To analyze the efficacy and safety of preventive analgesia in patients undergoing hip or knee arthroplasty due to osteoarthritis.
MethodsA systematic literature review was performed, using a defined a sensitive strategy on Medline, Embase and Cochrane Library up to May 2013. The inclusion criteria were: patients undergoing knee and/or hip arthroplasty, adults with moderate or severe pain (≥4 on a Visual Analog Scale). The intervention, the use (efficacy and safety) of pharmacological treatment (preventive) close to surgery was recorded. Oral, topical and skin patch drugs were included. Systematic reviews, meta-analysis, controlled trials and observational studies were selected.
ResultsA total of 36 articles, of moderate quality, were selected. The patients included were representative of those undergoing knee and/or hip arthroplasty in Spain. They had a mean age >50 years, higher number of women, and reporting moderate to severe pain (≥4 on a Visual Analog Scale). Post-surgical pain was mainly evaluated with a Visual Analog Scale. A wide variation was found as regards the drugs used in the preventive protocols, including acetaminophen, classic NSAID, Cox-2, opioids, corticosteroids, antidepressants, analgesics for neuropathic pain, as well as others, such as magnesium, ketamine, nimodipine or clonidine. In general, all of them decreased post-surgical pain without severe adverse events.
ConclusionsThe use or one or more pre-surgical analgesics decreases the use of post-surgical drugs, at least for short term pain.
Analizar la eficacia y la seguridad de la analgesia preventiva en pacientes que son sometidos a artroplastia de cadera o rodilla por artrosis.
MétodosSe realizó una revisión sistemática: se definió una estrategia de búsqueda bibliográfica sensible en Medline, Embase y Cochrane Library hasta mayo de 2013; se definió la población con los siguientes criterios: pacientes con indicación de artroplastia de cadera y/o rodilla, adultos, dolor moderado a intenso (≥4 en la Escala Visual Analógica), la intervención, el uso (eficacia y seguridad) del tratamiento farmacológico (preventivo) próximo a la cirugía. Se incluyeron formulaciones orales, tópicas y parches. Se incluyeron revisiones sistemáticas, metaanálisis, ensayos clínicos y estudios observacionales.
ResultadosSe incluyeron 36 artículos de calidad moderada. Incluían pacientes representativos de aquellos a los que se les indica una artroplastia de cadera o rodilla en nuestro país, adultos, con una edad media superior a 50 años, ligera mayor proporción de mujeres y que presentan dolor de moderado a grave (≥4 en la Escala Visual Analógica). El dolor posquirúrgico se evaluó sobre todo con la Escala Visual Analógica. Existe mucha variabilidad en cuanto a los fármacos utilizados incluyendo paracetamol, AINE clásicos, AINE selectivos de la Cox-2, opioides, corticoides, antidepresivos, analgésicos para el tratamiento del dolor neuropático y otros como sulfato magnésico, ketamina, nimodipino o clonidina. Todos en general parecen mejorar el dolor posquirúrgico sin presentar acontecimientos adversos graves.
ConclusionesEl uso de uno o varios analgésicos en el preoperatorio disminuye el consumo de analgésicos y el dolor en el posoperatorio, al menos el dolor agudo.
Preventive analgesia is defined as a set of pharmacological and non-pharmacological strategies that are implemented before creating a surgical wound with the goal of preventing or minimizing the pain caused by damaging surgical stimuli.1,2 The main objectives are to reduce acute pain due to tissue damage, prevent pathological modulation associated to pain on the central nervous system and inhibit the persistence of postoperative pain and the development of chronic pain. Preventive analgesia can also reduce the intake of analgesic drugs in the postoperative period.
Several experimental studies3,4 have confirmed that, at least in animals, the administration of analgesic drugs before tissue aggression is more effective to control pain than their administration subsequent to the damage.
However, these results have not been reproduced in a conclusive manner in everyday clinical practice. Several systematic reviews including the analysis of over 80 controlled clinical trials have shown that the starting time of analgesia did not affect the control of postoperative pain, regardless of the type of preventive analgesia employed.5 This conclusion is not completely categorical, since most of the existing studies are based on short-term interventions on postoperative pain, so their influence on the development of central hypersensitivity to pain6–8 cannot be reliably assessed. Moreover, there may be differences depending on the type of surgery.
The objective of the present work is to systematically review the literature to analyze the effectiveness and safety of preventive perioperative treatment using pharmacological measures in patients with an indication of hip or knee arthroplasty in relation to postoperative pain.
Materials and methodsA systematic literature review was conducted following the Cochrane Collaboration guide.9
Study selection criteriaThe studies selected included adult patients with an indication of knee and/or hip arthroplasty who suffered moderate to intense preoperative pain (≥4 in the analog visual scale). These studies should assess the use (effectiveness and safety) of a specific pharmacological treatment (preventive) soon before the intervention (not necessarily the previous 24–48h). The treatments included oral formulations (opioids, non-steroidal anti-inflammatory drugs [NSAIDs], analgesics, corticoids, anticonvulsants and antidepressants), topical (including capsaicin, topical lidocaine, topical NSAIDs and topical massage with vaseline), transdermal patches, etc. The studies should have compared the effect against active drugs, placebo or other procedures (exercise, etc.). The main indicator of the result (effectiveness) was postoperative pain, whilst secondary indicators of the result (effectiveness) included savings on opioids, days of hospital admission, quality of life, function, satisfaction, etc. Other variables analyzed included: digestive hemorrhage, constipation and cost.
Lastly, we only included studies with the following designs: metaanalyses, systematic reviews, clinical trials and observational studies.
We excluded studies conducted on animals, basic science, articles on prosthetic revisions and prostheses due to fractures, studies in which all the patients suffered a chronic inflammatory disease (rheumatoid arthritis, lupus, etc.), non-pharmacological measures, joint infiltrations (of every kind, including infiltrations of hyaluronic acid), symptomatic slow acting drugs for osteoarthritis (SYSADOAS), and articles in which the preventive treatment was based exclusively on anesthetic block.
Search strategyIn order to conduct this review, we screened through the following bibliographic databases: Medline (from the beginning until May 2013), Embase (from the beginning until May 2013), and Cochrane Library (from the beginning until May 2013). Given the volume of citations retrieved we did not search national and international congresses. Subsequently, we conducted a secondary manual search of the bibliography of the articles which were eventually included in the systematic review.
Table 1 shows the search strategies on Medline, as well as the number of citations retrieved. This search used the terms Mesh and terms in free text. As limitations, we only searched for articles which worked with human subjects and which were published in English or Spanish.
