The gold standard of carpal tunnel syndrome (CTS) treatment is the section of the transverse carpal ligament, the most common technique being the palmar cutaneous incision. Percutaneous techniques have been developed, although their risk/benefit ratio remains controversial.
ObjectiveTo analyse the functional outcome of patients undergoing CTS percutaneously ultrasound-guided and compare it with those of open surgery.
Material and methodProspective observational cohort study of 50 patients undergoing CTS (25 percutaneous with WALANT technique and 25 by open surgery with local anaesthesia and tourniquet). Open surgery was performed using a short palmar incision. The percutaneous technique was performed anterograde using the Kemis H3® scalpel (Newclip). A preoperative and postoperative assessment was performed at 2 weeks, 6 weeks and 3 months. Demographic data, presence of complications, grip strength and Levine test score (BCTQ) were collected.
ResultsThe sample consists of 14 men and 36 women with a mean age of 51.4 years (95% CI: 48.4–54.5). Percutaneous technique was performed anterograde using the Kemis H3® scalpel (Newclip). All patients improved from their CTS clinic without obtaining statistically significant differences in BCTQ score, nor in the presence of complications (p>0.05). Patients operated on percutaneously recovered faster grip strength at 6 weeks, but it was similar in the final review.
ConclusionsIn view of the results obtained, percutaneous ultrasound-guided surgery is a good alternative for the surgical treatment of CTS. Logically, this technique requires its learning curve and familiarisation with the ultrasound visualisation of the anatomical structures to be treated.
El gold standard del tratamiento del síndrome del túnel carpiano (STC) es la sección del ligamento transverso del carpo, cuya técnica más común es la incisión cutánea palmar. Se han desarrollados técnicas percutáneas, aunque su relación riesgo/beneficio sigue siendo controvertida.
ObjetivoAnalizar el resultado funcional de los pacientes intervenidos de STC de forma percutánea ecoguiada y compararlo con el de la cirugía abierta.
Material y métodoEstudio de cohortes observacional prospectivo de 50 pacientes intervenidos de STC (25 percutáneos con técnica Walant y 25 por cirugía abierta con anestesia local y manguito de isquemia). La cirugía abierta se efectuó mediante una incisión palmar corta. La técnica percutánea se efectuó de forma anterógrada utilizando el bisturí Kemis® H3 (Newclip). Se realizó una valoración preoperatoria y postoperatoria a las 2 semanas, 6 semanas y a los 3 meses. Se recogieron datos demográficos, presencia de complicaciones, fuerza de prensión y puntuación del test de Levine (BCTQ).
ResultadosLa muestra consta de 14 hombres y 36 mujeres con edad media de 51,4 años (IC 95%: 48,4-54,5). Todos los pacientes mejoraron de su clínica de STC sin obtener diferencias estadísticamente significativas en la puntuación BCTQ, ni en la presencia de complicaciones (p > 0,05). Los pacientes intervenidos de forma percutánea recuperaron más rápidamente la fuerza de prensión a las 6 semanas, pero fue similar en la revisión final.
ConclusionesEn vista de los resultados obtenidos, la cirugía percutánea ecoguiada es una buena alternativa para el tratamiento quirúrgico del STC. Esta técnica requiere una curva de aprendizaje y de familiarización con la visualización ecográfica de las estructuras anatómicas.
The surgical treatment of carpal tunnel syndrome involves sectioning the transverse carpal ligament (TCL). This ligament is usually sectioned through a palmar skin incision. This is a common, simple, safe, and effective procedure to treat this pathology, but it is not free of complications such as painful palmar scars, prolonged pain in the area of the incision, decreased grip strength, peri-incisional pain at the bases of the eminences (“pillar pain”), or prolonged return to former activities.1–5
Percutaneous techniques were developed in an attempt to reduce complications.1,2 As there is no open incision through the palm with these procedures, the incidence of discomfort and tenderness in the palmar area can be expected to be reduced. In addition, decreased postoperative morbidity, earlier return to former activities, and greater recovery of grip strength are also expected when using this type of technique.1,2,6 However, its risk-benefit ratio compared to that of open release remains controversial.7,8 These new limited approaches are associated with decreased visualisation of the median nerve and its terminal branches and vascular structures, which increases the risk of severe neurovascular injury during the procedure.1,2,8,9
Ultrasound has been used as a guide when performing these percutaneous techniques to minimise these risks, because ultrasound enables perfect visualisation of the median nerve, flexor tendons, and the transverse carpal ligament (TCL), as well as structures at risk, including the ulnar artery and the proximal palmar arch.1,2,8–11 Percutaneous ultrasound-guided section of the carpal tunnel is a mini-invasive technique developed over the past 20 years and has demonstrated efficacy and safety in several studies.1,2,8–12,14–22 It has also been reported that the efficacy of TCL section1,2,7–10,13–21 and postoperative complications depend on the type of instrument used for decompression.2,15,20,21
The aim of this work is to assess whether antegrade percutaneous ultrasound-guided carpal tunnel section provides, in our experience, more benefits than using a short palmar incision.
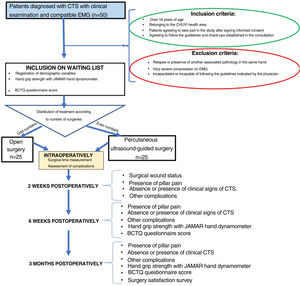
Material and methodsWe conducted a prospective observational analytical cohort study of patients in the health area of our hospital complex diagnosed with CTS by a specialist in orthopaedic and traumatology surgery at the centre, who met the inclusion and exclusion criteria.
Inclusion criteria: patients diagnosed with CTS by clinical examination and confirmed by complementary test (electromyogram [EMG]), patients over 18 years of age, patients belonging to our health area, who agreed to enter the study after signing informed consent and agreed to follow the recommendations and check-ups established in the consultation.
Exclusion criteria: patients who had previously undergone surgery for the same pathology in the same hand or with the presence of another associated pathology in the same hand, patients with very severe compression on EMG, and/or patients who were incapacitated or unable to follow the guidelines indicated by the physician.
Variables and measurement instrumentsThe main variable studied was the functional outcome of the patients measured using the validated Spanish version of the questionnaire specific to CTS (Levine test or Boston Carpal Tunnel Syndrome Questionnaire [BCTQ]23,24) preoperatively and postoperatively at six weeks and three months after surgery (Table 1). As secondary variables, the presence of complications (scar pain, peri-incisional pain or pillar pain, and other complications), the pre- and postoperative difference (six weeks and three months) in manual grip strength measured with the JAMAR manual dynamometer, and patient satisfaction with the surgery were analysed25 (Table 2). In addition, demographic variables such as age, sex, affected side, dominant hand, type of work (intense: manual work requiring weight bearing or repetitive gripping movements, moderate: manual work requiring moderate weight bearing or no repetitive gripping movements, and sedentary: patient with basic activities of daily living) and surgical time were recorded.
