varying across SRs. Charalambous et al.45 reported inconclusive results.
- -
Smoking: the three SRs included concluded that active smokers are at increased risk for developing a deep infection following primary joint replacement. Bedard et al.31 also evidenced an increased risk among ex-smokers relative to patients who had never smoked before. They also found that the risk of infection decreases in smokers with cessation of smoking.
- -
Uncontrolled diabetes: in the two SRs included, we observe an increased risk of prosthetic infection associated with elevated blood glucose or HbA1c values. The increased risk correlated with elevated perioperative blood glucose, but not with preoperative HbA1c. Heterogeneity across the studies included was high at 81%.46
- -
Other factors that were investigated: rheumatoid arthritis, asymptomatic bacteriuria, malnutrition, UTI, post-traumatic osteoarthritis, HCV, and preoperative opioid use were associated with poor prognosis in prosthetic patients. Six genetic polymorphisms out of 33 studied in the SR by Zhou et al.49 correlated with a higher risk of prosthetic infection, while others acted as protective factors, or the causal relationship could not be ascertained. Cementing of the joint prosthesis has also been found to be a poor prognostic factor for prosthetic infection; however, in the subgroup analysis, this relationship disappears if cementing is performed with antibiotics.37 Finally, two of the factors, such as the use of intravenous corticosteroids during surgery and simultaneous bilateral hip or knee replacement surgery, were not significantly associated with prosthetic infection.50,51 Although simultaneous bilateral hip or knee replacement surgery was initially presented as a protective factor, when analyzed according to comparable groups, we found no such relationship.50
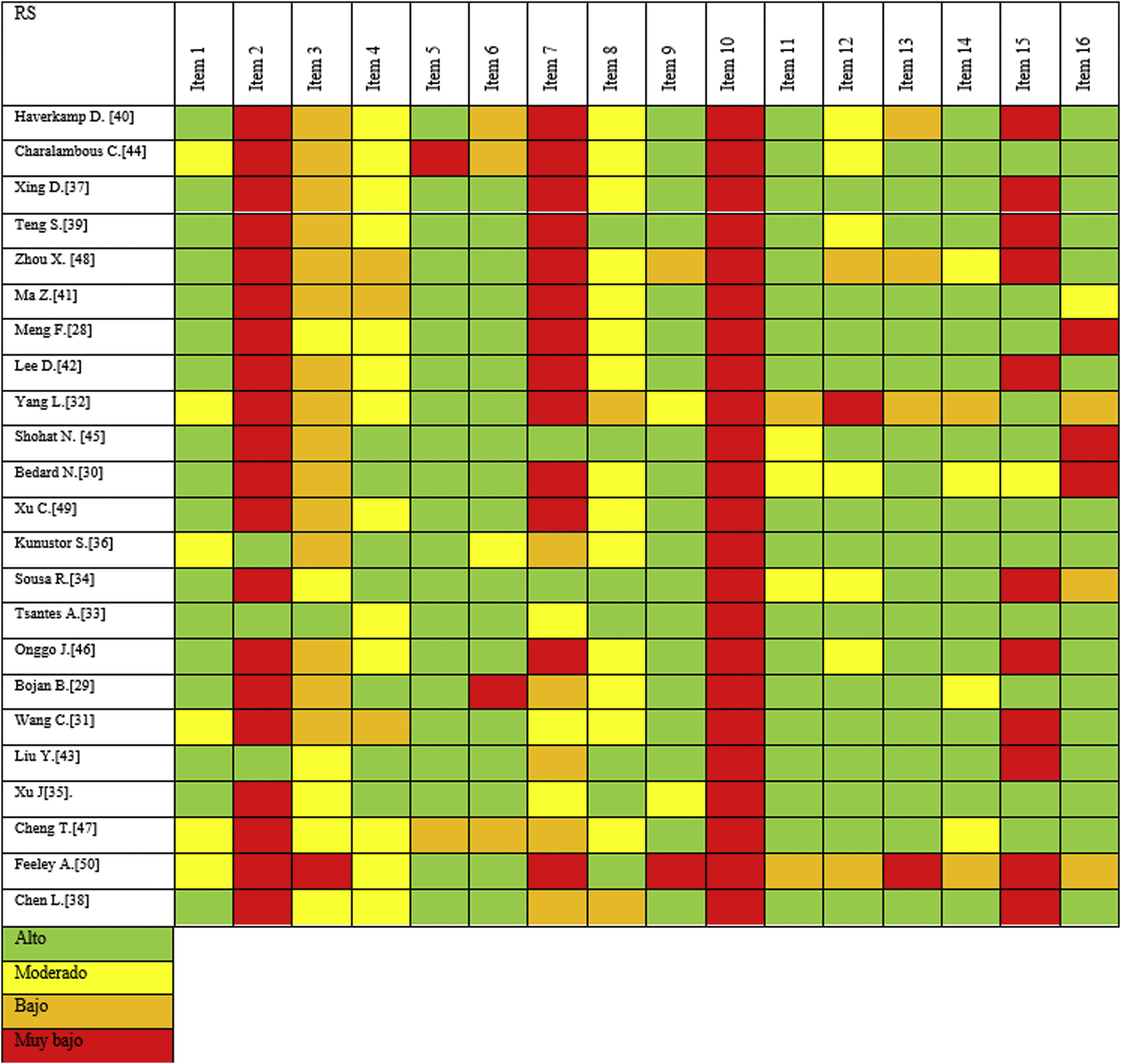
We performed the methodological evaluation using the AMSTAR-2 tool, modifying items 1 and 9. Fig. 3 depicts the revised AMSTAR-2 in the form of a graph. While in general all the SRs included exhibited important methodological gaps, the more recent SRs evidenced better methodological quality. For instance, as illustrated in item 7 regarding the reasons for excluding the primary studies, most of the studies either fail to report it or only do so very briefly; only two SRs provided detailed information concerning the reasons for exclusion of each study.31,33 The other two modified elements (i.e., items 1 and 9) were judged to be of moderate to adequate quality. Furthermore, another two items performed extremely poorly, with only three SRs reporting that a protocol had been implemented (2) and none of them reported the funding of the primary studies included (10).