Search strategy in Medline.
| # | Search terms | Citations |
|---|---|---|
| 7 | #6 Filters: Humans; English; Spanish | 2977 |
| 6 | #4 AND #5 | 3258 |
| 5 | ((((((((((((((((«Review»[Publication Type] OR Review, Systematic OR Review, Multicase OR Review Literature OR Review, Academic OR Review of Reported Cases OR Review)) OR (((«Clinical Trial»[Publication Type]) OR «Validation Studies»[Publication Type]) OR «Evaluation Studies»[Publication Type])) OR («Clinical Trial, Phase I»[Publication Type] OR Clinical Trial, Phase 1)) OR («Clinical Trial, Phase II»[Publication Type] OR Clinical Trial, Phase 2 OR Clinical Trial, Phase II)) OR («Clinical Trial, Phase III»[Publication Type] OR Clinical Trial, Phase 3 OR Clinical Trial, Phase III)) OR («Clinical Trial, Phase IV»[Publication Type] OR Clinical Trial, Phase 4 OR Clinical Trial, Phase IV)) OR («Controlled Clinical Trial»[Publication Type])) OR («Multicenter Study»[Publication Type] OR Multicenter Studies OR Multicenter Study)) OR («Randomized Controlled Trial»[Publication Type] OR Randomized Controlled Trial)) OR («Cohort Studies»[Mesh] OR Cohort Study OR Studies, Cohort OR Study, Cohort OR Concurrent Studies OR Studies, Concurrent OR Concurrent Study OR Study, Concurrent OR Historical Cohort Studies OR Studies, Historical Cohort OR Cohort Studies, Historical OR Cohort Study, Historical OR Historical Cohort Study OR Study, Historical Cohort OR Analysis, Cohort OR Analyses, Cohort OR Cohort Analyses OR Cohort Analysis OR Closed Cohort Studies OR Cohort Studies, Closed OR Closed Cohort Study OR Cohort Study, Closed OR Study, Closed Cohort OR Studies, Closed Cohort OR Incidence Studies OR Incidence Study OR Studies, Incidence OR Study, Incidence OR Cohort Studies)) OR («Cohort Studies»[Mesh] OR cohort study OR studies, cohort OR study, cohort OR concurrent studies OR studies, concurrent OR concurrent study OR study, concurrent OR historical cohort studies OR studies, historical cohort OR cohort studies, historical OR cohort study, historical OR historical cohort study OR study, historical cohort OR analysis, cohort OR analysis, cohort OR cohort analyses OR cohort analysis OR closed cohort studies OR cohort studies, closed OR closed cohort study OR cohort study, closed OR study, closed cohort OR studies, closed cohort OR incidence studies OR incidence study OR studies, incidence OR study, incidence OR cohort studies)) OR («Longitudinal Studies»[Mesh] OR Longitudinal Study OR Studies, Longitudinal OR Study, Longitudinal OR Longitudinal Survey OR Longitudinal Surveys OR Survey, Longitudinal OR Surveys, Longitudinal OR Longitudinal Studies)) OR («Follow-Up Studies»[Mesh] OR Follow Up Studies OR Follow-Up Study OR Studies, Follow-Up OR Study, Follow-Up OR Followup Studies OR Followup Study OR Studies, Followup OR Study, Followup OR Follow-Up Studies)) OR («Prospective Studies»[Mesh] OR Prospective Study OR Studies, Prospective OR Study, Prospective OR Prospective Studies)) OR («meta-analysis»[Publication Type] OR «meta-analysis as topic»[MeSH Terms] OR «meta-analysis»[All Fields])) | 4,426,305 |
| 4 | #1 AND #2 AND #3 | 4729 |