Validated Spanish version of the Boston Carpal Tunnel Syndrome Questionnaire.25
| A. Escala de severidad de los síntomas | |
| A. ¿Cómo es de grave su dolor en la muñeca o la mano por la noche? | 1. No tengo dolor en la muñeca o la mano |
| 2. Tengo un dolor leve | |
| 3. Tengo un dolor moderado | |
| 4. Tengo un dolor severo | |
| 5. Tengo un dolor muy severo | |
| B. ¿Con qué frecuencia se despierta durante una noche típica por dolor en la muñeca o en la mano (durante las dos últimas semanas)? | 1. Nunca |
| 2. Una vez | |
| 3. Dos o tres veces | |
| 4. De cuatro a cinco veces | |
| 5. Más de cinco veces | |
| C. ¿Normalmente, tiene dolor en la mano o en la muñeca durante el día? | 1. No |
| 2. Tengo un dolor leve | |
| 3. Tengo un dolor moderado | |
| 4. Tengo un dolor severo | |
| 5. Tengo un dolor muy severo | |
| D. ¿Con qué frecuencia usted tiene dolor en la muñeca o la mano durante el día? | 1. Nunca |
| 2. Una o dos veces | |
| 3. De tres a cinco veces | |
| 4. Más de cinco veces | |
| 5. El dolor es constante | |
| E. ¿Cuánto tiempo, aproximadamente, le duró su último episodio de dolor durante el día? | 1. Nunca tengo dolor durante el día |
| 2. 10min o menos | |
| 3. 10–60min | |
| 4. Más de 60min | |
| 5. El dolor es constante durante todo el día | |
| F. ¿Tiene adormecimiento (pérdida de sensibilidad) en la mano? | 1. No |
| 2. Tengo entumecimiento leve | |
| 3. Tengo entumecimiento moderado | |
| 4. Tengo entumecimiento severo | |
| 5. Tengo entumecimiento muy grave | |
| G. ¿Tiene debilidad en la mano o en la muñeca? | 1. No |
| 2. Tengo debilidad leve | |
| 3. Tengo debilidad moderada | |
| 4. Tengo debilidad severa | |
| 5. Tengo debilidad muy grave | |
| H. ¿Tiene sensación de hormigueo en la mano? | 1. No |
| 2. Tengo hormigueo leve | |
| 3. Tengo hormigueo moderado | |
| 4. Tengo hormigueo severo | |
| 5. Tengo hormigueo muy grave | |
| I. ¿Cómo es de grave el adormecimiento (pérdida de sensibilidad) o sensación de hormigueo en la noche? | 1. No tengo entumecimiento u hormigueo |
| 2. Tengo entumecimiento u hormigueo leve | |
| 3. Tengo entumecimiento u hormigueo moderado | |
| 4. Tengo entumecimiento u hormigueo severo | |
| 5. Tengo entumecimiento u hormigueo muy grave | |
| J. ¿Cuántas veces se despertó durante una noche típica por entumecimiento u hormigueo mano, en las últimas dos semanas? | 1. Nunca |
| 2. Una vez | |
| 3. Dos a tres veces | |
| 4. De cuatro a cinco veces | |
| 5. Más de cinco veces | |
| K. ¿Tiene dificultad con el agarre y el uso de objetos pequeños como llaves o bolígrafos? | 1. No |
| 2. Tengo dificultad leve | |
| 3. Tengo dificultad moderada | |
| 4. Tengo dificultad grave | |
| 5. Tengo dificultad muy grave | |
| Actividad | Sin dificultad | Poca dificultad | Moderada dificultad | Importante dificultad | No puedo realizar nada por los síntomas |
|---|---|---|---|---|---|
| B. Escala de situación funcional | |||||
| A. Escribir | 1 | 2 | 3 | 4 | 5 |
| B. Abotonarse la ropa | 1 | 2 | 3 | 4 | 5 |
| C. Sostener un libro mientras lee | 1 | 2 | 3 | 4 | 5 |
| D. Agarrar un teléfono | 1 | 2 | 3 | 4 | 5 |
| E. Abrir frascos | 1 | 2 | 3 | 4 | 5 |
| F. Hacer tareas domésticas | 1 | 2 | 3 | 4 | 5 |
| G. Llevar bolsas de la compra | 1 | 2 | 3 | 4 | 5 |
| H. Bañarse y vestirse | 1 | 2 | 3 | 4 | 5 |
Patient satisfaction survey as proposed by Weiner et al.25
| 1. Overall, how successful has your operation been? |
| a. Very successful. Complete or almost complete relief |
| b. Fairly successful, a good deal of relief |
| c. Not very successful, only a little relief |
| d. Failure. No relief |
| e. Worse than before |
| 2. If you had a friend with the same trouble you had, would you recommend the operation? |
| a. Yes |
| b. No |
Based on the study published by Kim et al.,26 who studied the minimal clinically important difference (MCID) for the BCTQ,23 and accepting an alpha risk of .05 and a beta risk of less than .2 in a bilateral contrast, a minimum of 20 subjects in the first group and 20 in the second group were needed to detect a difference equal to or greater than .94 units. The common standard deviation is assumed to be 1. A 10% loss to follow-up rate was estimated.
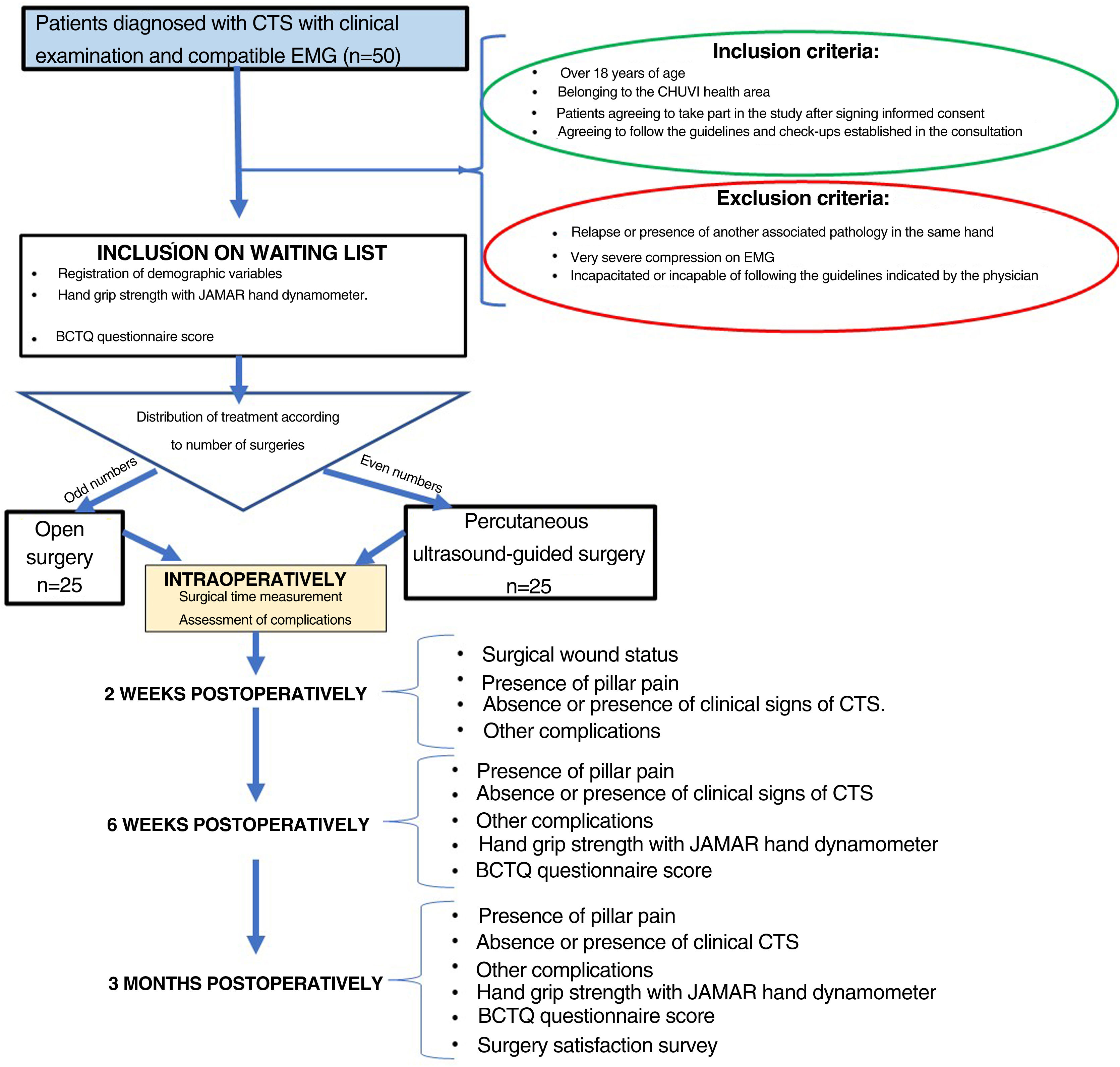
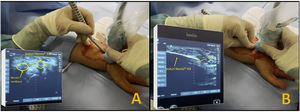
ProcedureFifty patients diagnosed with CTS and who met the described criteria were included in the study. These patients were informed of their pathology and, after signing the informed consent form, underwent surgery using the two methods proposed: 25 patients by short palmar incision and 25 patients by percutaneous ultrasound-guided surgery. All patients were operated by the same surgeon (MCM) (Fig. 1).