Other items had low or moderate scores, such as the rationale for the design (3), the completeness with which the literature search was conducted (4), and the detail with which information from the primary studies is transcribed (8). Eleven of the 23 included SRs did not investigate publication bias appropriately, as assessed by item 15.
DiscussionTo the best of our knowledge, this SR review is the first to evaluate the prognostic factors related to prosthetic infection and examine the methodological quality of the various prognostic SRs. We identified 13 prognostic factors associated with prosthetic infection: obesity, intra-articular corticosteroid injection, smoking, uncontrolled diabetes, rheumatoid arthritis, asymptomatic bacteriuria, malnutrition, UTI, post-traumatic osteoarthritis, HCV, preoperative opioid intake, certain genetic polymorphisms, and cementation without antibiotics. No association was found between prosthetic infection and two additional factors: perioperative intravenous corticosteroid use, and simultaneous bilateral surgery.
With this narrative synthesis we were able to classify the factors into modifiable and non-modifiable, individual, or procedural. This classification allows the clinician to detect those prognostic factors that can be modified in daily practice to provide better patient outcomes, such as intra-articular corticosteroid injection.
SRs enable us to summarize the results of several primary studies in a single study and thereby integrate the information available from several articles so as to bolster research. SR reviews contribute to a better understanding of overlapping studies between SRs addressing similar questions.24 Thus, we found three SRs that looked at the use of intra-articular corticosteroids and came to disparate results. Charalambous et al.45 demonstrated that there is no association between intra-articular corticosteroid injection prior to surgery and prosthetic infection. However, Meng et al.29 and Xing et al.38 did find an association. Looking at the overlap in the primary studies used in each of these three RS, we observe a very high degree of overlap (CCA=62%). It is worth noting that the two revisions that had a 100% overlap yielded contradictory results. One possible explanation is the use of different association estimators such as OR and RR: OR is more suitable for case–control studies, as in our case, and RR for cohort studies.55
The SRs included that studied obesity, smoking, or uncontrolled diabetes also displayed a very high or high degree of overlap, with a CCA of 13.3%, 19.4%, and 21%, respectively. In all of them, the results indicated that these factors correlate with prosthetic infection and are regarded as prognostic factors.