| 3 | Risk factors[MH] OR causalities OR multifactorial causality OR causalities, multifactorial OR causality, multifactorial OR multifactorial causalities OR multiple causation OR causation, multiple OR causations, multiple OR multiple causations OR reinforcing factors OR factors, reinforcing OR factors, reinforcing OR reinforcing factor OR causation OR causations OR enabling factors OR enabling factor OR factors, enabling OR factors, enabling OR predisposing factors OR factors, predisposing OR factors, predisposing OR predisposing factor OR determinant OR predictor OR predict* OR prognostic | 1,542,865 |
| 2 | Search «pain, postoperative»[MeSH Terms] OR («pain»[All Fields] AND «postoperative»[All Fields]) OR «postoperative pain»[All Fields] OR («postoperative»[All Fields] AND «pain»[All Fields]) OR («pain, postoperative»[MeSH Terms] OR («pain»[All Fields] AND «postoperative»[All Fields]) OR «postoperative pain»[All Fields] OR («postoperative»[All Fields] AND «pains»[All Fields]) OR «postoperative pains»[All Fields]) OR «pain»[MeSH Terms] OR «pain»[All Fields] | 536,118 |
| 1 | Osteoarthritides OR Osteoarthrosis OR Osteoarthroses OR Arthritis, Degenerative OR Arthritides, Degenerative OR Coxarthrosis OR Arthroplasties, Replacement, Hip OR Arthroplasties, Replacement, Hip OR Hip Prosthesis Implantation OR Hip Replacement, Total OR Hip Replacements, Total OR Arthroplasties, Replacement, Knee OR Knee Replacement, Total OR Knee Replacements, Total | 315,207 |
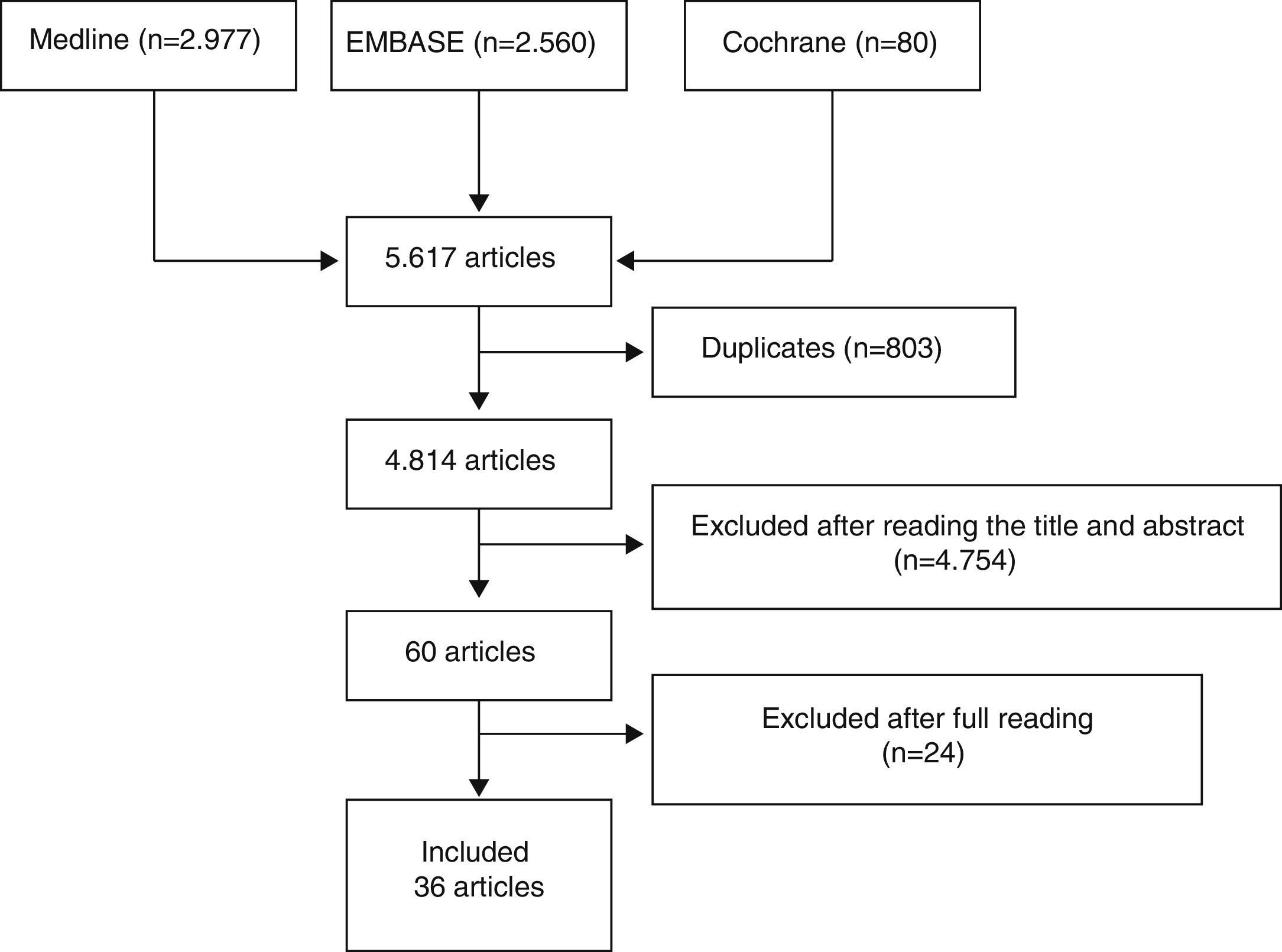
Three reviewers independently analyzed the articles retrieved with the search strategy in the different bibliographic databases to select those which fulfilled the criteria defined and analyzed the articles included in detail. Discrepancies were resolved by one of the reviewers, expert in methodology. The results of the searches were initially depurated by title and abstract or by the entire article in cases without an abstract, in sessions with a maximum duration of 20min. After this process, the articles selected were analyzed in detail (read in their entirety). Fig. 1 shows the flow diagram of the article selection process.
Lastly, we conducted a manual search of the references included in the articles selected for a detailed analysis. All references were retrieved through the Internet and entered into the EndNote software program to facilitate their management.
Data collection and quality assessment of the studiesThe three reviewers gathered data from the included studies using specific templates which were previously designed for this review. The Oxford quality scale10 was used to assess the methodological quality of the studies included.
Analysis and presentation of dataWe created evidence tables to describe the main features of the studies included. Some of the results were expressed as number and percentage (%), mean and standard deviation, median and interquartile range (p25–p75), others as odds ratio, relative risk or hazard ratio and 95% confidence intervals. We only assessed the possibility of conducting a metaanalysis in case of homogeneity.
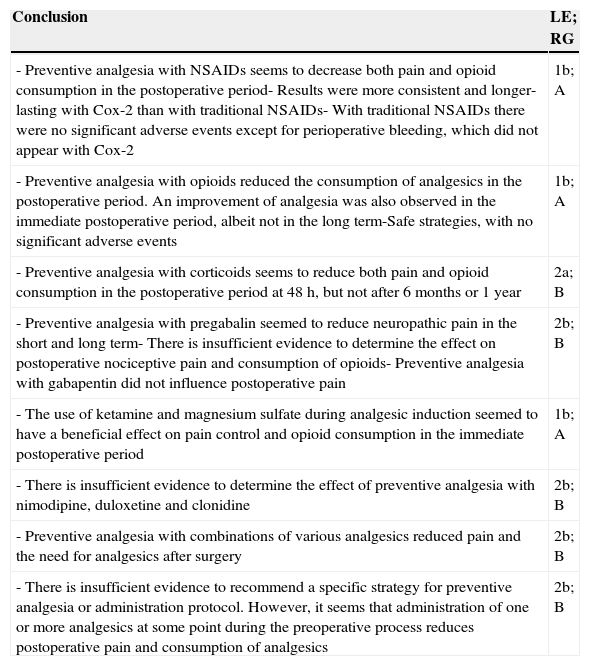
ResultsWe initially included 60 articles to be read in detail (Fig. 1). Out of these, we finally included 36 (Table 2). The articles excluded and the reasons for their exclusion are shown in Table 3, and their references in Annex 1. The main conclusions, along with their level of evidence and recommendation grade are shown in Table 4.