The type of surgery was selected randomly, with the first patient included in the study undergoing open surgery, the second patient undergoing percutaneous surgery, and so on.
The age, sex, side to be operated on, dominant hand, personal history, type of work performed (intense, moderate, or sedentary manual work), type of compression according to EMG (mild, moderate, marked, or severe), physical examination, hand grip strength measured with the JAMAR manual dynamometer and BCTQ score23,24 of the patients included in the study were recorded at the preoperative consultation. Surgical time, use of ischaemia cuff and presence of intraoperative complications were recorded at the time of surgery.
A two-week postoperative consultation was performed to assess the condition of the surgical wound, the presence of pillar pain, the absence or presence of clinical signs of CTS (paraesthesia or pain in the median nerve territory) and the presence of skin infection or other complications. Another consultation was performed six weeks and three months after surgery where, in addition to the previous assessment, hand grip strength measured with the JAMAR manual dynamometer and BCTQ score23 were recorded (Fig. 1). The questionnaire on their satisfaction with the surgery was added at the three-month review (Table 2).
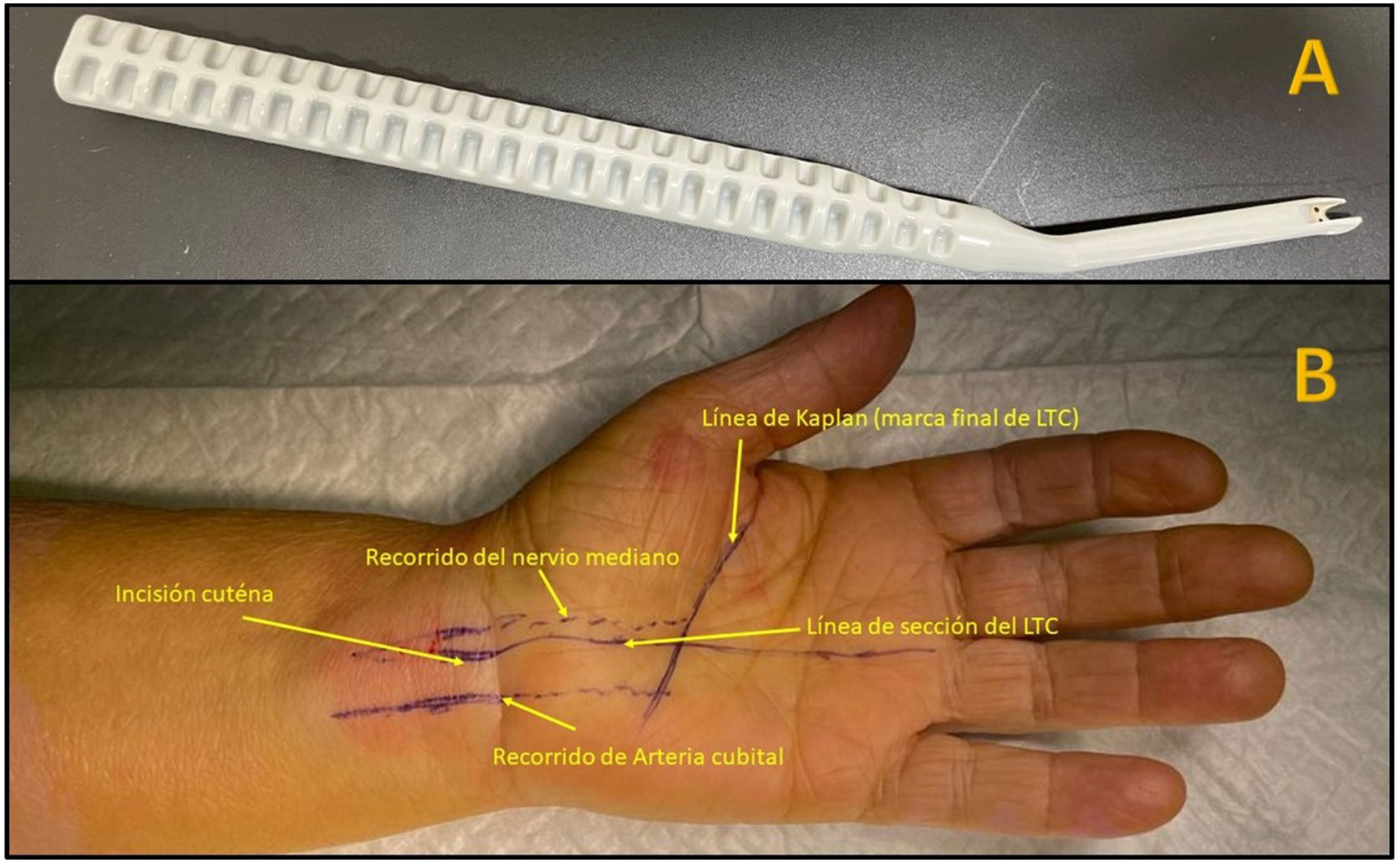
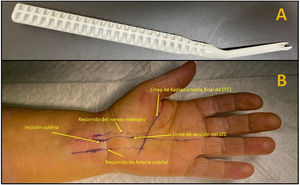
Surgical techniqueAll patients underwent surgery under local anaesthesia (8–10cc of mepivacaine 2% with 1cc of bicarbonate). A longitudinal palmar line following the radial border of the fourth radius and Kaplan's line was then drawn with a dermographic marker.
An ischaemia cuff without compression was used in the patients undergoing open surgery, inflated just before the skin incision was made. A palmar incision was made following the radial axis of the fourth radius from the proximal palmar crease to Kaplan's line. Skin closure was performed with Monocryl 3/0 and a compression bandage.