As in other primary studies, secondary research using SRs are supported by a series of guidelines and manuals to ensure proper reporting, avoid bias, and, thereby, improve methodological quality. Prognostic SRs have specific features compared to interventional or diagnostic SRs. When initiating a prognostic study, the research question must contemplate the study duration and design. Consequently, the questions are formulated according to the acronym PICOT-S (population, intervention or comparison, outcome, time, and study design).19 Other important points to ensure that the prognostic SR is adequately conducted are still under development, such as a specific repertoire for the search of primary studies or more sensitive search strategies.11 Furthermore, there is a higher risk of publication bias in this type of SR versus interventional SRs, inasmuch as observational studies are not prospectively registered in the same way as randomized trials. As for specific manuals, they only exist for interventional SRs. Several references have been published to aid in the elaboration of a prognostic SR, but a comprehensive guide has yet to be established.19 The assessment of risk of bias in prognostic SRs can be performed using the ROBIS tool, which is a specific tool for the evaluation of bias in all types of SRs (interventional, diagnostic, aetiological, or prognostic).22
In general, the risk of bias in a prognostic SR in the field of orthopedics is high. In the SRs included in the present study, this bias is largely attributable to incorrect data extraction or synthesis of information, the weakest point of which are studies of publication bias. In some cases, the non-performance of these explorations is justified by the small number of primary studies included in the SR.
Another gap in prognostic research is the need for a tool to assess methodological quality. AMSTAR-2 is a specific tool used for interventional SRs that includes both randomized and non-randomized studies.21 Against this background and in order to appraise the methodological quality of our prognostic SRs, we modified the tool. The changes consisted of rectifying the research question to include the terms “study time” and “study design,” according to the acronym PICOT-S, in item 1. The other change was made in one of the critical domains: item 9 to include bias assessment scales devoted to prognostic studies (case–control or cohort). When applying this modified AMSTAR-2 tool to prognostic studies, we detected poor methodological quality. Some of the elements of this tool improved over time. This can happen because it coincides with the publication of certain papers seeking to improve quality.19 Other items, however, have not improved, such as disclosing the funding of the primary studies included in the SRs. In item 3, our group felt that the rationale for the designs of the studies included was not robust enough. Although observational studies are the best design to address prognostic evidence, randomized clinical trials can be used in certain circumstances.37 This work provides a tool that, although it has yet to be validated, allows for the methodological evaluation of prognostic SRs: through the use of this tool, development and validation studies can be initiated.
This work has certain limitations.56 First, we limited the eligibility criteria to studies that addressed a single specific prognostic factor, excluding those that explored multiple factors or predictive models. Second, as in any secondary research, the quality of our results will depend on the quality of the SRs included and of the primary studies included; thus, given the risk of bias of most of the SRs included and the methodological limitations and possible overlaps, the conclusions of this review are limited.
Among the strengths of this work are the prospective protocol registration, rigorous assessment of risk of bias and methodological quality, and a search strategy designed by an experienced information specialist. Moreover, we have contributed to the review of AMSTAR-2 for prognostic SRs in a way that can be applied in future exploratory work.
It is important to attain better evidence regarding the factors that impact recovery from a surgical procedure, such as joint arthroplasty implantation. Therefore, the use of registries would make it possible to analyze more clearly not only the total number of arthroplasties implanted in a region, but also the various complications, such as infection, associated with such interventions. These registries are widely used in Nordic countries such as Sweden and Denmark but, while they have been discussed in various fora in Spain, they are not available.56
ConclusionsBy identifying modifiable prognostic factors, measures can be adopted in the clinical setting that contribute to improved outcomes for people undergoing joint replacement surgery. More importantly, pinpointing procedural modifiable factors can give our patients a better chance in this type of surgery. We encountered significant overlap among SRs, which indicates that our research in orthopedic surgery is redundant. Further efforts are needed to decrease the risk of bias and improve the methodological quality of this type of SR in our setting.
Level of evidenceLevel of evidence II.
FundingNone declared.
Conflict of interestsThe authors have no conflict of interests to declare.
The authors would like to thank Ivan Solà for his help for his assistance as documentalist for the bibliographic search.