Evidence table.
| # | Study | Population | Intervention | Measurement of outcome | Comments |
|---|---|---|---|---|---|
| 1 | Adam 2005,42 RCT, 3 month follow-up | • n=40 TKA• Mean age 68 years• 70% females• Criteria ASA I–III | • Ketamine- >0.05ml/kg, iv in bolus following anesthetic induction- 3μgkg−1min−1 iv (continuous infusion) intraoperator- 1.5μgkg−1min−1 iv (continuous infusion) during 48h after surgery• Saline solution (same volume) | • Postoperative pain• Postoperative opioid intake• Knee flexion• Days of hospital admission• Adverse events | • Quality 1b |
| 2 | Alexander 2002,12 RCT double blind placebo control, 24h follow-up | • n=99 TKA or THA• Mean age 64 years• 63% females• Moderate basal pain | • Diclofenac 75mg iv and ketorolac 60mg iv (before anesthetic induction) in a single dose• Placebo iv | • Postoperative opioid intake• Adverse events | • Quality 1b |
| 3 | Beaupre 2012,18 observational prospective, 12 weeks follow-up | • n=39 TKA• Mean age 65 years• 50% females• Moderate basal pain | • Oxycodone 10mg oral and celecoxib 100–200mg oral (2–12h before surgery)• Anesthetic block (femoral nerve) | • Postoperative pain up to 12 weeks• Adverse events | • Quality 2b• Very small sample size |
| 4 | Bergeron 2009,36 RCT double blind, 6 weeks follow-up, evaluation 1 year later | • n=50 TKA• do not provide more information | • Dexamethasone 40mg iv in anesthetic induction• Saline solution | • Postoperative pain at 6 weeks and 1 year | • Quality 1cmultiple biases |
| 5 | Bugter 200311, RCT double blind placebo control, 16 days follow-up | • n=36 THA• Mean age 61 years• 70% females | • Ibuprofen 600mg/8h oral 2 weeks before surgery• Placebo | • Postoperative pain at 24h• Postoperative opioid intake at 24h• Perioperative bleeding | • Quality 1c• multiple biases• Very small sample size |
| 6 | Buvanendran 2003,22 RCT double blind placebo control, 8 days follow-up | • n=70 TKA• Mean age 61 years• 67% females | • Rofecoxib 50mg oral preoperative 24h before and 2 weeks after surgery• Placebo | • Postoperative pain• Postoperative intake of opioids and other related drugs during hospital admission• Adverse events | • Quality 1b |
| 7 | Buvanendran 2010,39 RCT double blind placebo control, 6 months follow-up | • n=240 TKA• Mean age 21 years• 68% females | • Pregabalin 300mg oral 24h before and 2 weeks after surgery• Placebo | • Postoperative pain until 6 months• Postoperative opioid intake• Days of hospital admission• Adverse events | • Quality 1b |
| 8 | Casey 2006,44 RCT double blind placebo control, 2 days follow-up | • n=40 TKA• Mean age 71 years• 58% females | • Nimodipine 90mg oral 1h prior to surgery and for 48h more• Placebo | • Postoperative opioid intake | • Quality 2b |
| 9 | Clarke 2009_1,40 RCT placebo control, 6 months follow-up | • n=126 TKA• Mean age 62 years• 35% females | • Gabapentin 100–600mg 2h before and 2h after surgery• Placebo | • Postoperative pain until 6 months• Postoperative opioid intake until 6 months | • Quality 1b |
| 10 | Clarke 2009_2,41 RCT placebo control, 2 days follow-up | • n=40 TKA• Mean age 61 years• 61% females | • Gabapentin 100–600mg 2h before and 2h after surgery• Placebo | • Postoperative pain at 2 days• Postoperative opioid intake at 2 days | • Quality 2b |
| 11 | Duellman 2009,19 observational retrospective | • n=127 TKA or THA• Mean age 62 years• 48% females | • Oxycodone and celecoxib or valdecoxib (from surgery, different doses and patterns) and during postoperative period | • Postoperative opioid intake• Days of hospital admission• Adverse events | • Quality 2b |
| 12 | Eggers 1999,20 RCT placebo control, 9 days follow-up | • n=101 TKA• Mean age 67 years• <50% females | • Tenoxicam 40mg oral 1h before surgery +20mg iv at 24h+20mg oral 8 days• Tenoxicam 40mg iv 1h after surgery + 20mg iv at 24h + 20mg oral 8 days• Placebo | • Postoperative pain at 48h and 3–9 days• Postoperative opioid intake at 48h and at 3–9 days• Postoperative consumption of analgesics at 48h, 3–9 days• Adverse events | • Quality 1b |
| 13 | Feng 2008,23 RCT placebo control, 3 days follow-up | • n=34 TKA• Mean age 66 years• 69% females | • Rofecoxib 25mg 1h before surgery• Placebo | • Postoperative pain at 48–72h• Postoperative opioid intake at 48–72h• Adverse events | • Quality 2b• Very small sample size |
| 14 | Fletcher 1995,13 RCT double blind placebo control, 5 days follow-up | • n=60 THA• Mean age 64 years• 60% females | • Ketorolac 60mg some hours before surgery• Placebo | • Postoperative pain• Postoperative opioid intake• Perioperative bleeding | • Quality 1b |
| 15 | Hendolin 1996,27 RCT, follow-up 24h | • n=41 TKA• Mean age 70 years• 90% females | • Morphine 0.14mg/kg intramuscular 1h before surgery | • Pain postoperative• Postoperative opioid intake• Adverse events | • Quality 2b |
| 16 | Ho 2010,45 observational prospective, 2 days follow-up | • n=50 TKA | • Duloxetine 60mg 2h before surgery and following day• Placebo | • Postoperative pain• Postoperative opioid intake• Adverse events | • Quality 1b |
| 17 | Huang 2008,15 RCT, 7 days follow-up | • n=80 TKA• Mean age 70 years• Moderate basal pain | • Celecoxib 400mg 1h prior to surgery+200mg/12h 5 days | • Postoperative pain at 48–72h• Postoperative opioid intake at 48–72h• Adverse events | • Quality 2a |
| 18 | Inan 2007,25 RCT double blind placebo control, 2 days follow-up | • n=46 TKA• Elderly• >80% females | • Lornoxicam high dose 15min before surgery and a low postoperative dose• Placebo | • Postoperative pain• Postoperative opioid intake• Adverse events | • Quality 1b |
| 19 | Iohom 2002,14 RCT placebo control, 2 days follow-up | • n=30 THA• Mean age 62 years• ASA I–II | • Dexketoprofen 25mg 24h prior to surgery and up to 48h after surgery• Placebo | • Postoperative pain at 15h• Postoperative opioid intake• Adverse events | • Quality 1b• Very small sample size |
| 20 | Ittichaikulthol 2010,16 RCT placebo control, 2 days follow-up | • n=120 THA and TKA• Age 18–75 years• ASA I–II | • Parecoxib 40mg 1h before surgery• Celecoxib 400mg preoperative• Placebo | • Postoperative pain• Postoperative opioid intake• Adverse events | • Quality 1b |
| 21 | Hwang 2009,43 observational prospective, 2 days follow-up | • n=40 ARC• Mean age <50 years• 45% females• Criteria ASA I-II | • Magnesium sulfate- 50mg/kg infusion iv 15min before surgery- 15mg/kg infusion iv during surgery• Saline solution (same volume) | • Postoperative pain a 48h• Postoperative opioid intake 48h• Global satisfaction• Adverse events | • Quality 1b |
| 22 | Kardash 2008,34 RCT double blind placebo control, 2 days follow-up | • n=50 THA, unilateral or total• Mean age 68 years• 50% females | • Dexamethasone 40mg iv, 10min before surgery• Ibuprofen 400mg/6h oral (during 48h)• Paracetamol 650mg/6h oral (during 48h)• Placebo | • Postoperative pain• Postoperative opioid intake• CRP levels• Adverse events | • Quality 1b |