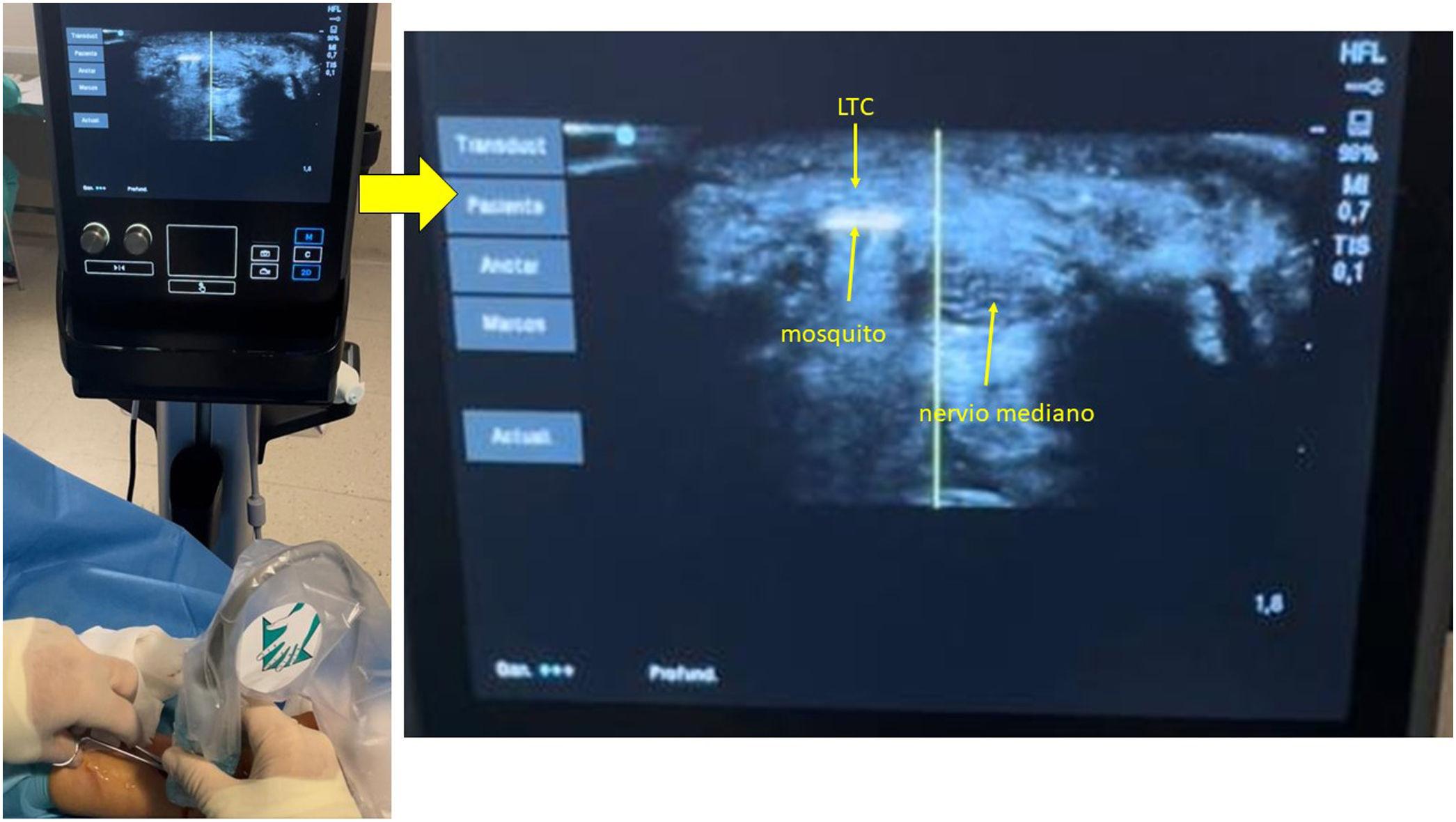
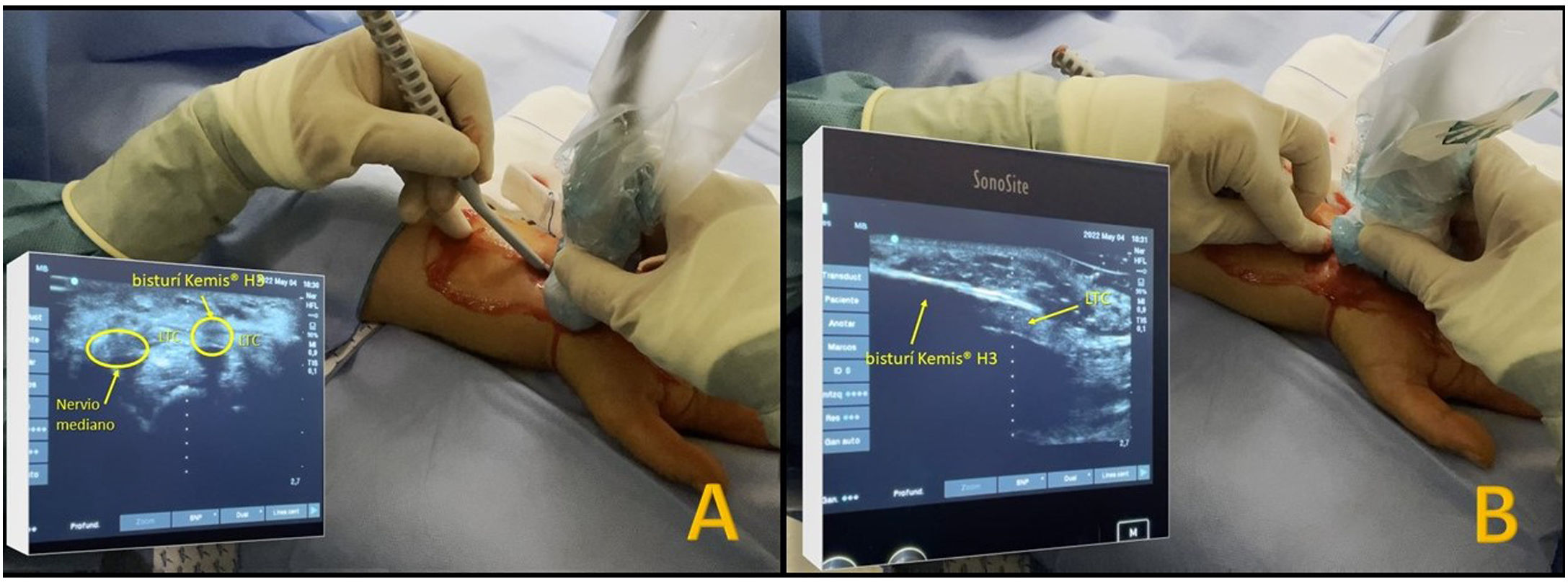
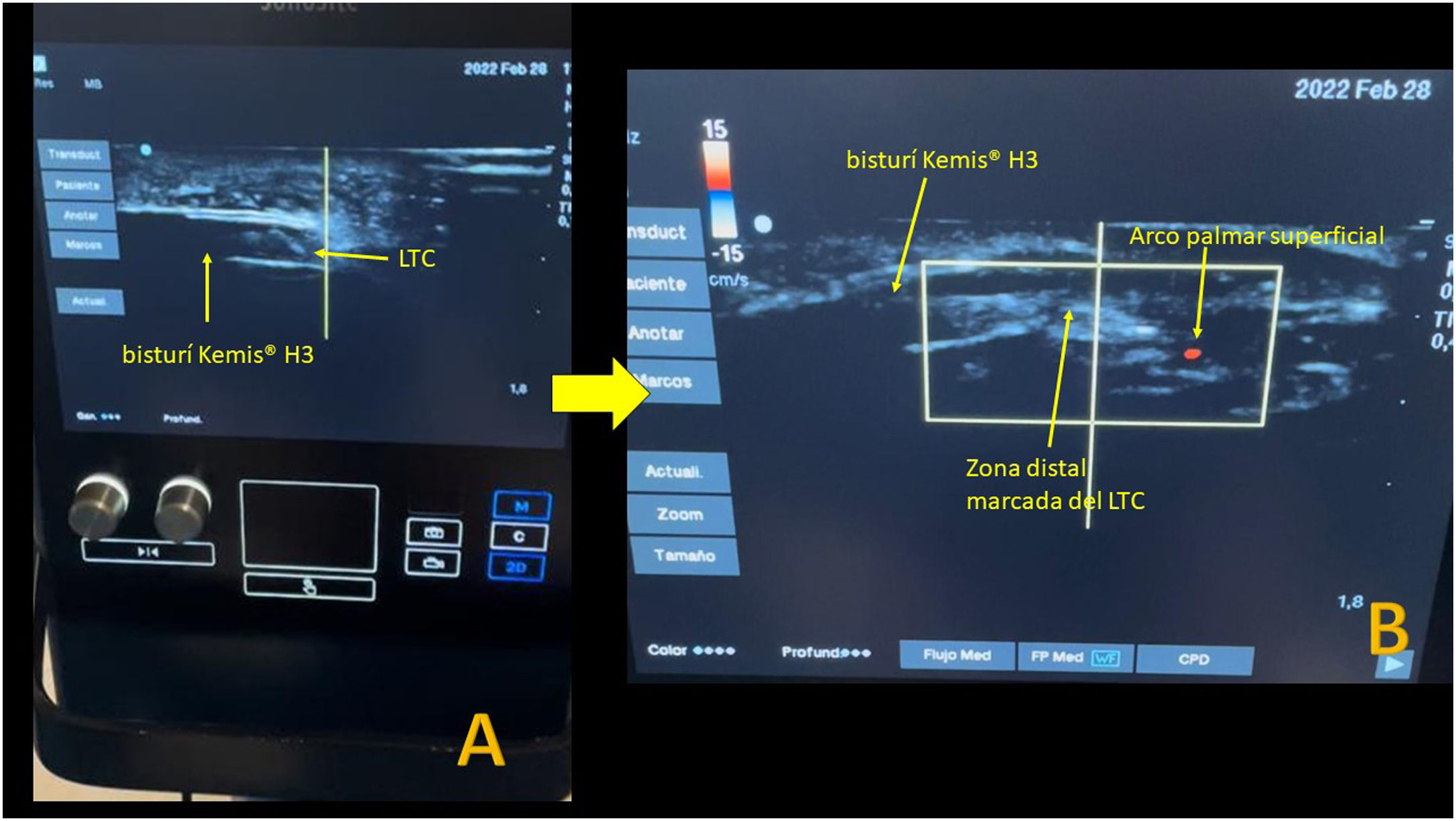
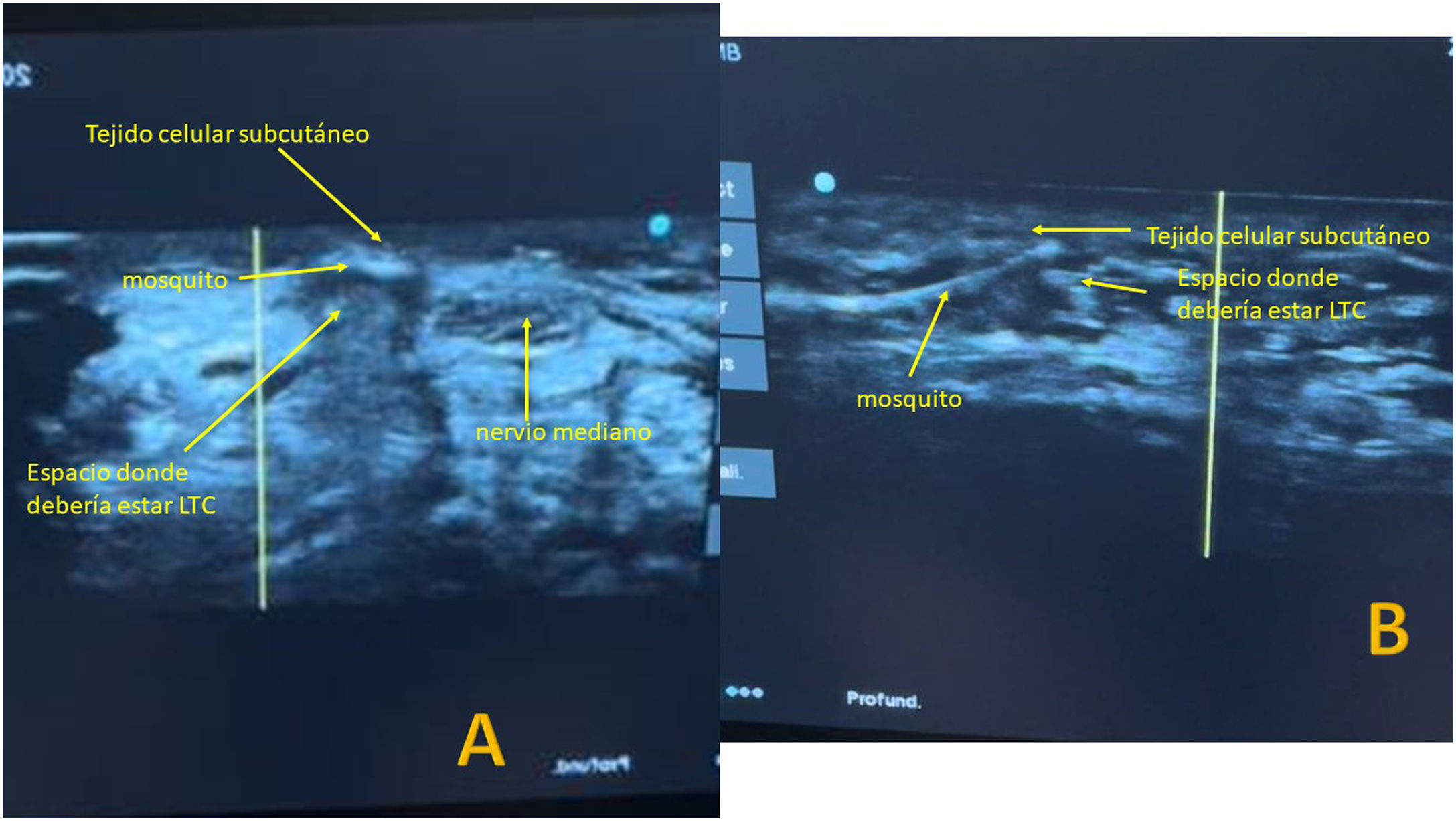
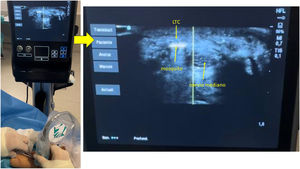
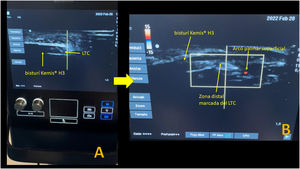
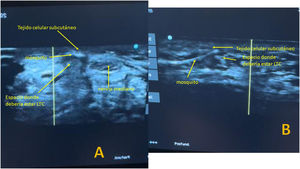
The Kemis H3® knife (Newclip) and Sonosite II ultrasound machine (Sonosite, Inc. Bothell, WA) with a 12MHZ probe were used in the patients undergoing percutaneous ultrasound-guided surgery (Fig. 2A). Walant anaesthetic technique was used (local anaesthesia without using an ischaemia cuff). A prior ultrasound study was performed to identify the median nerve, ulnar artery, and nerve, and superficial and deep palmar vascular arches. The most distal edge of the trapezium bone and the hook of hamate were also identified to determine the distal edge of the TCL.15 These landmarks are marked on the hand (Fig. 2B). An 8–10mm incision is made on the ulnar side of the palmaris longus tendon proximal to the wrist crease. An ultrasound-guided curved mosquito forceps is initially introduced above and below the TCL to aid section (Fig. 3). Then, also with ultrasound vision in the transverse plane (short axis), the Kemis H3® (Newclip) knife is introduced, having identified the median nerve and the ulnar artery, and ensuring that the two edges of the knife encompass the entire TCL (Fig. 4A). The knife is advanced, and the ultrasound machine is moved to the longitudinal view (long axis) to continue sectioning the TCL (Fig. 4B). Before reaching the marked distal area where the proximal vascular arch is located, the colour Doppler ultrasound is activated on the ultrasound machine to ensure that it is not injured (Fig. 5). After sectioning the TCL, a mosquito forceps is reintroduced under ultrasound visualisation in the short axis (Fig. 6A) and in the long axis (Fig. 6B) to check that the TCL has been completely sectioned. The skin is closed with one or two 3/0 Monocryl stitches and curettage is performed using the Mölndal technique.
We did a descriptive analysis of the variables with frequencies (percentages) and measures of central tendency (mean and 95% confidence intervals [95% CI]). We used univariate analysis to determine which variables might have an independent effect on functional outcomes (grip strength and the validated questionnaire for CTS: BCTQ24). For the comparison of quantitative variables between the two groups, to assess whether there were differences and whether these were significant, we analysed the normality of the distribution of the data in each of the cohorts and applied the parametric Student's t-test or the non-parametric test (Mann–Whitney U). We used the χ2 test to compare qualitative variables between the two cohorts. Then, including as independent variables those that showed significance in the univariate analysis, these were included in multivariate regression models.
The data were analysed with SPSS 24.0 software (IBM Corp., Armonk, NY, USA), taking .05 as the accepted α level of significance for all hypothesis tests.
Ethical aspectsThe investigators respected the fundamental principles of the Helsinki Declaration and the Council of Europe Convention on Human Rights and Biomedicine, and all current legislation related to the study. The study was authorised by the management of the health area and was approved by the network of research ethics committees (registration code: 2020/580).
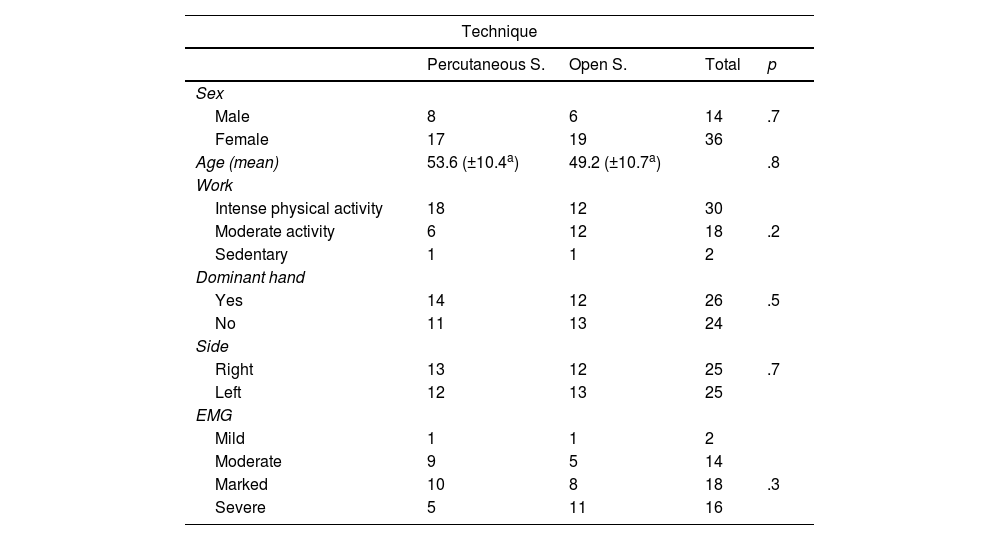
ResultsThe total number of patients analysed in our study was 50 (25 patients operated using short palmar incision and 25 using percutaneous ultrasound-guided surgery). The sample consisted of 14 men and 36 women with a mean age of 51.4 years (95% CI: 48.4–54.5). The demographic characteristics of the sample are shown in Table 3.
Descriptive data of the sample.
| Technique | ||||
|---|---|---|---|---|
| Percutaneous S. | Open S. | Total | p | |
| Sex | ||||
| Male | 8 | 6 | 14 | .7 |
| Female | 17 | 19 | 36 | |
| Age (mean) | 53.6 (±10.4a) | 49.2 (±10.7a) | .8 | |
| Work | ||||
| Intense physical activity | 18 | 12 | 30 | |
| Moderate activity | 6 | 12 | 18 | .2 |
| Sedentary | 1 | 1 | 2 | |
| Dominant hand | ||||
| Yes | 14 | 12 | 26 | .5 |
| No | 11 | 13 | 24 | |
| Side | ||||
| Right | 13 | 12 | 25 | .7 |
| Left | 12 | 13 | 25 | |
| EMG | ||||
| Mild | 1 | 1 | 2 | |
| Moderate | 9 | 5 | 14 | |
| Marked | 10 | 8 | 18 | .3 |
| Severe | 5 | 11 | 16 | |
EMG: electromyogram; S.: surgery.
The mean percutaneous surgery time was 14.4 (±5.2)min and open surgery 10 (±2.8)min, these differences being statistically significant (p=.000). No intraoperative complications were observed in any of the patients in the sample.