| 23 | Lunn 2011,38 RCT double blind placebo control, 2 days follow-up | • n=48 TKA• Mean age 66 years• 50% females• ASA I–II | • Methylprednisolone 125mg (2ml) iv, single dose before surgery• Placebo | • Postoperative pain at 48h• Opioid savings at 48h• Adverse events | • Quality 1b |
| 24 | Lunn 2013,37 double blind placebo control, 2 days follow-up | • n=48 THA• Mean age 66 years• 56% females• ASA I–II | • Methylprednisolone 125mg (2ml) iv, single dose before surgery• Placebo | • Postoperative pain at 48h• Opioid savings at 48h• Adverse events | • Quality 1b |
| 25 | Mallory 2002,24 observational prospective, 24 days follow-up | • n=317 TKA or THA• Mean age 64 years• 50% females | • Rofecoxib or celecoxib 2 weeks before surgery and for 10 days after | • Postoperative pain• Days of hospital admission• Adverse events | • Quality 2a |
| 26 | Martinez 2007,26 RCT placebo control, 5 days follow-up | • n=78 THA• Mean age 63 years• 50% females | • Pre group:- Parecoxib 40mg iv in induction and 12h after induction- Placebo 40mg iv. During surgical wound closure• Post group:- Parecoxib 40mg iv. During wound closure and 12h after induction- Placebo 40mg iv. During induction• Control group:- Placebo 40mg iv. During induction, wound closure and 12h after induction | • Postoperative pain• Postoperative opioid intake• Perioperative bleeding• Adverse events | • Quality 1b |
| 27 | Mc Swiney 199729, RCT double blind, 24h follow-up | • n=50 TKA• No explicit data provided on basal characteristics. Authors indicate that there were no differences between groups | • Experimental group:- Morphine 0.125mg/kg (60ml saline solution) iv• Control group:- Morphine 0.125mg/kg intramuscular in opposite leg | • Postoperative pain• Postoperative opioid intake | • Quality 1b |
| 28 | Meurnier 2007,17 RCT placebo control, 1 year follow-up | • n=44 TKA• Mean age 68 years• 55% females• ASA I–II | • Celecoxib 200mg oral 1h before surgery and during 3 weeks after (2 times per day)• Placebo 200mg oral 1h before surgery and during 3 weeks after (2 times per day)• Anesthetic technique: subarachnoid spinal with 17.5 isobaric bupivacaine at 20mg | • Postoperative pain until 28 days• Perioperative bleeding• Adverse events | • Quality 1b |
| 29 | Park 1996,46 RCT double blind placebo control, 36h follow-up | • Population (n=39) TKA• Mean age 67 years• 60% females• ASA I–III | • Clonidine 5μgkg−1 oral 1.5h before surgery, 12h and 24h after initial dose• Placebo same dose oral | • Postoperative pain• Postoperative opioid intake• Adverse events | • Quality 1b |
| 30 | Porter 1983,33 RCT double blind | • n=26 THA or TKA• Mean age 63 years• 50% females | • Group I: methadone 10mg iv just after induction of anesthesia• Group P: methadone 10mg, iv. After intervention (3h after induction)• Group I: neuromuscular block pancuronium 0.1mg/kg• Group P: bupivacaine 0.5% extradural | • Postoperative opioid intake• Adverse events | • Quality 1b• Very small sample size |
| 31 | Rasmussen 2010,35 RCT double blind placebo control, 24h follow-up | • n=42 THA• Mean age 71 years• 57% females• ASA I–III• Moderate basal pain | • Experimental group:- Gabapentin 1200mg 1h before anesthesia- Dexamethasone 8mg iv before anesthetic induction- Ketamine 0.15mg/kg preoperative- Paracetamol 1g q hour before anesthesia- Ketorolac 15mg at the end of surgery• Control group:- Placebo 1200mg 1h before anesthesia- Placebo 8mg iv before anesthetic induction- Paracetamol 1g 1h before anesthesia- Ketorolac 15mg at the end of surgery | • Postoperative pain• Postoperative opioid intake• Adverse events | • Quality 1b |
| 32 | Reiter 2003,32 RCT double blind placebo control, 24h follow-up | • n=98 TKA or THA• Mean age 62 years• 60% females• ASA I–III | • Morphine 20mg oral 1h before surgery• Placebo 20mg oral 1h before surgery• Anesthesia with phentanyl 3μg/kg iv, thiopental 3–5mg/kg iv and vecuronium 0.1mg/kg iv. Maintained with isoflurane and nitrous oxide at 60% in O2 | • Pain postoperative• Postoperative opioid intake• Adverse events | • Quality 1b |
| 33 | Renner 2011,21 RCT double blind, 24h follow-up | • n=11 THA• Mean age 68 years• 83% females | • Etoricoxib 120mg oral 2h before surgery; 120mg oral 1 day after surgery• Placebo oral 2h before surgery, oral 1 day after surgery | • Inhibition of prostaglandin production• Suppression of IL-6 increase• Postoperative pain• Postoperative opioid intake• Overall satisfaction• Adverse events | • Quality 1b• Very small sample size |
| 34 | Skinner 2004,47 observational prospective | • n=102 TKA or THA• Mean age 63 years• 65% females | • Experimental group:- Preoperative (immediately before surgery): rofecoxib 50mg oral; tramadol 50mg oral; paracetamol 650mg oral; dexamethasone 2mg oral- Postoperative (hospital): rofecoxib 50mg/day oral; tramadol 50mg/6h oral; paracetamol 650mg/6h oral; hydrocodone 5mg/paracetamol 500mg oral, 1–2 tablets/4h and iv opioids on demand- Postoperative (following discharge): rofecoxib 50mg/day oral; tramadol 50mg/6h oral; hydrocodone 5mg/paracetamol 500mg oral 1–2 comp/4h- Postoperative (following heparin, 14 days): rofecoxib 50mg/day oral; tramadol 50mg/6h oral; aspirin 350mg/day- Bupivacaine 0.25% intraarticular 2ml/h during 48h (for TKA patients)• Control group: conventional therapy | • Postoperative pain• Adverse events | Quality 2b |
| 35 | Slowey 1985,30 RCT, 24h follow-up | • n=30 THA• Mean age 65 years• 60% females• ASA I–II | • Intramuscular group: morphine 15mg intramuscular 1h before surgery; placebo oral 1h before surgery (3 tablets)• Oral group-60: placebo intramuscular 1h before surgery; morphine 30mg LC oral 1h before surgery (2 tablets); placebo oral 1h before surgery (1 tablet)• Oral group-90: placebo intramuscular 1h before surgery; morphine 30mg LC oral 1h before surgery (3 tablets) | • Postoperative pain• Adverse events | • Quality 1b• Very small sample size |
| 36 | Wong 1997,31 observational prospective 3 days follow-up | • n=45 TKA• Mean age 61 years• 50% females• ASA I–II | • Group G:- 30min before surgery and upon incision: saline solution 15ml iv- 30 and 60min postoperative: saline solution 10ml• Group EA:- 30min before surgery and upon incision: lidocaine 2% 15ml iv- 30 and 60min postoperative: lidocaine 2% 10ml; morphine 1.5mg, ketamine 20mg• Group EB:-30min before surgery and upon incision: lidocaine 2% 15ml iv, morphine 1.5mg, ketamine 20mg-30 and 60min postoperative: lidocaine 2% 10ml• Anesthetic technique: general anesthesia (group G), epidural lidocaine (groups EA, EB) | • Postoperative pain• Postoperative opioid intake• Overall satisfaction• Adverse events | • Quality 1b |
h: hours; iv: intravenous; kg: kilogram; mg: milligram; min: minute; ml: milliliter; RCT: randomized clinical trial; THA: total hip arthroplasty; TKA: total knee arthroplasty.