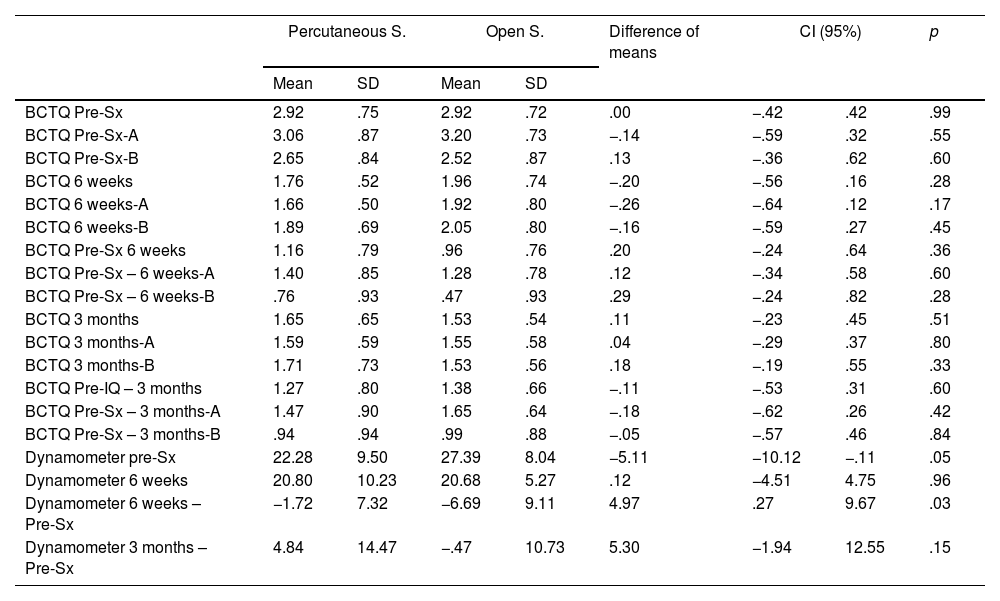
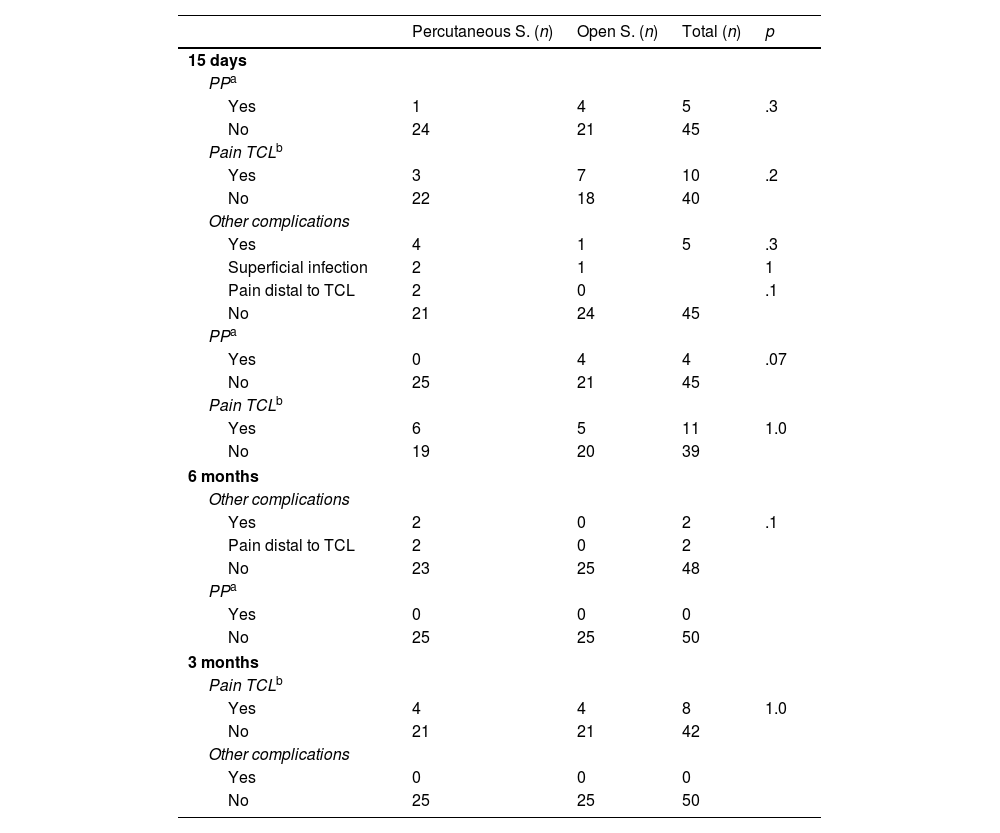
At the postoperative check-ups, CTS symptoms (paraesthesia and hand pain) had improved in all the patients, without obtaining statistically significant differences (p>.05) in the BCTQ23,24 score, both analysed as a whole and segregated by parts (symptom intensity and functional status) (Table 4). There were also no significant differences in the presence of complications (pillar pain, scar pain, or infection) (Table 5). In the first review, slight reddening of the incision was observed (two in the percutaneous surgery group and one in the open surgery group), which was considered a superficial infection of the surgical wound and treated for five days with oral antibiotics. Two patients in the percutaneous group had pain in the ulnar area distal to the TCL at the first two post-operative check-ups, but not at the three-month check-up. No statistically significant association was observed between the presence of complications and type of physical activity (p=.8), or type of compression according to the preoperative EMG (p=1).
Descriptive data of the sample.
| Percutaneous S. | Open S. | Difference of means | CI (95%) | p | ||||
|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | |||||
| BCTQ Pre-Sx | 2.92 | .75 | 2.92 | .72 | .00 | −.42 | .42 | .99 |
| BCTQ Pre-Sx-A | 3.06 | .87 | 3.20 | .73 | −.14 | −.59 | .32 | .55 |
| BCTQ Pre-Sx-B | 2.65 | .84 | 2.52 | .87 | .13 | −.36 | .62 | .60 |
| BCTQ 6 weeks | 1.76 | .52 | 1.96 | .74 | −.20 | −.56 | .16 | .28 |
| BCTQ 6 weeks-A | 1.66 | .50 | 1.92 | .80 | −.26 | −.64 | .12 | .17 |
| BCTQ 6 weeks-B | 1.89 | .69 | 2.05 | .80 | −.16 | −.59 | .27 | .45 |
| BCTQ Pre-Sx 6 weeks | 1.16 | .79 | .96 | .76 | .20 | −.24 | .64 | .36 |
| BCTQ Pre-Sx – 6 weeks-A | 1.40 | .85 | 1.28 | .78 | .12 | −.34 | .58 | .60 |
| BCTQ Pre-Sx – 6 weeks-B | .76 | .93 | .47 | .93 | .29 | −.24 | .82 | .28 |
| BCTQ 3 months | 1.65 | .65 | 1.53 | .54 | .11 | −.23 | .45 | .51 |
| BCTQ 3 months-A | 1.59 | .59 | 1.55 | .58 | .04 | −.29 | .37 | .80 |
| BCTQ 3 months-B | 1.71 | .73 | 1.53 | .56 | .18 | −.19 | .55 | .33 |
| BCTQ Pre-IQ – 3 months | 1.27 | .80 | 1.38 | .66 | −.11 | −.53 | .31 | .60 |
| BCTQ Pre-Sx – 3 months-A | 1.47 | .90 | 1.65 | .64 | −.18 | −.62 | .26 | .42 |
| BCTQ Pre-Sx – 3 months-B | .94 | .94 | .99 | .88 | −.05 | −.57 | .46 | .84 |
| Dynamometer pre-Sx | 22.28 | 9.50 | 27.39 | 8.04 | −5.11 | −10.12 | −.11 | .05 |
| Dynamometer 6 weeks | 20.80 | 10.23 | 20.68 | 5.27 | .12 | −4.51 | 4.75 | .96 |
| Dynamometer 6 weeks – Pre-Sx | −1.72 | 7.32 | −6.69 | 9.11 | 4.97 | .27 | 9.67 | .03 |
| Dynamometer 3 months – Pre-Sx | 4.84 | 14.47 | −.47 | 10.73 | 5.30 | −1.94 | 12.55 | .15 |
A: symptom severity scale; B: functional status scale; BCTQ: Boston Carpal Tunnel Syndrome Questionnaire; Pre-Sx: preoperative.
Postoperative complications divided according to surgical technique.
| Percutaneous S. (n) | Open S. (n) | Total (n) | p | |
|---|---|---|---|---|
| 15 days | ||||
| PPa | ||||
| Yes | 1 | 4 | 5 | .3 |
| No | 24 | 21 | 45 | |
| Pain TCLb | ||||
| Yes | 3 | 7 | 10 | .2 |
| No | 22 | 18 | 40 | |
| Other complications | ||||
| Yes | 4 | 1 | 5 | .3 |
| Superficial infection | 2 | 1 | 1 | |
| Pain distal to TCL | 2 | 0 | .1 | |
| No | 21 | 24 | 45 | |
| PPa | ||||
| Yes | 0 | 4 | 4 | .07 |
| No | 25 | 21 | 45 | |
| Pain TCLb | ||||
| Yes | 6 | 5 | 11 | 1.0 |
| No | 19 | 20 | 39 | |
| 6 months | ||||
| Other complications | ||||
| Yes | 2 | 0 | 2 | .1 |
| Pain distal to TCL | 2 | 0 | 2 | |
| No | 23 | 25 | 48 | |
| PPa | ||||
| Yes | 0 | 0 | 0 | |
| No | 25 | 25 | 50 | |
| 3 months | ||||
| Pain TCLb | ||||
| Yes | 4 | 4 | 8 | 1.0 |
| No | 21 | 21 | 42 | |
| Other complications | ||||
| Yes | 0 | 0 | 0 | |
| No | 25 | 25 | 50 | |
(n): number of cases.
Significant differences were found in the difference in grip strength between the preoperative measurement and that obtained at six weeks (p=.03), but not in the check-up at three months (p=.15) (Table 4).
Regarding the patient satisfaction survey at the final check-up, 19 patients in the percutaneous surgery group rated the outcome of their surgery as very successful and the rest as fairly successful. In the open surgery group 20 patients rated the surgery as very successful and 5 as fairly successful. These differences were not statistically significant between the groups (p=1); 100% of patients would recommend this type of surgery to a family member or friend. No statistically significant association was found between satisfaction with the surgery and the degree of compression according to EMG (p=.3), nor with type of physical activity (p=.7), nor with the presence of complications at 15 days and 6 weeks (pillar pain [p=.9/p=.8], pain at TCL level [p=.4/p=.2], other complications [p=.06/p=.3]).
DiscussionFrom the results obtained, percutaneous ultrasound-guided antegrade surgery with the Kemis H3® scalpel is a good alternative for the surgical treatment of CTS, achieving similar results to open surgery without intraoperative complications.
As previously demonstrated in cadaver studies,2,8,10,11,15,21,27 ultrasound allows nerve structures to be visualised and minimises the complications of percutaneous techniques. Logically, this technique requires a learning curve and familiarisation with ultrasound visualisation of the anatomical structures to be treated. In this study, the duration of surgery was statistically significantly longer for the percutaneous technique than for the conventional technique, but we must take into consideration that this study included the first 25 patients to undergo this percutaneous technique. Rojo-Manaute et al.27 in their cadaver study concluded that percutaneous ultrasound-guided surgery can be difficult for a surgeon with no previous experience in ultrasound-guided procedures and recommend developing these skills first with ultrasound-guided infiltrations. The authors of this paper used cadaver practice beforehand to familiarise themselves with the technique, in addition to routinely performing ultrasound-guided hand and wrist blocks and infiltrations.
Some authors have recorded greater improvement in disability and a faster return to normal daily activities with an ultrasound-guided carpal tunnel release technique than with a blind mini-open carpal tunnel release.16–18,27 In this study, we found no statistically significant differences in the BCTQ score between the two techniques. Faster recovery of grip strength was achieved in the percutaneous surgery group at the 6-week check-up (p=.03), however, these differences equalised at the 3-month check-up. Although these differences were not statistically significant, it should be noted that the mean grip strength of the patients undergoing percutaneous surgery was higher than preoperatively, whereas this was not the case with the open surgery group. In this study, we did not analyse when the patient returned to work, as we are not involved in an occupational setting. All patients, irrespective of the surgery used, achieved effortless mobility from the first day and resumed routine activities from the sixth week onwards.
One of the most frequent complications of percutaneous procedures is not completely severing the TCL and not fully decompressing the nerve,7,8,15 especially distally for fear of injuring the palmar vascular arch. In their cadaver study, Burnham et al.15consistently confirmed that the most distal edge of the transverse carpal ligament joins the most distal edges of the hook of hamate and trapezius, and recommend using the most distal edge of the hook of hamate and trapezius as a landmark to delineate the edge of the TCL, a landmark we use in this work to delineate the distal edge of the TCL. We obtained a clinical improvement in BCTQ score, with complete relief of pain and paraesthesia in the median nerve territory except in one patient who underwent percutaneous surgery. This patient had severe median nerve compression on preoperative EMG. Although the paraesthesia did not completely disappear, the patient's clinical pain and BCTQ score improved with no worsening of clinical or BCTQ score at the final examination.
Wu et al.19 also recommend the use of static bone landmarks for a safe approach to the TCL. In the transverse axis, they define the “safe zone” as the area between the median nerve and the hamate hook and in the longitudinal axis from 5mm proximal to the midlunate to 5mm distal to the metacarpal metadiaphyseal junction. In a cadaver study, the width of the transverse safe zone averaged 5mm (range 4–8mm), which allowed insertion of surgical instruments and supported the possibility of percutaneous surgery.10 These authors recognise that there are anatomical variations where the ulnar neurovascular bundle is located radial to the hamate hook, and therefore they recommend a prior hyperdissection to restore the safety of the safe zones. In this study, an ultrasound-guided median nerve block is performed and then 3 or 4 cc of 2% mepivacaine is introduced above the TCL.
Another factor that may make sectioning the TCL with this knife difficult is the thickness of the ligament. Studies report that the thickness of the TCL does not usually exceed 3mm.27,28 The blade width of the Kemis H3® is 3.1mm (Fig. 2A). Although it would be advisable to have a preoperative measurement of the thickness of the TCL, it was not analysed in this study, as we do not have an ultrasound scanner in the office, and we decided not to overload the radiology department. To avoid very thick TCLs, patients with very severe compression on EMG were ruled out, assuming that these patients’ TCL could have a thickness greater than the size of the knife blade. With this criterion, we did not have to convert any percutaneous surgery to open surgery. Manaute et al.27 recommend a second pass with the knife to free any volar remnants in cases where the TCL is abnormally thick. The Kemis H3® knife is designed to include the entire TCL between the ends that support the blade (Fig. 2A), and therefore we believe it would be very difficult to perform a partial TCL section, had this been the case, we would have converted the procedure to open surgery.
As previously mentioned, another risk with these techniques is injury to the superficial palmar arterial arch.10,15 According to Wu et al.,19 the safety zone described in the longitudinal axis avoids injury to the superficial palmar arch, which is normally located more than 5mm from the distal edge of the TCL. To avoid this iatrogenesis, we activate colour Doppler when we approach the marked line previously identified as the distal border of the TCL. In our series, we observed no injury to this structure.
The other major complication is median nerve injury7,8,19 due to its nerve abnormalities, as described by Lanz.29 Wu et al.19 comment that the type C1 proposed by Lanz29 may challenge the concept of safe zones. By releasing the median nerve as ulnar as possible, such potential injuries are minimised. At present, no such complications have been reported in the literature, nor did we observe them in our study. However, for this reason, it is possible that two patients presented with ulnar pain distal to the TCL in the first two check-ups, although without motor or sensory dysfunction.
Firstly, the number of patients. Although in the calculation of the sample size, 20 patients are sufficient to find an MCID in the BCTQ,23,24 we believe it is appropriate to increase the sample size to confirm or rule out whether the data obtained in the rest of the variables present statistically significant differences. We consider this study to be a preliminary study. Also, the follow-up time is limited, and it may be that clinical recurrences may be found by increasing it. However, we believe that a strength of this study is that all surgeries were performed by the same surgeon in the same health centre.
In conclusion, we can say that, in view of the results, anterograde percutaneous ultrasound-guided section of the transverse carpal ligament with the Kemis H3® knife is a good alternative for the surgical treatment of CTS. Logically, this technique requires a learning curve and familiarisation with ultrasound visualisation of the anatomical structures to be treated.
Level of evidenceLevel of evidence II.
FundingNo funding was received for this paper.
Conflict of interestsThe authors have no conflict of interests to declare.