Studies excluded after full reading and reasons for exclusion.
| # | Study | Reasons for exclusion |
|---|---|---|
| 1 | Barreveld 2013 | Systematic review including studies of hip and knee arthroplasties, as well as other types of surgery |
| 2 | Becchi 2007 | Main objective was analgesia with continuous psoas compartment block (no preventive analgesia with drug treatment) |
| 3 | Berger 2009 | No comparison group |
| 4 | Brooks 2003 | Case report describing an epidural catheter |
| 5 | Bullingham 1984 | Description of a treatment with sublingual buprenorphine initially administered perioperatively. The preemptive effect was not evaluated |
| 6 | Buvanendran 2010 | Study evaluating plasma concentrations of pregabalin in blood and in LCR. Pain was not evaluated |
| 7 | Clarke 2012 | Review of articles on the use of gabapentin and pregabalin in different pathologies not exclusively TKA and THA |
| 8 | De Oliveira 2012 | Metaanalysis on the preoperative use of ketorolac. Mixing articles from various specialties |
| 9 | Du Manoir 2003 | Use of nefopam in postoperative treatment |
| 10 | Fransen 2004 | Inclusion of revision surgeries |
| 11 | Fu 2010 | Inclusion of intraarticular infiltrations |
| 12 | Kilickan 2000 | Evolution of epidural analgesia |
| 13 | Moretti 2012 | Evaluation of a postoperative treatment |
| 14 | Hebl 2008 | Three patients in the intervention group and four patients in the control group presented a diagnosis of rheumatoid arthritis |
| 15 | Koinig 1988 | Arthroscopy |
| 16 | Notarnicola 2011 | Only anesthetic block |
| 17 | Perrin 2009 | Series of cases, pilot study with a very small sample size |
| 18 | Reuben 2002 | The author retracted |
| 19 | Reuben 2007 | The author retracted |
| 20 | Reuben 2008 | The author retracted |
| 21 | Rosenberg 2006 | Summary of several studies, insufficient data to complete data collection form |
| 22 | Schroer 2011 | Preventive treatment was the same for all patients, placebo was administered in the postoperative period |
| 23 | Southworth 2009 | Inclusion of other types of surgeries |
| 24 | Straube 2005 | Inclusion of other types of surgeries |
Main conclusions along with level of evidence and recommendation gradea
| Conclusion | LE; RG |
|---|---|
| - Preventive analgesia with NSAIDs seems to decrease both pain and opioid consumption in the postoperative period- Results were more consistent and longer-lasting with Cox-2 than with traditional NSAIDs- With traditional NSAIDs there were no significant adverse events except for perioperative bleeding, which did not appear with Cox-2 | 1b; A |
| - Preventive analgesia with opioids reduced the consumption of analgesics in the postoperative period. An improvement of analgesia was also observed in the immediate postoperative period, albeit not in the long term-Safe strategies, with no significant adverse events | 1b; A |
| - Preventive analgesia with corticoids seems to reduce both pain and opioid consumption in the postoperative period at 48h, but not after 6 months or 1 year | 2a; B |
| - Preventive analgesia with pregabalin seemed to reduce neuropathic pain in the short and long term- There is insufficient evidence to determine the effect on postoperative nociceptive pain and consumption of opioids- Preventive analgesia with gabapentin did not influence postoperative pain | 2b; B |
| - The use of ketamine and magnesium sulfate during analgesic induction seemed to have a beneficial effect on pain control and opioid consumption in the immediate postoperative period | 1b; A |
| - There is insufficient evidence to determine the effect of preventive analgesia with nimodipine, duloxetine and clonidine | 2b; B |
| - Preventive analgesia with combinations of various analgesics reduced pain and the need for analgesics after surgery | 2b; B |
| - There is insufficient evidence to recommend a specific strategy for preventive analgesia or administration protocol. However, it seems that administration of one or more analgesics at some point during the preoperative process reduces postoperative pain and consumption of analgesics | 2b; B |
LE: level of evidence; RG: recommendation grade.
The epidemiological data of the study populations are shown in Table 1. The patients were adults with a mean age over 50 years, with a slightly higher proportion of females, who presented moderate to severe pain ≥4 in the analog visual scale. Postoperative pain was mainly assessed through an analog visual scale. In general, the quality of the studies was moderate, with some isolated studies of higher quality but with a small “n”.
There was considerable variability in terms of the drugs used, which included paracetamol, non-selective NSAIDs, Cox-2 (Coxib) selective NSAIDs, opioids, corticoids, antidepressants, anticonvulsants and others, such as magnesium sulfate, ketamine, nimodipine and clonidine.
The preventive analgesia strategy was also highly variable. We included one study which defined “preventive” administration as that administered in the 2 weeks prior to the surgery,11 but most of the studies referred to analgesia administered 24–48h prior to the intervention or at the time of inducing anesthesia. In many studies, the strategy not only consisted preoperative administration, but also included analgesic treatment during the surgery and/or in the following hours.
Overall, we observed that, in most studies, preventive analgesia decreased postoperative pain and the intake of opioids during the first hours after surgery. Below are the results obtained, grouped by analgesia strategy.
Preventive treatment with NSAIDsIn general, the administration of NSAIDs as a preventive strategy was effective and seemed to decrease both pain and consumption of opioids in the immediate postoperative period. Coxib presented the advantage of not causing alterations in platelet aggregation, which did not alter the rate of hemorrhagic complications.
The use of ibuprofen11 2 weeks prior to the surgery was not found to influence pain control in the postoperative period. A single dose of diclofenac and ketorolac12,13 before inducing anesthesia decreased the consumption of morphine and its secondary effects compared to placebo in the first 24h after the intervention. On the other hand, dexketoprofen14 administered 24h prior to the surgery and up to 48h after the intervention improved pain at 15h and decreased the consumption of opioids.
A total of five studies used celecoxib preventively,15–19 administered between 1h and 2 weeks before surgery. The result was a decrease in pain and consumption of opioids in the immediate postoperative period (the first 72h). Long-term results were not as consistent. One study17 which used celecoxib 1h before and for 3 weeks after the surgery observed an improvement of postoperative pain compared to placebo up to 28 days later. The use of tenoxicam20 1h before the intervention did not influence pain improvement and consumption of opioids in the short term. After 9 postoperative days, despite no differences in pain compared to placebo being observed, the consumption of opioids was lower among the treated group.
The effect of etoricoxib 1 and 2h prior to the surgery was also assessed,21 and a decrease in the levels of interleukin-6 and prostaglandin E-2 in the blood compared to the control group was observed, as well as a lower level of pain, which was maintained until the third or fourth day. The consumption of opioids was higher among the control group during the first postoperative 12h. The use of rofecoxib22–24 1h before the intervention decreased the length of hospital admission and improved postoperative pain and analgesic intake after 2 days. These results were not maintained after discharge.
It was observed that lornoxicam25 at high doses 15min before the surgery and at low doses in the postoperative period decreased the consumption of opioids, but this was not associated to a clear decrease of postoperative pain. Parecoxib16,26 during induction of anesthesia and 12h after it improved postoperative pain and decreased the intake of morphine. The analgesic effects were still evident after 24h when two injections separated by 12h were administered.
Preventive analgesia strategies with NSAIDs were safe, except for an increase of perioperative bleeding, which was observed with ibuprofen11 and ketorolac.13 This effect was not observed with parecoxib16,26 and celecoxib.15,17–19
Preventive treatment with opioidsIn general, preventive administration of opioids is safe and decreases the consumption of opioids in the immediate postoperative period. Several studies also reported an improvement in immediate postoperative analgesia. There are no data regarding their long-term effects.
Several studies have used morphine21,27–32 in oral, intravenous and intramuscular formulations applied from 1h before the surgery up to the moment of anesthetic induction and have observed a decrease in the levels of pain and consumption of opioids during the first postoperative hours, except in the case of a low quality study,27 in which the pain increased among patients treated with oral morphine 1h prior to the surgery.
The use of oxycodone18,19 was assessed in two low-quality studies and obtained contradictory results both for pain control and opioid intake in the postoperative period. The use of methadone33 just after the induction and before the intervention decreased the postoperative requirement for analgesia.
Preventive treatment with corticoidsIn general, preventive use of corticoids seems to decrease both pain and opioid consumption in the postoperative period at 48h, but not after 6 months or 1 year.
The use of dexamethasone34–36 and methylprednisolone37,38 were also assessed. One low-quality study36 used dexamethasone during anesthetic induction and the results, assessed at 6 months and 1 year, showed no improvement in pain and opioid consumption. Another study, in which dexamethasone was used minutes before surgery,34 reported an improvement of dynamic pain during rehabilitation and a decrease in opioid consumption during the postoperative period, albeit with no effect on pain at rest. One study35 associated dexamethasone with gabapentin and ketamine in a multimodal analgesia protocol and observed an improvement in the levels of postoperative pain, but no decrease in opioid intake. Two studies26,27 reported that the use of methylprednisolone prior to surgery improved postoperative pain at 48h and decreased opioid intake.
Preventive treatment with anticonvulsantsIn general, preventive administration of pregabalin seemed to decrease neuropathic pain and opioid consumption in the immediate and long-term postoperative period. However, there is not enough evidence of its effect on postoperative nociceptive pain. Its secondary effects could represent a limitation. There is not enough conclusive evidence about gabapentin.
One study39 assessed neuropathic pain following knee arthroplasty after administering pregabalin 24h before the surgery and 2 weeks after the intervention and reported a decrease in neuropathic pain up to 6 months after the surgery, as well as reduced intrahospital consumption of opioids, shorter hospital stay and increased range of movement during the first 30 days of rehabilitation (compared to placebo). However, the secondary effects, particularly somnolence and obnubilation, were more pronounced with pregabalin.
Gabapentin has been used on its own and as part of multimodal analgesia protocols. Isolated gabapentin administered 2h prior to the surgery was used in two studies.40,41 The first of them40 concluded that it did not decrease the level of pain and opioid consumption compared to placebo in the immediate postoperative period and after 6 months, whereas the second study41 did observe a reduced consumption of opioids among patients treated with gabapentin. However, the latter was a low-quality study, with a short follow-up period. Gabapentin35 used as part of a multimodal analgesia protocol including dexamethasone, ketamine and NSAIDs improved postoperative pain with no differences regarding opioid intake compared to the use of isolated NSAIDs.
Preventive treatment with other drugsThe use of ketamine and magnesium sulfate during induction of anesthesia seemed to have a beneficial effect on pain control and opioid intake during the immediate postoperative period. There is insufficient evidence about nimodipine, duloxetine and clonidine as a preventive strategy for postoperative analgesia and opioid consumption.
Ketamine administered during anesthetic induction could have a preventive effect. One study42 administered it on its own and reported that, although no variations in the level of pain were observed during the first 48h after the intervention, there was a decrease in opioid intake and knee flexion was recovered faster. Another study31 associated ketamine and morphine and reported an improvement in postoperative analgesia.
The use of magnesium sulfate43 in anesthetic induction reduced postoperative pain and opioid consumption during the first 48h after the intervention. Nimodipine44 administered 1h prior to the surgery and for 48h in the postoperative period did not reduce the level of pain and increased the use of morphine 12h after the surgery. On the other hand, the use of duloxetine45 2h before the surgery and on the morning after did not change postoperative pain, but decreased the use of opioids during hospital admission. Clonidine46 administered 1.5h before the surgery, and 12 and 24h after the initial dose, did not improve postoperative pain, but reduced the use of morphine.
Preventive treatment with various interventionsIt seems that the use of combinations of several analgesics as a preventive strategy has a beneficial effect and reduces postoperative pain and analgesic requirements.
One study47 using a preoperative protocol with rofecoxib, tramadol, paracetamol and dexamethasone and a postoperative combination of rofecoxib, tramadol, paracetamol, hydrocodone and opioids reported a significant reduction in the level of pain compared to placebo.
It has not been proven that adding anesthetic blocks (femoral nerve) to oxycodone and celecoxib (2–12h before the surgery) improved postoperative analgesia up to 12 weeks.18 The preventive use of oxycodone and celecoxib or valdecoxib from the time of surgery and in the postoperative period decreased the consumption of opioids, nausea, vomiting and the length of hospital admission.19
Furthermore, the preoperative use of gabapentin, ketamine and dexamethasone, combined with paracetamol and ketorolac improved postoperative pain compared to the use of paracetamol and ketorolac alone. There were no differences regarding morphine consumption.35 The use of ketamine with morphine and epidural anesthesia with lidocaine prior to the intervention provided better postoperative analgesia compared to general anesthesia.31
DiscussionPreventive analgesia refers to treatments started on the days before the intervention or during anesthetic induction with the objective of reducing pain and drug intake in the postoperative period. This strategy is particularly relevant among patients undergoing hip and knee arthroplasty, as these are aggressive procedures with extensive tissue damage which are usually performed on patients with previously established chronic pain.48
There are no clinical guidelines which determine the most adequate medication and pattern. In a similar review to the present one which included various types of interventions, Buvanendran49 highlighted that NSAIDs had consistently proven their capacity to reduce postoperative pain levels and opioid consumption. On the other hand, the study also recommended the preoperative use of gabapentin and local anesthetic during surgery as part of multimodal analgesia protocols. NSAIDs proved their effectiveness in a metaanalysis conducted by Ong50 which evaluated studies including all types of surgeries.
This systematic literature review analyzed the effectiveness and safety of drug treatments proposed in different studies, as well as the patterns described for patients undergoing knee or hip arthroplasty.
There are no homogeneous, high-quality studies providing sufficient evidence to recommend a specific preventive strategy or administration protocol. However, it seems that the administration of one or several analgesics at some point during the preoperative process reduces the consumption of analgesics and pain during the postoperative period.18,19
Both traditional11–14 and Cox-215–17,20–23,25,26 NSAIDs, as well as opioids27–33 and corticoids34,37,38 have been shown to be effective. The use of neuroleptics39,41 could have some beneficial effect on the management of postoperative neuropathic pain, with no evidence of its effect on nociceptive pain.35,40 The use of ketamine42 and magnesium sulfate43 during anesthetic induction seems to have a beneficial effect. There is insufficient evidence to determine the effects of nimodipine,44 duloxetine45 and clonidine46 as part of a preventive strategy. It appears that using combinations of several analgesics as part of a preventive strategy decreases pain and the need for postoperative analgesics.19,35,47
As a limitation of our review, we could highlight that the considerable variability of drugs and preventive strategies described in the literature have notably hindered a generalization of results. We also observed the presence of various different types of bias which affected the validity of numerous studies, therefore conditioning the reproducibility of results. However, the epidemiological characteristics of the population included in the studies selected were similar to those of patients in whom knee or hip arthroplasty is indicated in our country.51 Another limitation of the present study is that it only conducts a systematic review, without delving into a metaanalysis enabling the results of the different studies to be synthesized and thus allowing a better assessment of the strength of the treatments. However, the wide variability in drugs and doses used in the studies made this approach unfeasible.
In conclusion, there is insufficient evidence to recommend a specific preventive strategy or administration protocol. However, we believe that administration of Cox-2 and/or opioids during the weeks prior to the intervention, associated to the use of drugs such as corticoids, ketamine and magnesium sulfate during anesthetic induction could be safe and effective strategies for the management of postoperative pain in patients undergoing hip or knee arthroplasty.
FundingThe present article obtained funding from MSD. MSD did not participate in the definition of the topic, development of the review, conclusions and drafting of the work.
Conflict of interestDr. Díaz Heredia declares a conflict of interest directly related to the present original: “I have received payment from MSD as consultant to elaborate the present work”, as well as not directly related to the present original: “I have received payment for teaching activities from the following companies: Biomet, Grunenthal, MSD, Pfizer, Smith and Nephew; I have received financing for research projects from the following companies: Biomet, Grunenthal and MSD”.
Dr. Loza Santamaría declares a conflict of interest directly related to the present original: “I have received payment from MSD as methodologist to conduct the present study”, as well as not directly related to the present original: “I have received financing for research projects from the following companies: Pfizer, Roche, Abbvie, Novartis and MSD”.
Dr. Cebreiro declares a conflict of interest directly related to the present original: “I have received payment from MSD as consultant to elaborate the present study”.
Dr. Ruiz Iban declares a conflict of interest directly related to the present original: “I have received payment from MSD as consultant to elaborate the present study”, as well as not directly related to the present original: “I have received payment as a consultant from the following companies: Biomet, Bristol-Myers Squibb, Grunenthal, MSD; I have received payment for teaching activities from the following companies: Astelas, Biomet, Bristol-Myers Squibb, Grunenthal, MSD, Pfizer, Smith and Nephew and Zambon; I have received financing for research projects from the following companies: Biomet, Grunenthal and MSD”.
Level of evidenceLevel of evidence II.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that no patient data appear in this article.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
The authors wish to thank Cristina Benitez and Eduardo Junco for their contribution to the development of the systematic review.
The authors also wish to thank Merck Sharp and Dohme for their funding to conduct the present systematic review.
Please cite this article as: Díaz-Heredia J, Loza E, Cebreiro I, Ruiz Iban MÁ. Analgesia preventiva en artroplastia de cadera o rodilla: una revisión sistemática. Rev Esp Cir Ortop Traumatol. 2015;59:73–90.